Abstract
Context: Tarragon [Artemisia dracunculus L. (Asteraceae)] is used as a commercial flavoring and in perfumery. In traditional folk medicine, tarragon has been used for treatment of pain and gastrointestinal disturbances.
Objective: This study investigated the antinociceptive effect of the essential oil of A. dracunculus (EOAD) in various experimental models.
Materials and methods: The median lethal dose (LD50) of EOAD was estimated using the method of Lorke. The antinociceptive effect was assessed using chemical (formalin and acetic acid) and thermal (hot-plate) nociceptive tests in rats and mice. In all experiments, EOAD was administered intraperitoneally at the doses of 10, 30, 100 and 300 mg/kg.
Results: In the acute toxicity test, the value of estimated LD50 for EOAD was 1250 mg/kg. EOAD (100 and 300 mg/kg) significantly reduced (p < 0.001) the pain response in the first (59.5 and 91.4%) and second (52.5 and 86.3%) phases of the formalin test, respectively. Central involvement in analgesic profile was confirmed by the hot-plate test, in which the EOAD showed a significant analgesic activity by increasing latency time. EOAD (10, 30, 100 and 300 mg/kg) significantly (p < 0.001) inhibited (89, 95, 97 and 97%) the nociception produced by acetic acid. Naloxone failed to antagonize the antinociceptive effect of the essential oil in the acetic acid-induced writhing test. It seems that mechanism(s) other than opioid receptors is (are) involved in the analgesic effect of EOAD.
Conclusions: This study reported the peripheral and central antinociceptive activity of the EOAD and rationalized the traditional use of the plant in the treatment of different painful conditions.
Introduction
Artemisia dracunculus L. (Asteraceae) is an herbaceous plant with a very wide distribution in Eurasia and North America. The herb is principally used as a seasoning for food and as a source of essential oil. Medicinally, aerial parts are used to treat symptoms of various digestive ailments and as an adjunctive therapy for the painful component of spasmodic colitis. Additional medicinal properties that are claimed for the plant include antiseptic, antipyretic, emmenagogue, anthelmintic, diuretic and calming effects (Deans & Simpson, Citation2002). The Paipai Indians of northern Baja California alleviate stomach ailments by drinking the herbal tea and in the San Luis Valley of Colorado and adjacent New Mexico, the dried plant is used to flavor “biscochitos” (sweet breads) and the infusion is drunk for stomachaches (Linares & Bye, Citation1987). In Iranian traditional medicine, this plant is used as a laxative, eupeptic, carminative, stomachic, antispasmodic, vermifuge, emmenagogue and to treat gastritis (Miraldi et al., Citation2001; Mosaddegh et al., Citation2012). The leaves are also used topically for relieving rheumatic pains (Zargari, Citation1992).
Various phytochemical and biological activity studies have been carried out with the plant. From aerial parts of A. dracunculus dihydroflavonols (Balza & Towers, Citation1984), alkamides (Saadali et al., Citation2001) and phenylpropanoids (Lopes-Lutz et al., Citation2008) have been isolated. The major components of the essential oil are menthol, anethole, anisole, anisic acid, d-sabinene, estragole, limonene, myrcene, ocimene, α-phellandrene, anisaldehyde, β-pinene, 1-methoxy-4-(2-propenyl)-benzene and 1R-α-pinene (Bora & Sharma, Citation2011).
Toxicological evaluation of Tarralin™, an ethanol extract of A. dracunculus, revealed that it is non-toxic, and no observed adverse effect level (NOAEL) for the 90-day study was considered to be 1000 mg/kg/d (Ribnicky et al., Citation2004). Antimicrobial (Benli et al., Citation2007), antifungal (Meepagala et al., Citation2002), antioxidant (Teixeira et al., Citation2013), antidiabetic (Eisenman et al., Citation2011; Scherp et al., Citation2012), antiplatelet (Shahriyary & Yazdanparast, Citation2007) and anticonvulsant (Sayyah et al., Citation2004) activities have been reported for A. dracunculus. This article reports the antinociceptive activity of the essential oil of A. dracunculus (EOAD) on which there are no previous studies.
