Abstract
Background. Injecting drug use (IDU) may lead to exposure to a range of carcinogenic agents. We investigated the risk and distribution of cancers among individuals with a history of IDU in Sweden. Material and Methods. The cancer incidence in a cohort of longitudinally followed participants in a needle exchange program (NEP), recruited between 1987 and 2007, was compared to that in the Swedish general population, matching for age group and gender. Baseline demographic and drug use data were collected and longitudinal testing of serological markers for HIV, hepatitis B and C virus was performed during NEP participation. Standardized incidence ratios (SIR) for types of cancer found in the study cohort were calculated, using data from the Swedish National Cancer Registry for reference. Results. The mean follow-up time for the 3255 participants was 11.8 years, constituting 38 419 person years at risk. The mean age at end of follow-up was 42.7 years, and 75% of participants were men. Seventy-eight cases of cancer were observed (SIR 1.1 [95% CI = 0.9–1.4]). The SIR was significantly increased for five cancer types among men; primary liver, laryngeal, lung, oropharyngeal and non-melanoma skin cancer (respective SIR 12.8 [95% CI = 4.2–30.0], 9.2 [95% CI = 1.9–26.8], 3.2 [95% CI = 1.5–6.1], 7.3 [95% CI = 1.5–21.2], and 3.5 [95% CI = 1.1–8.2]), and for cancers of endocrine organs among women (5.3 [95% CI = 1.7–12.4]). Conclusion. Although the standardized overall cancer incidence in this relatively young IDU cohort was similar to that in the general population, the risk of specific types of cancer was significantly increased, suggesting that IDU confers elevated risks for certain malignancies. These findings prompt further studies to investigate causative factors and suggest the need for surveillance among persons with a history of IDU.
The etiology of cancer is complex and a multitude of factors, both genetic and environmental, may be involved. Injection drug users (IDUs) are at risk of frequent exposure to carcinogens, in particular toxic substances in narcotic drugs and blood-borne viruses (HIV, hepatitis B and hepatitis C viruses) [Citation1–3]. Furthermore, environmental factors known to be associated with cancer, such as tobacco, alcohol and micronutrient deficiencies, may be more common in IDUs [Citation4,Citation5]. The distribution of cancer types occurring with higher frequency among IDUs could also suggest the presence of hitherto unknown mechanisms in carcinogenesis, and direct targeted investigations for causative agents.
Few studies have examined the cancer risk related to injection drug use (IDU), and most have focused on cancer among HIV-infected IDUs [Citation1,Citation6]. Since IDUs are at an overall increased risk of death, it is possible that any biological impact of IDU on cancer development could be obscured by premature death from other causes, such as trauma, intoxication and acute infections [Citation7–9]. Detection of cancer among IDUs may also be reduced, due to lower access to screening and diagnostic investigations for suspected cancer than in the general population [Citation10].
We hypothesized that IDU could predispose to a long-term increased risk of cancer. Cancer incidence among persons exposed to IDU is difficult to investigate due to the lack of representative cohorts that may be followed over time. In this study we have compared the overall incidence of cancer and the distribution of cancer types among Swedish IDUs with those in the Swedish general population, matching for age group and gender. For this purpose, we have followed an unselected cohort of IDUs participating in a needle exchange program (NEP), using national identity numbers for linking to national registries.
Setting
The Malmö NEP was initiated in 1987 as a targeted intervention to prevent HIV transmission, and provides clean needles and syringes to active IDUs, as well as a package of medical and social services. It is based in the Department of Infectious Diseases, Skåne University Hospital, Malmö, the third largest city in Sweden with 286 000 inhabitants. All services are provided free of charge. Criteria for participation in the NEP are age above 20 years and signs of venepuncture as evidence of ongoing IDU. The number of heavy drug addicts (mostly IDUs) in Malmö was estimated to be around 1600 in 1998 [Citation11]. In that year 1136 persons visited the NEP, suggesting that about 70% of the heavy drug addicts in Malmö are registered in the NEP.
At registration, a structured interview is conducted by an experienced nurse or staff nurse and a detailed questionnaire concerning personal data, drug habits and social conditions is completed. For serosurveillance purposes, blood samples are obtained at approximately three monthly intervals. Participants are longitudinally tested for serological markers of HIV, hepatitis B and C virus (HBV and HCV) for as long as they remain negative for the respective infection. Testing for HIV is required for participation, but can be done under code. In contrast, testing for viral hepatitis is voluntary, but must be done under national identity number. All collected data is coded and entered into a computerized database.
