Abstract
Aim:
A bacterial genetics-guided approach was utilized for the discovery of new compounds affecting bacterial genome stability.
Materials & methods:
Fungal extracts and fractions were tested for genome instability-mediated antibacterial activity. Interaction assays and RT-qPCR were used to identify compounds that boost the activity of sub-minimum inhibitory concentration streptomycin and obtain insights on the molecular mechanisms of the primary hit compound, respectively.
Results:
Several extracts and fractions caused bacterial genome instability. Codeine, in synergy with streptomycin, regulates double-strand break (DSB) repair and causes bacterial ribosome dysfunction in the absence of DSBs, and dysregulation of ribosome biogenesis in a DSB-dependent manner.
Conclusion:
This study demonstrates a potential viable strategy that we are exploring for the discovery of new chemical entities with activities against Escherichia coli and other bacterial pathogens.
Graphical abstract

Infections caused by Gram-negative bacteria are usually difficult to treat in clinics [Citation1,Citation2]. This phenomenon is partly caused by intrinsic antimicrobial resistance due to presence of an outer membrane consisting of lipopolysaccharides, and the acquisition of resistance phenotypes from the indiscriminate usage of antibiotics [Citation3,Citation4]. Currently, restrictive and supervised usage of antibiotics in clinics is anticipated to minimize emergence and spread of antimicrobial-resistant bacterial strains [Citation5]. However, it is also imperative to constantly screen for new bioactive molecules endowed with potent antibacterial activities and new mechanisms of action. Moreover, the development of relevant approaches for enhancing the efficiency of existing antibiotics long term is expedient [Citation6].
Fluoroquinolones are notable antibiotics for the treatment of diverse bacterial infections [Citation7,Citation8]. The bactericidal activity of fluoroquinolones is as a result of the formation of DNA double-strand breaks (DSBs) in bacterial chromosomes, which is a concomitant outcome of inhibition of the ligase activity of bacterial type II topoisomerases following chromosomal cleavage by the same enzyme [Citation9,Citation10]. In Escherichia coli, DSBs are mainly repaired via homologous recombination when the RecBCD protein complex binds and processes the ends of the DSBs for downstream recombination events [Citation11]. Owing to the lethality of a single unrepaired DSB, fluoroquinolones exhibit rapid bactericidal activity at very low concentrations of the antibiotic in susceptible strains.
Fungi are important natural sources of new and potent antibacterial compounds that exhibit new mechanisms of action. They are notable for the production of diverse secondary metabolites including peptides (nonribosomal), alkaloids, terpenoids and polyketides [Citation12,Citation13]. The production of diverse secondary metabolites is usually attributed to the rich genome and the adaptable metabolic states of these fungi [Citation13]. The enormous chemical diversity of fungal secondary metabolites may imply their spectrum of biological activities could be applicable to the treatment of a wide scope of disease conditions, including both infectious and noncommunicable diseases [Citation14–19]. Hence, it is not surprising that secondary metabolites from fermentation cultures of wood-decaying fungi are endowed with antibacterial, antifungal and anticancer activities [Citation20,Citation21]. In addition, these fungal metabolites are valuable resources for screening and identification of new bioactive compounds that could be enrolled as drug candidates for the development of novel chemotherapeutic agents. In spite of the versatility of fungal secondary metabolites, they have not been exhaustively explored for novel drug development, especially in the current era where new antibiotics are urgently required for controlling nosocomial infections [Citation22].
The continual reliance on the prototype model for screening of natural products for antibacterial candidates would most likely identify the same classes of compounds that are already known for their antibacterial activities. In our previous perspective, we discussed a new paradigm for the discovery of new classes of antibiotics via the strategy of targeting bacterial genome stability [Citation6]. E. coli strains containing specific insertions of genetic elements that were originally designed to study the cellular mechanisms of DSB repair were used in this study for the screening of fungal natural products for drug discovery [Citation6,Citation23]. Since natural products derived from fungal secondary metabolites are currently under-explored for their potential role in novel drug development, we utilized our collection of extracts and fractions from fungal fermentation cultures for identification of compounds that could potentially target bacterial genome stability. A phenotypic-based screening approach was also optimized to investigate cellular targets in E. coli that are affected by a novel mechanism that increases the susceptibility of the bacteria to DSB formation.