Materials and methods
Drugs and chemicals
Morphine hydrochloride and naloxone hydrochloride were purchased from Sigma Chemical Co., St. Louis, MO. All chemicals, unless otherwise stated, were purchased from Merck, Darmstadt, Germany. All drugs were dissolved in saline. The essential oil was prepared in 1% v/v Tween 80 in sterile saline.
Plant material and essential oil
The aerial parts of A. dracunculus were purchased at the vegetative stage from a local market, near the city of Urmia, Iran, in August 2010. It was authenticated by Dr. Sh. Kazempour Osaloo, Department of Plant Biology, Tarbiat Modares University (Tehran, Iran). Voucher specimens (3647) were deposited in the herbarium of the Department of Medicinal and Industrial Plants, Urmia University. EOAD was extracted from the fresh aerial parts by the steam distillation method in a Clevenger-type apparatus. EOAD analysis was as previously described (Jalilzadeh-Amin et al., Citation2012).
Animals
Swiss albino mice of both sexes (23–28 g) and male Wistar rats (160–210 g) were used. Rats and mice were housed six per cage and maintained in a room temperature (22 ± 2 °C) and submitted to a 12 h light/dark cycle. The animals were left for 14 days for acclimatization to animal room conditions and were provided certified rodent diet and tap water ad libitum. Animals were acclimatized to the laboratory for at least 2 h before testing and were used once throughout the experiments. Groups of six animals were used in each test group, and control animals received vehicle only. All test solutions were administered in a volume of 10 ml/kg body weight (Luchese et al., Citation2010). All experiments reported in this study were carried out in accordance with current guidelines for the care of laboratory animals and ethical guidelines for investigation of experimental pain in conscious animals (Zimmermann, Citation1983). Prior to the biological experimentation on animals, the protocols were approved by the Institutional Animals Ethical Committee of the University.
Acute toxicity test
The intraperitoneal (i.p.) acute toxicity (lethal dose; LD50) of the EOAD was evaluated in mice (Lorke, Citation1983). The experiment was carried out in two phases; in the first phase, geometric doses of the EOAD (10, 100 and 1000 mg/kg) were administered i.p. to three groups of mice, and the control group received normal saline (10 ml/kg, i.p.). In the second phase, other doses of the EOAD (1600, 2900 and 5000 mg/kg) were administered. Signs of toxicity and mortality within 24 h were noted. The LD50 was then calculated based on the pattern of death observed in the second phase using the Probit-log analysis from the graph of percent mortality against log dose of the EOAD.
Antinociceptive tests
Formalin test
Rats were injected with 50 µl of 2.5% formalin (in normal saline) into the subplantar space of the right hind paw (Dubuission & Dennis, Citation1977). After injection, a mirror was positioned behind the cage and gave an unobstructed view of the hind-paw. Rats were then observed for 30 min, and the time spent licking the injected paw was recorded in two phases. The first phase, 0–5 min post-formalin injection, is known as the early phase, and the period between 15 and 30 min as the late phase. EOAD was injected intraperitoneally at doses of 10, 30, 100 and 300 mg/kg, 30 min before formalin injection in a volume of 10 ml/kg. Control animals received an equal volume of vehicle. Morphine (10 mg/kg, i.p.) pretreated animals were included in the study for comparison. The test was performed at room temperature of 22–26 °C and care was taken to exclude environmental disturbances (high temperature, noise and excessive movement) that might interfere with the animal’s response (Tjolsen et al., Citation1992).
Hot-plate test
The hot plate assay method was employed for the purpose of preferential assessment of possible centrally mediated analgesic effects of the EOAD. Rats were preselected on the hot-plate at 53 ± 0.1 °C; any showing a reaction time (latency for licking the hind feet or jumping) greater than 30 s were discarded (MacDonald et al., Citation1946). The rats were then treated with vehicle (1% Tween 80 in normal saline, 10 ml/kg, i.p.), graded doses of EOAD (10, 30, 100 and 300 mg/kg, i.p.) or morphine (10 mg/kg, i.p.). The reaction time(s) for each rat was determined on the hot-plate before and after drug administration at intervals of 30 min for a total period of 120 min. To avoid possible injury, a cut-off period of 60 s was followed while measuring the reaction time.
