Abstract
Background. Little is known about how well guidelines about adjuvant chemotherapy in colon cancer are followed in daily practice. We evaluated the current guideline, which is based on the MOSAIC trial, by examining implementation, treatment patterns and disease-free survival. Material and methods. We analysed a population-based cohort of 391 patients treated with adjuvant chemotherapy for stage III colon cancer in 2005–2006. Data were gathered from the Dutch Cancer Registry and medical records of 19 hospitals. Patients were classified according to whether or not they fulfilled MOSAIC trial eligibility criteria. Results. The administered regimens were: fluorouracil-leucovorin (17 patients), capecitabine (93), fluorouracil-leucovorin plus oxaliplatin (145), and capecitabine plus oxaliplatin (136). After its inclusion in national guidelines, oxaliplatin was prescribed in 16 hospitals within six months. Patients receiving oxaliplatin were younger and had less comorbidity than other patients. Dose schedules corresponded well with guidelines. Two-year disease-free survival probability of oxaliplatin patients meeting MOSAIC eligibility criteria was 78.4% (95% CI 72.5–84.3), which was comparable to MOSAIC trial results. Conclusion. Guidelines for adjuvant chemotherapy in stage III colon cancer are generally well followed in daily practice. However, uncertainty remains regarding the optimal treatment of elderly patients and patients with comorbidities, which underscores the need for practical clinical trials including these patients.
Colon and rectal cancers are the second most common cause of death in Western countries [Citation1]. Nearly half of the patients who undergo curative surgery will ultimately relapse and die of metastatic disease [Citation2]. During the 1990s the survival rates of patients with stage III colon cancer significantly improved by the introduction of adjuvant chemotherapy with 5-fluorouracil and leucovorin (5FU/LV) [Citation3].
As result of the publication of the MOSAIC trial in 2004, which demonstrated that adding oxaliplatin to 5FU/LV improved the adjuvant treatment, clinical practice guidelines in The Netherlands were changed early 2005 [Citation4]. National guidelines since then have recommended the use of six months of treatment with 5FU/LV combined with oxaliplatin (FOLFOX) as the primary treatment option for stage III and possibly high-risk stage II colon cancer patients. In addition, the oral fluoropyrimidine capecitabine was indicated for patients who are not eligible or who refuse treatment with oxaliplatin, based on the X-ACT trial [Citation5]. Also the use of capecitabine combined with oxaliplatin (CAPOX) as an alternative to FOLFOX was supported by the Dutch association for Medical Oncology (NVMO), as these treatments had shown comparable efficacy in metastatic colorectal cancer [Citation6–8]. Data on the efficacy of CAPOX in the adjuvant setting were not available at that time.
In light of more recent evidence from RCTs, this strategy proved to be valid and in line with the current international clinical practice guidelines [Citation9–12].
However, the nationwide level of implementation of the primarily RCT-based guidelines and its impact on population-based clinical outcomes is unknown. For instance, differences between RCTs and daily practice may exist in the patient selection criteria, dosing regimens, the use of supportive care, and the intensity of follow-up [Citation13]. Observational studies including detailed information on chemotherapy use in daily practice can complement findings from RCTs and allow post-implementation evaluation of guidelines [Citation14].
In our study we retrospectively analysed population- based data of stage III colon cancer patients treated with adjuvant chemotherapy in the first two years after the change of the Dutch clinical practice guideline. The aim was to examine the speed of guideline implementation and to compare the guideline to chemotherapy use in daily practice with respect to treatment choice, patient characteristics and dosage quantities. In addition, we compared the disease-free survival (DFS) outcomes of patients receiving FOLFOX and CAPOX in Dutch daily practice with the outcomes of patients receiving FOLFOX in the MOSAIC trial.