Methods
Fermentation of fungi & extraction of secondary metabolites
Pure fungal isolates (soil-borne fungi, terrestrial endophytic fungi and marine endophytic fungi) were inoculated into 500-ml culture bottles containing 200 ml of broth. The constituent of the broth was yeast extract, peptone, malt extract and dextrose (5 g/l, 5 g/l, 5 g/l and 30 g/l, respectively). The inoculated broths were incubated at 30°C for 2 months with brief swirling of the culture bottles daily. Secondary metabolites produced in each fermentation culture were extracted using an equal volume of ethyl acetate and dried at 45°C under reduced pressure using a rotary evaporator [Citation20,Citation21]. The concentrated metabolites were reconstituted in methanol.
Fractionation of fungal extracts using preparative TLC
Preparative thin-layer chromatography (TLC) fractionation of fungal extracts was conducted using silica gel 60 coated with the F254 fluorescent indicator on aluminium plates [Citation20]. The first round of TLC fractionation was performed using a mobile phase consisting of 35 ml ethyl acetate, 10 ml acetonitrile and 5 ml petroleum ether. After fractionation, TLC plates were dried and visualized under UV light at high (365 nm) and low (254 nm) wavelengths. TLC bands that were obtained were eluted from the silica gel using methanol and referred to as 1D-TLC fractions. The 1D-TLC fractions were fractionated again by preparative TLC, as described previously [Citation21], and the bands obtained were eluted and referred to as 2D-TLC fractions. The final round of TLC fractionation was performed using the 2D-TLC fractions to obtain 3D-TLC fractions.
Analysis of the antibacterial activities of the fungal extracts & fractions
The fungal extracts were all initially screened for the presence of antibacterial agents using the disc diffusion assay. Sterile Whatman paper discs (6 mm in diameter) were infused with 40 μl (60 mg) of extract under aseptic conditions and air dried. E. coli strains were grown overnight in nutrient broth and diluted to optical density at 600 nm (OD600) of 0.2. The nutrient broth used for dilution of the overnight E. coli cultures was supplemented with 0.2% arabinose to ensure induction of the site-specific DSB at the chromosomal lacZ locus of the E. coli::DSB strain [Citation11; Supplementary data). In addition, sterile cotton swabs were used to uniformly spread 50 μl of 0.2% arabinose solution on Mueller–Hinton agar plates and air dry under aseptic conditions for 30 min. The arabinose-coated agar plates were subsequently inoculated with the diluted cultures using sterile cotton swaps. The paper discs infused with the fungal extracts were uniformly placed on the inoculated agar plates. Sterile paper discs infused with either ethyl acetate or methanol were air dried and used as negative controls. All agar plates were incubated at 37°C for 18 h and examined for the presence of zones of inhibition [Citation20]. disc diffusion assays were performed in duplicates. A set of 19 commercial antibiotics were also used for quality control purposes for all disc diffusion assays, as previously reported [Citation6].
Fungal fractions (1D-TLC and 3D-TLC fractions) were also tested via disc diffusion assay at 10 mg/disc.
For pairwise (synergistic) analysis of antibacterial activities, the nutrient broth used for dilution of the overnight E. coli culture (the control strain) was not supplemented with arabinose. Moreover, the Mueller–Hinton agar plates were not precoated with arabinose prior to the disc diffusion assay. Paper discs were infused with 20 μl (30 mg) of inducers of DNA DSBs (iDSB) or DSB repair inhibitors (DSBRi) extracts and an iDSB extract-containing paper disc was placed alongside a DSBRi extract-infused paper disc on the inoculated agar plates prior to incubation at 37°C for 18 h.
Compound interaction assays using the disc diffusion method
For the initial screening of the library of 56 compounds (Supplementary data), 50 μl of 0.5 mg/ml of streptomycin (25 μg) was uniformly spread onto Mueller–Hinton agar plates precoated with 0.2% arabinose under aseptic conditions. The arabinose and streptomycin-coated agar plates were air dried under aseptic conditions for 30 min before inoculation with the diluted E. coli cultures at OD600 of 0.2; the nutrient broth used for dilution of the overnight E. coli cultures was supplemented with 0.2% arabinose. The 56 compounds were tested at 10 μg/disc using the disc diffusion assay, with the exception of 5-fluorouracil, which was tested at 1 μg/disc.