Acetic acid-induced writhing test
Acetic acid (0.6% v/v) was administered i.p. in a volume of 10 ml/kg (Koster et al., Citation1959). Vehicle (1% Tween 80 in normal saline, i.p.), morphine (10 mg/kg, i.p.) and EOAD (10, 30, 100 and 300 mg/kg, i.p.) were administered 30 min before acetic acid injection. The number of abdominal constrictions produced in each group for the succeeding 20 min was counted and compared to the response in the control group. Analgesia was calculated as the percentage inhibition of abdominal constriction.
To determine the possible participation of the opioid system in the antinociceptive effect, separate groups of EOAD (30, 100 and 300 mg/kg, i.p.) and morphine (10 mg/kg, i.p.) animals were pretreated with naloxone (2 mg/kg, s.c.) 10 min prior to acetic acid injection.
Statistical analysis
The data represent the mean ± SEM and were analyzed for statistical significance by one-way analysis of variance followed by Dunnett’s test. The minimum level of significance considered was p < 0.05.
Results
Acute toxicity tests of essential oil were done in mice. The i.p. LD50 of the EOAD in mice was calculated to be 1250 mg/kg. Administration of EOAD intraperitoneally in lower doses produced no mortality and visible signs of delayed toxicity 14 days post-treatment. Severe depression, abnormal gait, ataxia, increased respiration and decreased activity were observed at dose higher than 1000 mg/kg.
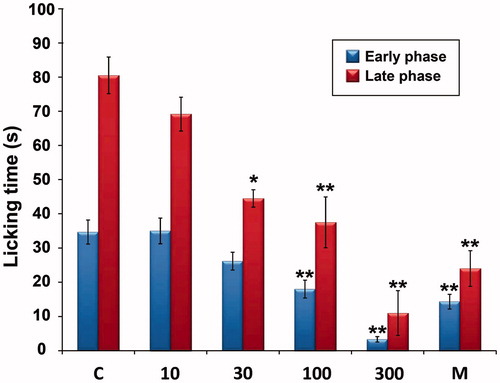
Subplantar injection of 2.5% formalin evoked a characteristic biphasic licking response. As shown in , the EOAD in doses of 100 and 300 mg/kg significantly reduced the licking time in both the early and late phases. At the dose of 30 mg/kg, the EOAD reduced only the second phase of the test. Whereas, morphine, as a standard drug, also inhibited both phases of formalin-induced pain.
Figure 1. Effect of the essential oil of A. dracunculus (EOAD) on formalin-induced nociception in rats. The total time spent in licking the injected hind-paw was measured in the early phase (0–5 min) and the late phase (15–30 min). The vehicle (C, 10 ml/kg), the EOAD (10, 30, 100 and 300 mg/kg) or morphine (M, 10 mg/kg) were administered intraperitoneally. Asterisks indicate significant difference from control. Each column represented the mean ± SEM, n = 6, **p < 0.001, *p < 0.01 (ANOVA followed by Dunnett’s test).
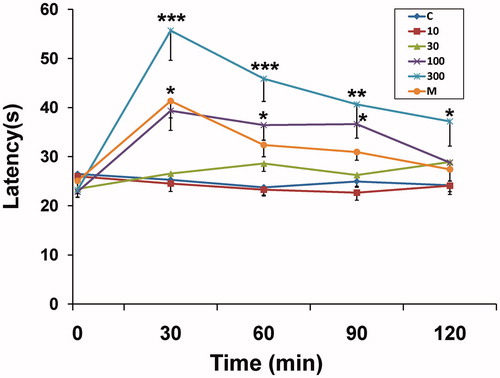
In hot-plate test, EOAD in dose of 300 mg/kg, increased the latency time for nociception significantly (p < 0.01) above the control value throughout the period (30–120 min) of observation (). Administration of 100 mg/kg EOAD increased the latency to the hot-plate test at 30–90 min (p < 0.05).