Methods
Data and cohort construction
The primary data source for the population-based study was The Netherlands Cancer Registry (NCR), which registers information on demographics, tumour characteristics and survival outcomes of more than 95% of all new cancer cases in The Netherlands. All stage III colon cancer patients (pTanyN1, 2M0, ICD-O C18-C19.9) who were diagnosed in 2005 or 2006, and who received adjuvant chemotherapy were identified in the NCR. To gather additional information, we approached 72% of the hospitals in The Netherlands and included the 19 first responding hospitals into our study. The medical files of all identified patients were reviewed in these 19 selected hospitals (three university hospitals, nine large teaching hospitals, and seven general hospitals dispersed over The Netherlands), which together were considered to be a good representation of clinical daily practice in The Netherlands. Data were collected on baseline characteristics, eligibility criteria used in the MOSAIC trial, treatment schedules and DFS. We recorded comorbid conditions using a slightly adapted version of the Charlson index, which classifies all serious comorbid conditions based on possible prognostic impact into eight groups (i.e. previous malignancies, chronic obstructive pulmonary diseases, cardiovascular diseases, cerebrovascular diseases, hypertension, diabetes mellitus, digestive tract diseases and other) [Citation15,Citation16]. Reasons for not prescribing oxaliplatin were also recorded. Additional information on treatment schedules, dose reduction, delay and/or interruptions of treatment and its reasons was recorded in a randomly selected subset of patients.
Statistical analyses
To check the representativeness of the 19 selected hospitals for The Netherlands, we first compared the average percentage of patients receiving adjuvant chemotherapy among hospitals included in our study versus other hospitals in The Netherlands by means of the Students t-test. Also their median age was compared. Next, we assessed the frequency of administration of treatment in the selected hospitals. Two groups of patients were created (“receiving oxaliplatin based regimens” and “receiving regimens without oxaliplatin”) and the baseline characteristics of these two groups were compared using the Students t-test for continuous variables and the χ2-test for dichotomous or nominal values. Next, we investigated the uptake of new treatments as recommended by the new guidelines by the hospitals. We used the Cochran-Armitage trend test to test for time trends in the use of different treatment regimens. Reasons for not prescribing oxaliplatin were explored using descriptive statistics. A multivariate logistic regression analysis was performed to identify independent predictors of non-prescription of oxaliplatin. Dose schedules and modifications were compared using the tests for continuous and categorical variables mentioned above. Subsequently, DFS was calculated from the date that chemotherapy started until relapse or death or censored on the date last known to be alive. Based on the published MOSAIC trial, patients receiving oxaliplatin were grouped into “fulfilling MOSAIC eligibility criteria” and “not fulfilling MOSAIC eligibility criteria” [Citation4]. Per group, the two-year DFS rate was estimated using the Kaplan-Meier method and compared to the two-year DFS rate of the stage III patients receiving oxaliplatin in the MOSAIC trial by means of the χ2-test. The MOSAIC two-year DFS rate and standard error were derived from the published Kaplan-Meier curve and number of patients at risk at 24 months [Citation11]. In all analyses, statistical significance was assumed if the two-tailed probability value was less than 0.05. The SAS computer package (version 8.2) was used for all statistical analyses (SAS Institute Inc., Cary, NC, USA, 1999).
Results
Patients and treatments
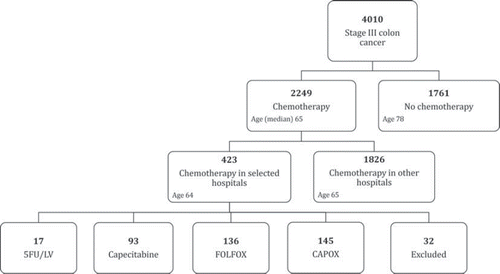
Between January 2005 and December 2006, 4010 patients were diagnosed with stage III colon cancer in The Netherlands, of whom 2249 were treated with adjuvant chemotherapy (). A total of 423 patients were treated at one of the 19 hospitals included in our study. The average percentage of patients receiving adjuvant chemotherapy in the 19 included hospitals was 53% versus 57% in 92 not included hospitals (p = 0.17). Also the median age of the selected versus non-selected patients was comparable (64 vs. 65 years). The four most commonly administered regimens were: 5FU/LV (17 patients), capecitabine (93), FOLFOX (145), and CAPOX (136). Five patients were excluded from further analysis: three patients received bevacizumab and two patients UFT as adjuvant chemotherapy (< 2%). Furthermore, 27 patients were excluded because of inclusion in clinical trials (16), diagnosis of a second malignancy in the past five years (9), and missing files (2).