The five compounds (codeine, gliclazide, hydrochlorothiazide, serine hydroxamate and N-acetylglucosamine) that were selected from the library of 56 compounds were used for the preparation of 10-mg/ml solutions. An aliquot of 100 μl of the 10-mg/ml solutions was uniformly spread onto the Mueller–Hinton agar plates precoated with arabinose and air dried prior to determination of the MIC of streptomycin via the disc diffusion method.
For analysis of the super-regulation effect of codeine, the Mueller–Hinton agar plates were initially coated with 50 μl of 0.2% arabinose, air dried for 30 min and recoated with 50 μl of 0.5 mg/ml of streptomycin (25 μg). The agar plates were air dried and coated again with 100 μl of a 10 mg/ml codeine solution. After air drying for 30 min, the agar plates were inoculated with the diluted E. coli cultures at OD600 of 0.2; the nutrient broth used for dilution of the overnight E. coli cultures was supplemented with 0.2% arabinose. The five compounds (codeine, gliclazide, hydrochlorothiazide, serine hydroxamate and N-acetylglucosamine) were tested for antibacterial activity at 10 μg/disc.
Viability assay using the broth microdilution method
The broth microdilution assay was performed using nutrient broth supplemented with 0.2% arabinose at a total assay volume of 200 μl per well. Overnight cultures were diluted to a final OD600 of 0.05 per well. Codeine was used at a final concentration of 0.05 mg/ml (10 μg per well) and streptomycin was utilized at twofold serial dilutions, starting with an initial amount of 5 μg per well (0.025 mg/ml). The 96-well plates containing the E. coli cells were initially incubated at 37°C for 24 h and the absorbance at 600 nm was recorded using a Varioskan plate reader. For fluorescence measurements, 10 μl of 0.2 mg/ml of resazurin dye was added to each well of the 96-well plate and incubated at 37°C in the absence of light. Fluorescence measurements were recorded using a Varioskan plate reader at 18, 20 and 24 h after addition of the dye.
RNA extraction & RT-qPCR analysis
Overnight cultures of the E. coli strains were diluted to a final OD600 of 0.05 using nutrient broth supplemented with 0.2% arabinose. Streptomycin and codeine were added, where required, at final concentrations of 0.05 mg/ml (2.5 mg per 50 ml of bacterial culture) and 1 × 10-4 mg/ml (7.8125 μg per 50 ml bacterial culture), respectively. The diluted cultures were incubated at 37°C for 24 h and total RNA was extracted from the bacterial cell pellets using the Zymo Research Fungal/Bacterial RNA MiniPrep kit, according to the manufacturer’s instructions. The total RNA was eluted using nuclease-free water, quantified, and its purity determined using a nanodrop prior to storage at -80°C.
The RT-qPCR assay was performed at a total reaction volume of 20 μl using the Luna® Universal One-Step RT-qPCR Kit, according to the manufacturer’s instructions. Total RNA was diluted using nuclease-free water and used at an amount of 87.5 μg per reaction. The reaction cycle was: reverse transcription at 55°C for 10 min, initial denaturation at 95°C for 1 min; 40 cycles of denaturation at 95°C for 10 s, annealing at 50°C for 30 s and extension at 60°C for 30 s. Primers are listed in the Supplementary data. The E. coli mazE gene was used as a housekeeping gene for normalization of the raw Ct values via the 2-ΔΔCT method.
Results
Abundance of fungal natural products exhibiting iDSB & DSBRi activities in E. coli
We have previously described a new strategy for the discovery of new classes of antibiotics targeting bacterial genome stability [Citation6]. In this study, E. coli strains containing insertions of specific genetic elements were used for the screening of natural products for drug discovery [Citation6].