Figure 2. Effect of the essential oil of A. dracunculus (EOAD) in the hot-plate test in rats. The reaction time was measured in seconds (s) before (0 min) and 30, 60, 90 and 120 min after drug treatment. Horizontal axis shows time intervals (min), and the lines represent reaction time (s) in each animal group treated with the vehicle (C, 10 ml/kg), the EOAD (10, 30, 100 and 300 mg/kg) or morphine (M, 10 mg/kg). Asterisks indicate significant difference from control. Values are mean ± SEM, n = 6, ***p < 0.001, **p < 0.01, *p < 0.05 (ANOVA followed by Dunnett's test).
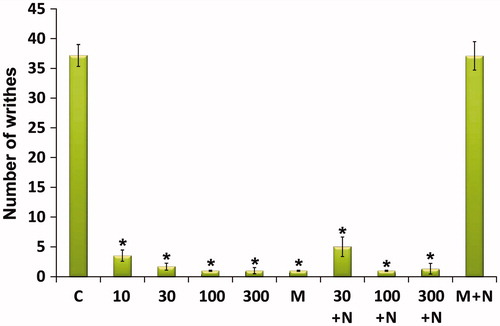
EOAD in doses of 10–300 mg/kg reduced the acetic acid-induced writhing count. The effect was dose-dependent and showed a significant effect (p < 0.001). Pretreatment of animals with naloxone, an opioid antagonist, decreased the antinociceptive effect produced by morphine, but did not affect the action caused by EOAD ().
Figure 3. Effect of the essential oil of A. dracunculus (EOAD) on acetic acid-induced writhing in mice. The vehicle (C, 10 ml/kg), the EOAD (10, 30, 100 and 300 mg/kg) and morphine (M, 10 mg/kg) were administered 30 min before the intraperitoneal administration of acetic acid and the number of writhes were counted over a period of 20 min. The effects of naloxone on EOAD and morphine antinociception are shown in the right side of the panel. Naloxone (N, 2 mg/kg s.c.) was administered 10 min before EOAD or morphine. Asterisks indicate significant difference from control. Values are mean ± SEM, n = 6, *p < 0.001 (ANOVA followed by Dunnett’s test).
Discussion
The antinociceptive effects of the EOAD were investigated in this study. The antinociceptive activity was evaluated with three animal models, which could provide a response to different grades of noxious stimuli (Le Bars et al., Citation2001). EOAD produced a potent analgesic effect in mice and rats as assessed by chemically (acetic acid and formalin) and thermal (hot-plate) induced nociception.
Administrations of graded doses of EOAD (i.p.) in mice gave a LD50 value of 1250 mg/kg. This finding probably suggests that the plant essential oil is relatively safe or non-toxic to mice.
The formalin test is believed to represent a more valid model for clinical pain (Le Bars et al., Citation2001). In this test, the early phase is thought to result from direct chemical activation of myelinated and unmyelinated nociceptive afferent fibers and the late phase as a consequence of noxious stimulus-evoked long-term changes in the properties of dorsal horn neurons (Abbadie et al., Citation1997). Centrally acting drugs such as opioids exert an inhibition in both phases (Shibata et al., Citation1989), as it is clearly consistent with our morphine results in the formalin test, whereas peripherally acting drugs such as indomethacin, aspirin and hydrocortisone only inhibit the late phase, which seems to be a result of an anti-inflammatory response (Elisabetsky et al., Citation1995; Hunskaar & Hole, Citation1987). Our data show that the plant essential oil is capable of exerting antinociception by acting at both phases suggesting that it may exert a central action.
The hot-plate test was selected to investigate central analgesic activity, because it has several advantages, particularly the sensitivity to strong analgesics and limited tissue damage (Al-Ghamdi, Citation2001). A significant antinociceptive effect with the hot-plate test was shown after EOAD (100–300 mg/kg, i.p.) administration indicating central analgesic effect.