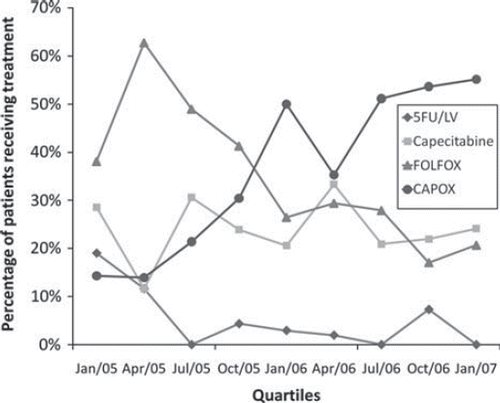
We observed a rapid adoption of oxaliplatin in the period shortly after Dutch national guidelines recommended it for the adjuvant treatment of colon cancer at the start of 2005 (). Of the 19 hospitals included in our survey, eight were already using oxaliplatin in the first quarter of 2005, followed by a total of 16 hospitals using oxaliplatin during the second quarter of 2005. By January 2006, oxaliplatin was standard therapy in all 19 hospitals. Furthermore, a significant trend from FOLFOX use to CAPOX use was observed between January 2005 and December 2006 (ptrend < 0.001). In the second quarter of 2005, 82% of the patients receiving oxaliplatin were treated with FOLFOX versus 18% with CAPOX. By the start of 2007 only 27% were being treated with FOLFOX versus 73% with CAPOX. However, despite the rapid adoption of oxaliplatin use on a hospital level, a substantial proportion of the patients did not receive oxaliplatin-based regimens. The percentage of patients not receiving oxaliplatin was 28% and this percentage did not change over time (ptrend = 0.77). Already since the first quarter of 2005, the majority of these patients were treated with capecitabine instead of 5FU/LV.
Figure 2. Distribution of regimen use from the first quartile of 2005 to the first quartile of 2007, by treatment group.
The baseline characteristics of the patients in the four treatment groups are summarised in . Patients receiving oxaliplatin were significantly younger (p < 0.0001) and had fewer comorbidities (p = 0.001) than patients who did not receive oxaliplatin. Furthermore, patients receiving oxaliplatin more often had well-differentiated tumour histology (p = 0.007) and higher serum carcinoembryonic antigen (CEA) levels (p = 0.028) than other patients. Additional stratification by age (older vs. younger than 70 years of age) revealed that these differences in tumour differentiation and CEA levels could be explained by the different age distribution in the two groups.
Table I. Baseline characteristics of stage III colon cancer patients receiving chemotherapy in Dutch daily clinical practice.
We next explored reasons why some patients did not receive oxaliplatin (111 patients). The reasons for non-prescription were: prescription not in line with hospital policy (18%), advanced age (21%), patient refusal (19%), comorbidity or poor health status (10%), specific contra-indications for oxaliplatin (2%), combination of these factors (7%), and unknown (23%). To identify independent predictors of non-prescription of oxaliplatin, we performed a multivariate logistic regression on baseline characteristics and included the variables age, presence of comorbid conditions, gender, depth of invasion of primary tumour (T-stage), lymph node involvement (N-stage), differentiation and serum CEA level. The multivariate analysis identified only age and comorbidity as being independent predictors of non-prescription of oxaliplatin (OR [95 CI] of 0.765 [0.708–0.826] and 0.426 [0.169–1.075], respectively).
Dose schedules
To evaluate dose schedules, additional data were collected from the medical records of a randomly selected subset of 206 patients. This selection was also stratified by hospital and oxaliplatin use to ensure equal numbers of patients that did and did not receive oxaliplatin.
provides an overview of the patterns of use of the different treatment regimens in daily practice. With six months of chemotherapy being accepted as the standard duration of adjuvant treatment, and a treatment cycle of two weeks for FOLFOX and three weeks for CAPOX and capecitabine, the median number of planned cycles was 12, eight and eight, for FOLFOX, CAPOX and capecitabine, respectively. The median number of cycles received equalled the planned number of cycles in FOLFOX and capecitabine, indicating that at least 50% of the patients completed the number of cycles that was expected according to the protocol. The median number of oxaliplatin cycles for patients receiving the CAPOX regimen was seven.