For this study, all participants registered with correct national identity numbers in the NEP between 1987 and 2007 were included. No exclusion criteria were applied.
Methods
The NEP database was first matched with a national death and emigration registry from Statistics Sweden to obtain dates for possible deaths or emigrations from Sweden among the participants. The database was then matched to the National Cancer Registry from the National Board of Health and Welfare.
The National Cancer Registry is based on the registration of malignant tumors, and 99% of the reported tumors are histopathologically confirmed. The most recent assessment of the Swedish cancer registry, published in 2009, reported a high degree of coverage of malignant tumors in Sweden [Citation12]. The data obtained from these registries included all registrations until 31 December 2007. The seventh and ninth International Classification of Diseases (ICD-7 and ICD-9) were used for categorization of different cancer types.
To calculate standardized incidence ratios (SIR) for different cancer types found in the study cohort, a method using person-years at risk was chosen. SIRs were calculated for all cancer types found in the IDU cohort. The time period a participant in the NEP was considered to be at risk for cancer was from the year of entry in the NEP until the year of death, emigration, or the end of study follow-up. From the National Board of Health and Welfare gender, age and calendar year specific cancer incidence data were obtained for all reported cancers in Sweden. To avoid influence of natural fluctuations in incidence during the follow-up period the mean incidence from 1988 until 2007 was used.
Data on HIV, HBV and HCV serological markers was obtained from the available test results in each participant case record.
Statistical analysis
The data was divided into five year age groups starting from 10 years old to 79 years old. For each cancer type diagnosed in the cohort an expected number was computed based on the mean incidence data mentioned above. The 95% confidence intervals (CI) for the SIR were determined assuming Poisson distribution for the observed cases [Citation13]. For the statistical analysis and matching of the database to the different registries SPSS statistics 17.0 (IBM corporation) and Microsoft Office Excel 2007 (Microsoft) were used.
Ethics
The study was approved by the Ethics Committee at the University of Lund. All information in the database that could be used to identify individual subjects, including the national identity numbers, were removed prior to statistical analysis.
Results
Study population
The database from the NEP contained 4139 individuals during the study period. National identity numbers were missing from 882 subjects, and two individuals appeared in the death and emigration registries prior to their enrollment in the NEP. Thus, a total of 3255 individuals (79%) could be included (). Comparing several baseline variables among the excluded individuals with those included in the study revealed minor non-significant differences between the two groups, with the exception that participants without accessible national identity number were, to a large extent, one-time visitors in the program (). The mean age of the study participants at the end of follow-up was 42.7 years. Male and female participants had similar age distributions, but the median age of women at the end of follow-up was lower than that of male participants (41 vs. 44 years) ().
Figure 1. Flow diagram of the potential participants in the study, reasons for exclusion and outcomes for the included participants.
Figure 2. Box plots showing the age distribution among study participants, divided into male and female.
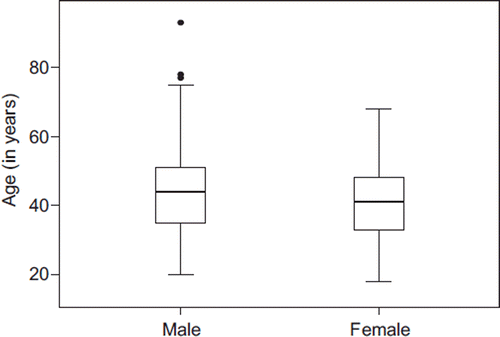
Table I. Baseline characteristics of needle-exchange program participants.
Standardized incidence ratios for cancer among IDUs
The follow-up time at risk for the entire cohort was 38 419 years, yielding a mean follow-up time of 11.8 years. Ninety cases of cancer were detected, 12 of which had been registered prior to the year of entry in the NEP, resulting in a total of 78 cases of cancer for SIR analysis (). The mean age at cancer diagnosis was 49.7 years (men 50.8 years; women 47.2 years).
Table II. Observed and expected number of cancer cases, standardized incidence ratios, and 95% confidence intervals among 3255 injecting drug users in a needle exchange program in Malmö, Sweden (follow-up until 12 December 2007).
The overall cancer incidence in the NEP cohort was not significantly increased (SIR 1.1 [95% CI = 0.9–1.4]). However, among male participants, the SIR was increased for primary liver cancer (12.8 [95% CI = 4.3–30.0]), laryngeal cancer (9.2 [95% CI = 1.9–26.8]), cancers of trachea, bronchus and lung (3.2 [95% CI = 1.5–6.1]), oropharyngeal cancer (7.3 [95% CI = 1.5–21.2]) and non-melanoma skin cancer (3.5 [95% CI = 1.1–8.2]). Among women, a significantly increased SIR was found for cancers of endocrine organs (5.3 [95% CI = 1.7–12.4]. This group included two thyroid cancers, one parathyroid cancer, one cancer of the pituitary gland and one cancer of the aortic body.