EOAD significantly inhibited the abdominal constriction induced by acetic acid in mice. Acetic acid causes an increase in peritoneal fluids of PGE2 and PGF2α, serotonin and histamine. This model is commonly used for screening peripheral analgesics (Collier et al., Citation1968; Deraedt et al., Citation1980). Although the writhing test has poor specificity (Le Bars et al., Citation2001), it is a very sensitive method of screening antinociceptive effects of compounds and with a good correlation between ED50 values obtained in animals using this test and analgesic doses in humans (Collier et al., Citation1968). The antinociceptive effect of the essential oil appears to be due to mechanisms independent of activation of opioid receptors, since naloxone, an opioid antagonist, failed to reverse its action in the acetic acid-induced writhing test.
Two principal chemical components of EOAD, which have been identified in our study, were pulegone and estragole (Jalilzadeh-Amin et al., Citation2012). It is more likely that the antinociceptive effect of the EOAD is caused by the presence of pulegone. This naturally occurring monoterpene ketone was able to reduce the number of writhings induced by acetic acid (De Sousa et al., Citation2007). It also reduces the linking time induced by formalin and increases the latency time in the hot-plate test with non-participation of the opioid system (De Sousa et al., Citation2011). In vitro studies revealed that pulegone is a selective cyclooxygenase-2 inhibitor (Kawata et al., Citation2008). Although no mechanism of action has been investigated, probably (+) pulegone acts through inhibition of the production of inflammatory mediators from the cascade of cyclooxygenase.
In addition, there is probably an involvement of estragole, which has been shown to block nerve excitability (Leal-Cardoso et al., Citation2004) and relax isolated smooth muscle (Coelho-de-Souza et al., Citation1997). Finally, it is interesting to highlight that α-pinene and limonene, two monoterpenes of the EOAD, could also enhance the antinociceptive activity of EOAD, since they have shown anti-inflammatory and antinociceptive effects in several animal models (Guimarães et al., Citation2013). Other EOAD constituents also appear to be involved in its antinociceptive action. Further studies are needed to clarify the mechanism of action and the components responsible for these pharmacological effects.
Conclusion
Results suggest that EOAD possesses a potent antinociceptive effect. The overall activity of this plant suggests both central and peripheral antinociceptive activity of A. dracunculus. This finding supports the use of A. dracunculus in traditional medicine for the treatment of painful disorders.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This study was supported by the Faculty of Veterinary Medicine, Urmia University, Iran.
References
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI. (1997). Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: Studies with remifentanil and lidocaine. Pain 69:101–10
- Al-Ghamdi MS. (2001). The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol 76:45–8
- Balza F, Towers GHN. (1984). Dihydroflavonols of Artemisia dracunculus. Phytochemistry 23:2333–7
- Benli M, Kaya I, Yigit N. (2007). Screening antimicrobial activity of various extracts of Artemisia dracunculus L. Cell Biochem Func 25:681–6
- Bora KS, Sharma A. (2011). The genus Artemisia: A comprehensive review. Pharm Biol 49:101–9
- Coelho-de-Souza AN, Barata EL, Magalhães PJC, et al. (1997). Effects of the essential oil of Croton zehntneri, and its constituent estragole on intestinal smooth muscle. Phytother Res 11:299–304
- Collier HOJ, Dinneen LC, Johnson CA, Schneider C. (1968). The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother 32:295–310
- De Sousa DP, Júnior EV, Oliveira FS, et al. (2007). Antinociceptive activity of structural analogues of rotundifolone: Structure--activity relationship. Z Naturforsch C 62:39–42
- De Sousa DP, Nóbrega FFF, De Lima MRV, Almeida RN. (2011). Pharmacological activity of (R)-(+)-pulegone, a chemical constituent of essential oils. Z Naturforsch 66:353–9