The planned dose for each regimen was equal to the dosing recommendations of the national guidelines. The mean dosages in milligrams per square metre per week across all cycles administered were slightly lower than the planned dosages. When we calculated the mean dose in the administered cycles as a percentage of the mean dose advised in the guidelines, we found that 83% of the recommended dose was given in capecitabine monotherapy and even 98% of the oxaliplatin in the FOLFOX and CAPOX regimens. However, regarding the mean dose over all planned cycles, we found that the mean dose of oxaliplatin in CAPOX was significantly lower than that in FOLFOX, with 30 mg/m2/wk vs. 36 mg/m2/wk, respectively (p = 0.0213). This also resulted in a significantly lower mean cumulative dose of oxaliplatin (CAPOX: 780 mg/m2 vs. FOLFOX: 936 mg/m2, p = 0.002). In total, 71% of the planned doses amongst CAPOX-treated patients were administered vs. 84% amongst FOLFOX-treated patients (p = 0.0896).
Table II. Planned and actually delivered dose in Dutch daily clinical practice.
Disease-free survival (DFS)
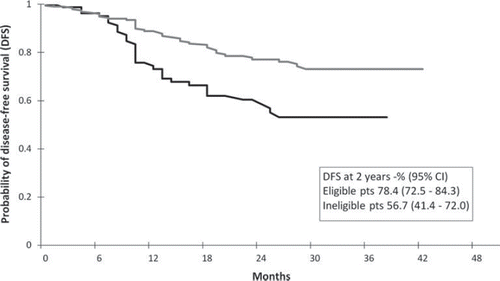
The recommendation to use oxaliplatin in the adjuvant treatment of stage III colon cancer in The Netherlands was based on the results of the MOSAIC trial. For this reason we compared the two-year DFS rates of the patients receiving oxaliplatin-based regimens in Dutch daily practice to the results of the MOSAIC trial.
Of the 281 patients receiving oxaliplatin-based regimens, 200 met the MOSAIC eligibility criteria, 43 did not and 38 could not be classified due to missing data. They were excluded from further analysis. Reasons for ineligibility were: chemotherapy treatment did not start within seven weeks after surgery (27 patients), CEA levels above 10 ng/ml (12), age older than 75 years (one), and combination of mentioned reasons (3). The two-year probability of DFS of eligible and ineligible patients was 78.4% (95% CI 72.5–84.3) and 56.7% (95% CI 41.4–72.0), respectively (). The published Kaplan-Meier curve for stage III patients receiving oxaliplatin in the MOSAIC trial showed a two-year DFS probability of 79.5% (95% CI 75.6–83.4). This probability was not significantly different from the two-year DFS of eligible oxaliplatin patients from Dutch daily practice (p = 0.32).
Discussion
In this study analysing population-based data from Dutch daily practice in 2005–2006, we evaluated the clinical practice guideline for the adjuvant treatment of stage III colon cancer.
When treatment with oxaliplatin, either in the FOLFOX or CAPOX regimen, became the new standard therapy in early 2005, we observed a quick implementation with the majority of the hospitals already using oxaliplatin in the second quarter of 2005. This rapid adoption is most likely due to the extensive experience that the physicians already had with oxaliplatin as an important treatment in advanced colorectal cancer [Citation6]. Over time, we found an increasing preference for the use of CAPOX over FOLFOX. This preference was probably due to the need for intravenous access devices in the administration of 5FU/LV in the FOLFOX regimen.
We also observed a rapid adoption of capecitabine monotherapy as an alternative to 5FU/LV. Like in oxaliplatin, this was most likely due to the extensive experience already obtained in advanced colorectal cancer and because of the ease of the oral capecitabine administration [Citation6].