Serological results for HIV, HBV and HCV are presented in . HIV prevalence has remained low among participants, whereas a majority of IDUs have markers of HCV infection [Citation14,Citation15]. Data on HBV markers were missing from several participants.
Table III. Prevalence of serological markers for HIV, HBV and HCV.
Discussion
Although an overall increased risk of cancer was not detected among injection drug users in this longitudinal study, we did observe elevated risks for certain types of cancer with regard to gender. Men with a history of IDU were at higher risk for developing cancers of the liver, respiratory tract and skin, whereas an increased risk of cancers in various endocrine organs was found among women.
The reason for the gender differences observed could depend on gender-specific risk exposures or biological factors. Furthermore, the small number of female participants could explain why the high SIRs for certain cancer types failed to reach statistical significance.
HIV infection is common in many IDU populations, and has strong associations with the development of several types of cancer [Citation16], but its impact in our cohort was negligible. This is in contrast to most other published studies on this subject, which have focused on the specific role of HIV in this context [Citation1].
The association between hepatocellular carcinoma (HCC) and chronic viral hepatitis is well established [Citation17]. Since exposure to HCV was nearly universal in our cohort (87%) and data on HBV markers were lacking from 28% of participants, we were unable to determine particular risk factors for HCC development. In a recent Swedish study investigating the incidence of HCC among persons notified with hepatitis C in Sweden between 1990–2004, the reported SIR was higher than in our cohort (35 [95% CI of 31–40]) [Citation3]. The most common route of infection reported for HCV-infected subjects in that study was IDU (57%), and the mean age at diagnosis was 60 years. In our cohort, the corresponding age was 42 years, suggesting that the incidence of HCC is likely to rise over time with aging of the cohort, in accordance with recent observations from cohorts of veterans in USA [Citation18]. Similar phenomena might occur for other types of malignancies associated with HCV, such as lymphoproliferative malignancies [Citation19].
We also detected increased SIRs among men for oropharyngeal, laryngeal and lung cancers. An increased risk of lung cancer was observed among French and Italian IDUs, both for those with and without HIV infection [Citation1], and an association between cannabis use and lung cancer has been reported [Citation20]. Cigarette smoking – a recognized risk factor for these malignancies [Citation21,Citation22] – was reported at baseline by 97% of participants in our cohort from whom this information was available. We did not have access to accurate data on alcohol use during follow-up, which could contribute to the increased risk of oropharyngeal carcinoma [Citation23]. The presence of potential carcinogenic substances in narcotic drugs could account for an increased cancer incidence among persons exposed to IDU; however, scientific information on this is extremely scarce [Citation20].
Interestingly, several of the cancer types with elevated SIR in this cohort, such as non-melanoma skin cancer, oropharyngeal cancer and cervical cancer, are known to be associated with human papillomavirus (HPV) infection [Citation24,Citation25], although an increased risk of HPV has not been clearly linked to IDU.
Our finding of an increased incidence of various cancers of endocrine organs among women has not been reported previously; the reasons for this are unknown and merit further investigation.
Our study was limited by a relatively small cohort size, with a low proportion of women. The real risk of cancer related to IDU may have been underestimated both due to premature death from drug-related causes, and a mean follow-up time of 11.8 years, which could have been insufficient to detect differences in incidence for other types of cancer. For these reasons, we were unable to investigate risk factors for cancer development among participants and to perform stratification relating to age group and duration of IDU.
All IDUs in our uptake area were not included in the cohort, which therefore might not be fully representative of this population. However, we believe that the design of this study has advantages, by using unselected inclusion of participants during active IDU with longitudinal follow-up and linking to high-quality national registers. IDUs may be of special relevance for studying cancer incidence and identification of potential carcinogenic agents – both toxic substances, as well as unrecognized blood-borne viruses that may be implicated in carcinogenesis [Citation24]. The linking of data from the National Cancer Registry with participant data from NEP demonstrate a new and promising way to further assess the long-term risk of cancer related to IDU exposure.
Acknowledgements
Supported by an ALF grant from the Medical Faculty of Lund University, and from donation grants from Malmö Cancer Foundation and Alfred Österlund Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Serraino D, Boschini A, Carrieri P, Pradier C, Dorrucci M, Maso LD, . Cancer risk among men with, or at risk of, HIV infection in southern Europe. AIDS 2000;14:553–9.