- Deans SG, Simpson EJM. (2002). Artemisia dracunculus. In: Wright CW, ed. Medicinal and Aromatic Plants – Industrial Profiles. London: Taylor and Francis Inc, 91–7
- Deraedt R, Jougney S, Delevalee F, Flahaut M. (1980). Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 61:17–24
- Dubuission D, Dennis SG. (1977). The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4:167–74
- Eisenman SW, Poulev A, Struwe L, et al. (2011). Qualitative variation of anti-diabetic compounds in different tarragon (Artemisia dracunculus L.) cytotypes. Fitoterapia 82:1062–74
- Elisabetsky E, Arnador TA, Albuquerque RR, et al. (1995). Analgesic activity of Psychotria colorata (Willd. ex R. and S.) Muell. Arg. alkaloids. J Ethnopharmacol 48:77–83
- Guimarães AG, Quintans JS, Quintans LJ Jr. (2013). Monoterpenes with analgesic activity – A systematic review. Phytother Res 27:1–15
- Hunskaar S, Hole K. (1987). The formalin test in mice: Dissotiation between inflammatory and non-inflammatory pain. Pain 30:103–14
- Jalilzadeh-Amin G, Maham M, Dalir-Naghadeh B, Kheiri F. (2012). In vitro effects of Artemisia dracunculus essential oil on ruminal and abomasal smooth muscle in sheep. Comp Clin Pathol 21:673–80
- Kawata J, Kameda M, Miyazawa M. (2008). Cyclooxygenase-2 inhibitory effects of monoterpenoids with a p-menthane skeleton. Int J Essen Oil Therapeut 2:145–8
- Koster R, Anderson M, de Beer EJ. (1959). Acetic acid and analgesic screening. Fed Proc 18:412–15
- Le Bars D, Gozariu M, Cadden SW. (2001). Animal models of nociception. Pharmacol Rev 53:597–652
- Leal-Cardoso JH, Matos-Brito BG, Lopes-Junior JE, et al. (2004). Effects of estragole on the compound action potential of the rat sciatic nerve. Braz J Med Biol Res 37:1193–8
- Linares E, Bye JrRA. (1987). A study of four medicinal plant complexes of Mexico and adjacent United States. J Ethnopharmacol 19:153–83
- Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. (2008). Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69:1732–8
- Lorke, D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol 54:275–87
- Luchese C, Prigol M, Acker CI, Nogueira CW. (2010). Antinociceptive effect of butyl (2-phenylethynyl) selenide on formalin test in mice: Evidences for the involvement of serotonergic and adenosinergic systems. Eur J Pharmacol 644:49–54
- MacDonald AD, Woolfe G, Bergel F, et al. (1946). Analgesic action of pethidine derivatives and related compounds. Br J Pharmacol 1:4–14
- Meepagala KM, Sturtz G, Wedge DE. (2002). Antifungal constituents of the essential oil fraction of Artemisia dracunculus L. var. dracunculus. J Agr Food Chem 50:6989–92
- Miraldi E, Ferri S, Mostaghimi SV. (2001). Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran). J Ethnopharmacol 75:77–87
- Mosaddegh M, Naghibi F, Moazzeni H, et al. (2012). Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J Ethnopharmacol 141:80–95
- Ribnicky DM, Poulev A, O'Neal J, et al. (2004). Toxicological evaluation of the ethanolic extract of Artemisia dracunculus L. for use as a dietary supplement and in functional foods. Food Chem Toxicol 42:585–98
- Saadali A, Boriky D, Blaghen M, et al. (2001). Alkamides from Artemisia dracunculus. Phytochemistry 58:1083–6
- Sayyah M, Nadjafnia L, Kamalinejad M. (2004). Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J Ethnopharmacol 94:283–7
- Scherp P, Putluri N, LeBlanc GJ, et al. (2012). Proteomic analysis reveals cellular pathways regulating carbohydrate metabolism that are modulated in primary human skeletal muscle culture due to treatment with bioactives from Artemisia dracunculus L. J Proteomics 75:3199–210
- Shahriyary L, Yazdanparast R. (2007). Inhibition of blood platelet adhesion, aggregation and secretion by Artemisia dracunculus leaves extracts. J Ethnopharmacol 114:194–8
- Shibata M, Ohkubo T, Takahashi H, Inoki R. (1989). Modified formalin test: Characteristic biphasic pain response. Pain 38:347–52
- Teixeira B, Marques A, Ramos C, et al. (2013). Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind Crops Prod 43:587–95
- Tjolsen A, Berge OG, Hunskaar S, et al. (1992). The formalin test: An evaluation of the method. Pain 51:5–17
- Zargari A. (1992). Medicinal Plants. Vol. 3. Tehran, Iran: Tehran University Publications
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–10