The Dutch guideline does not specifically indicate who is eligible for oxaliplatin and who is not, leaving this decision up to the judgement of the physicians. We observed that physicians were reluctant to prescribe oxaliplatin to patients with advanced age or serious comorbidities. One other population-based study reported on prescription of oxaliplatin as adjuvant chemotherapy and found similar results [Citation17]. The median age of the patients not receiving oxaliplatin was 73, whereas it was 61 in patients receiving oxaliplatin in daily practice. The latter number equals the median age of the patients randomised in the MOSAIC trial suggesting that physicians considered the MOSAIC criteria when deciding on the prescription of oxaliplatin. Over the past years several studies have reported conflicting results regarding the efficacy and safety of oxaliplatin-based chemotherapy in older patients [Citation18–20]. As a consequence, uncertainty remains regarding the question whether the lower rates of oxaliplatin treatment we observed in older patients represent wise clinical judgement or undertreatment.
Specific guideline recommendations are also lacking regarding the eligibility for capecitabine or 5FU/LV without oxaliplatin as adjuvant treatment option. We observed that a substantial part of the stage III colon cancer population in The Netherlands did not receive any adjuvant chemotherapy in 2005 and 2006. They were older than patients receiving chemotherapy (median age 78). This finding is in line with results from studies that found that age and comorbidities were associated with the decision to prescribe 5FU/LV or no adjuvant therapy in stage III colon cancer [Citation21,Citation22]. However, since patients not receiving any adjuvant chemotherapy were not included for additional data collection, this paper provides no further insights in this elderly patient population.
In general, the observed dose schedules demonstrated a good adherence to existing guidelines. The mean dosages in milligrams per square metre per week across all cycles administered were only slightly lower than those recommended by the guidelines. However, more than half of the patients in all regimens also needed dose modifications resulting in lower total cumulative dosages than could theoretically have been administered. But even in trials the administered dose is usually lower than the planned dose. For FOLFOX, the dosages of oxaliplatin given in daily practice were similar to the dosages reported in the MOSAIC trial [Citation4]. Also for capecitabine monotherapy these dosages were similar in RCT findings [Citation8]. However, regarding CAPOX, patients in daily practice received on average 71% of the planned dose, whilst the literature reports this to be 87%, although part of this difference can probably be explained by the fact that the latter number reflects the median percentage rather than the average [Citation10]. Also when comparing the FOLFOX and CAPOX regimens in our study, we found a lower mean dose of oxaliplatin, more dose modifications and a lower percentage of planned dose given in de CAPOX regimen, all together suggesting that oxaliplatin is less well tolerated in CAPOX versus FOLFOX. A pooled analysis in advanced colorectal cancer also found that some toxicities were slightly but consistently more prominent in capecitabine containing regimens [Citation23]. However, dosage comparisons in our study need to be interpret with caution since the decision to select patients using random sampling that was stratified by hospital and oxaliplatin use, resulted in the selection of only 37 patients who received FOLFOX.
The DFS of the eligible patients receiving oxaliplatin was comparable to that of the MOSAIC patients receiving oxaliplatin. Our result supports the external validity of the MOSAIC trial results, which in general has been a matter of concern in RCTs [Citation13]. A limitation of our study here is that although we used the same definition of DFS as presented in the MOSAIC trial, we cannot guarantee that the same method was used in both studies. The estimated time to occurrence depends on the monitoring of the patients during follow-up which might have been less intense in daily practice as compared to trial monitoring. This might have resulted in a delayed diagnosis of relapse in daily practice. However, the proposed follow-up schedule in the Dutch guideline does not differ from the follow-up schedule followed in the MOSAIC trial. Moreover, we calculated that a delay of three months would not have an effect on the conclusion that the DFS was similar in both studies. Between 2005 and 2010, two other RCTs reported similar outcomes when using oxaliplatin in the adjuvant treatment of colon cancer [Citation9,Citation24].
Our finding of a decreased DFS in patients who did not meet the trial eligibility criteria underscores the fact that trial results should not be extrapolated to other patient categories in daily practice [Citation25]. The less favourable prognosis in non-eligible patients can mainly be explained by the significantly higher CEA values of these patients. Although it is uncertain whether adjuvant therapy with oxaliplatin has an added value here, it is unlikely that oxaliplatin would be unfavourable for this group of patients as oxaliplatin also plays an important role in the treatment of metastatic colon carcinoma.