- Lehman EM, Wilson ML. Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: A systematic review and meta-analysis. Int J Cancer 2009;124:690–7.
- Strauss R, Torner A, Duberg A-S, Hultcrantz R, Ekdahl K. Hepatocellular carcinoma and other primary liver cancers in hepatitis C patients in Sweden – a low endemic country. J Viral Hepat 2008;15:531–7.
- Campbell JV, Hagan H, Latka MH, Garfein RS, Golub ET, Coady MH, . High prevalence of alcohol use among hepatitis C virus antibody positive injection drug users in three US cities. Drug Alcohol Depend 2006;81:259–65.
- Trump DL, Johnson CS, Giovannucci E. The epidemiology of vitamin D and cancer risk. In: Vitamin D and cancer. New York: Springer; 2011. pp. 73–97.
- Dal Maso L, Franceschi S, Polesel J, Braga C, Piselli P, Crocetti E, . Risk of cancer in persons with AIDS in Italy, 1985–1998. Br J Cancer 2003;89:94–100.
- Jonsson AK, Holmgren P, Druid H, Ahlner J. Cause of death and drug use pattern in deceased drug addicts in Sweden, 2002–2003. Forensic Sci Int 2007;169:101–7.
- Bargagli AM, Hickman M, Davoli M, Perucci CA, Schifano P, Buster M, . Drug-related mortality and its impact on adult mortality in eight European countries. Eur J Public Health 2006;16:198–202.
- Miller C, Kerr T, Strathdee S, Li K, Wood E. Factors associated with premature mortality among young injection drug users in Vancouver. Harm Reduct J 2007;4:1.
- McKnight B, McKnight I, Kerr T, Li K, Montaner J, Wood E. Prevalence and correlates of cervical cancer screening among injection drug users. J Obstet Gynaeocol Can 2006; 28: 695–9.
- Olsson B, Adamsson-Wahren C, Byqvist S. Det tunga narkotikamissbrukets omfattning i Sverige 1998 (MAX-projektet, delrapport 3 Nr 61) (The extent of heavy drug abuse in Sweden 1998 [MAX-project, report 3 Nr 61]). Stockholm: Centralförbundet för alkohol- och narkotikaupplysning (CAN); 2001.
- Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register – a sample survey for year 1998. Acta Oncol 2009;48:27–33.
- Lidell FDK. Simple exact analysis of the standardised mortality ratio. J Epidemiol Community Health 1984;38:85–8.
- Månsson A-S, Moestrup T, Nordenfelt E. Continued transmission of Hepatitis B and C viruses, but no transmission of human immunodeficiency virus among intravenous drug users participating in a syringe/needle exchange program. Scand J Infect Dis 2000;32:253–8.
- Alanko Blomé M, Björkman P, Flamholc L, Jacobsson H, Molnegren V, Widell A. Minimal transmission of HIV despite persistently high transmission of hepatitis C virus in a Swedish needle exchange program. J Viral Hepat Epub 2010.
- Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, . Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008;148: 728–36.
- Widell A, Verbaan H, Wejstål R, Kaczynski J, Kidd-Ljunggren K, Wallerstedt S. Hepatocellular carcinoma in Sweden: Its association with viral hepatitis, especially with Hepatitis C viral genotypes. Scand J Infect Dis 2000;32:147–52.
- Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, . Increasing prevalence of HCC and cirrhosis in patients with chronic Hepatitis C virus infection. Gastroenterology 2011;140:1182–8.
- Duberg A-S, Nordstrom M, Torner A, Reichard O, Strauss R, Janzon R, . Non-Hodgkin’s lymphoma and other nonhepatic malignancies in Swedish patients with hepatitis C virus infection. Hepatology (Baltimore, Md.), 2005;41:652–9.
- Aldington S, Harwood M, Cox B, Weatherall M, Beckert L, Hansell A, . Cannabis use and risk of lung cancer: A case-control study. Eur Respir J 2008;31:280–6.
- Bosetti C, Gallus S, Franceschi S, Levi F, Bertuzzi M, Negri E, . Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer 2002;87:516–8.
- Kabat GC. Aspects of the epidemiology of lung cancer in smokers and nonsmokers in the United States. Lung Cancer 1996;15:1–20.
- Rodriguez T, Altieri A, Chatenoud L, Gallus S, Bosetti C, Negri E, . Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol 2004;40:207–13.
- McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 2008;1782:127–50.
- Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol 2005;32(Suppl 1):S59–66.