In conclusion, our results point towards a quick nation-wide implementation of the stage III colon cancer clinical guideline after its change early 2005. We observed a good concordance of practice with the RCT-based treatment recommendations and similar DFS outcomes of trial eligible patients receiving oxaliplatin in daily practice vs. patients receiving oxaliplatin in RCTs. However, uncertainty regarding the optimal treatment for elderly patients or patients with serious comorbidities is still present today. The lack of specific guideline recommendations for this large and increasing patient population underscores that practical clinical trials for elderly patients with stage III colon cancer are needed.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
- Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum 1997;40:15–24.
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, . Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8.
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, . Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.
- Twelves C, Wong A, Nowacki MP, Abt M, Burris H, , 3rdCarrato A, . Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
- Punt CJ. New options and old dilemmas in the treatment of patients with advanced colorectal cancer. Ann Oncol 2004;15:1453–9.
- Shields AF, Zalupski MM, Marshall JL, Meropol NJ. Treatment of advanced colorectal carcinoma with oxaliplatin and capecitabine: A phase II trial. Cancer 2004;100:531–7.
- Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, . XELOX (capecitabine plus oxaliplatin]: Active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 2004;22:2084–91.
- Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, . Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol 2007;25:2198–204.
- Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J, . Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: A planned safety analysis in 1,864 patients. J Clin Oncol 2007;25: 102–9.
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, . Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC Trial. J Clin Oncol 2009;27:3109–16.
- Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A, ESMO Guidelines Working Group. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2010;21(Suppl 5): v70–7.
- Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet 2005;365:82–93.
- Silverman SL. From randomized controlled trials to observational studies. Am J Med 2009;122:114–20.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–83.
- Lemmens VE, van Halteren AH, Janssen-Heijnen ML, Vreugdenhil G, Repelaer van Driel OJ, Coebergh JW. Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands is affected by socioeconomic status, gender, and comorbidity. Ann Oncol 2005;16:767–72.
- Kahn KL, Adams JL, Weeks JC, Chrischilles EA, Schrag D, Ayanian JZ, . Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 2010;303:1037–45.
- Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, . Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
- McCleary JN, Meyerhardt J, Green E, Yothers G, de Gramont A, Van Cutsem E, . Impact of older age on the efficacy of newer adjuvant therapies in > 12,500 patients (pts) with stage II/III colon cancer: Findings from the ACCENT Database (abstract). J Clin Oncol 2009;27(170s).
- Haller DG, Cassidy J, Tabernero J, Maroun JA, De Braud FG, Price TJ, . Efficacy findings from a randomized phase III trial of capecitabine plus oxaliplatin verus bolus 5-FU/LV for stage III colon cancer (NO16968): No impact of age on disease-free survival (DFS). (abstract). Presented at the 2010 ASCO gastrointestinal cancers Symposium, Orlando, FL, January 22. 2010.
- Etzioni DA, El-Khoueiry AB, Beart RW, Jr. Rates and predictors of chemotherapy use for stage III colon cancer: A systematic review. Cancer 2008;113:3279–89.
- van Steenbergen LN, Rutten HJ, Creemers GJ, Pruijt JF, Coebergh JW, Lemmens VE. Large age and hospital-dependent variation in administration of adjuvant chemotherapy for stage III colon cancer in southern Netherlands. Ann Oncol 2010;21:1273–8.
- Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, . Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: A pooled analysis of randomized trials. J Clin Oncol 2008;26:5910–7.
- Haller D, Tabernero J, Maroun J, De Braud F, Price T, Van Cutsem E, . First efficacy findings from a randomized phase III trial of capecitabine + oxaliplatin vs. bolus 5-FU/LV for stage III colon cancer (NO16968/XELODA study). Presented at the joint ECCO/ESMO multidisciplinary congress, (abstr 5LBA), Berlin, 20–24 September, 2009.
- Mol L, Punt CJA, van Gils CWM, Ottevanger PB, Koopman M. Trial participation in a multicentre phase III trial (CAIRO) in advanced colorectal cancer patients in The Netherlands, and a comparison of outcome between trial and non-trial patients. (abstract 581PD). Presented at the 35th ESMO Congress Milan, Italy 8–12 October, 2010.