A library of 628 extracts from fungal fermentation cultures were screened to identify candidates that exhibit antibacterial activities affecting genome stability in E. coli. These extracts were obtained from fermentation cultures of soil-borne fungi (SBF; 201 extracts), terrestrial endophytic fungi (TEF; 306 extracts) and marine endophytic fungi (121 MEF extracts). Extracts that selectively exhibited antibacterial activity against a recB-mutant strain of E. coli (E. coli::recB) were inferred as putative iDSBs (A). Extracts that selectively exhibited activity against an E. coli strain containing a genetic system for induction of a site-specific DSB at the chromosomal lacZ locus (E. coli::DSB) were inferred as putative inhibitors of DSB repair (DSBR; B). An E. coli strain that was proficient for DSB repair (had no mutation in the recB gene) and was also incapable of site-specific DSB induction at the chromosomal lacZ locus was used as a control strain (wild-type; WT) for identification of extracts that exhibited general antibacterial activities via mechanisms that were not dependent on either DSB formation or inhibition of DSB repair (general growth inhibitors; C). A total of 25 extracts were identified as iDSB while 28 other extracts were identified as DSBRi (D & E). It is inferred that these 53 extracts contain antibacterial compounds that affect the integrity of the genome of E. coli. Five extracts exhibited antibacterial activity against WT but not the E. coli::recB strain, while eight extracts inhibited the growth of WT but not the E. coli::DSB strain. Moreover, 108 extracts exhibited antibacterial activities against both the WT and E. coli::recB strains while 103 extracts inhibited growth of both the WT and E. coli::DSB strains. Interestingly, these 103 extracts also exhibited antibacterial activity against the E. coli::recB strain, implying they might contain compounds that nonselectively perturb other vital bacterial cellular processes (general growth inhibitors). Collectively, these initial observations demonstrate fungal natural products affecting bacterial genome stability is only twofold less than the general growth inhibitors and highlight the unique and critical role of fungal secondary metabolites in the development of novel antibacterial compounds targeting bacterial genome integrity.
(A) Representative image illustrating the antibacterial activity of DSB-inducing (iDSB) extracts. (B) Representative image illustrating the antibacterial activity of DSB repair-inhibiting (DSBRi) extracts. (C) Representative image illustrating extracts exhibiting antibacterial activity against the control Escherichia coli strain. (D) Extracts exhibiting antibacterial activity via induction of DSBs. (E) Extracts exhibiting antibacterial activity via inhibition of DSB repair. E. coli::recB represents an E. coli strain containing a mutation in the recB gene, which renders the strain susceptible to DNA DSBs. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB, which is also the WT strain, represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus and has no mutation in the recB gene. White arrows show visible zones of inhibition.
DSB: Double-strand break; WT: Wild-type.
![Figure 1. Screening for double-strand break-inducing and double-strand break repair-inhibiting extracts against Escherichia coli.(A) Representative image illustrating the antibacterial activity of DSB-inducing (iDSB) extracts. (B) Representative image illustrating the antibacterial activity of DSB repair-inhibiting (DSBRi) extracts. (C) Representative image illustrating extracts exhibiting antibacterial activity against the control Escherichia coli strain. (D) Extracts exhibiting antibacterial activity via induction of DSBs. (E) Extracts exhibiting antibacterial activity via inhibition of DSB repair. E. coli::recB represents an E. coli strain containing a mutation in the recB gene, which renders the strain susceptible to DNA DSBs. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB, which is also the WT strain, represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus and has no mutation in the recB gene. White arrows show visible zones of inhibition.DSB: Double-strand break; WT: Wild-type.](/cms/asset/20c80bb5-3b6e-4764-9281-c53036f09f2c/ifdd_a_12367015_f0002.jpg)
Pairs of iDSB & DSBRi exhibit potent antibacterial activities against WT E. coli
A crucial proof required to demonstrate the feasibility of using fungal metabolites for targeting genome stability of bacterial pathogens is the pairwise testing of iDSB and DSBRi candidates against the WT strain. We reckoned that this proof of concept is vital, even though several iDSB and DSBRi fungal extracts were obtained from the initial screening procedure. As a result, the iDSB and DSBRi extracts were ranked according to the magnitude of their antibacterial activities and the top five candidates from each category were utilized for pairwise testing against only the WT strain. Nine of the 25 pairwise combinations of an iDSB with a DSBRi exhibited synergistic antibacterial activity against the WT strain, which is indicative of a genome instability-mediated antibacterial activity (). The MEF184 extract did not exhibit antibacterial activity when paired with any of the five selected iDSBs. In contrast, synergistic antibacterial activity against the WT strain was observed when the MEF178 extract was paired with each of the five selected iDSBs. This observation might indicate that the MEF178 extract is a highly potent inhibitor of DSB repair, hence its ability to effectively inhibit growth of the WT strain in the presence of DSBs that were potentially generated by the five iDSBs. Collectively, these data confirm the potential role of fungal secondary metabolites as useful resources for the development of combination antibiotic chemotherapy targeting the genome integrity of E. coli, as we have previously reported [Citation6].
Table 1. Additive antibacterial activities of double-strand break-inducing and double-strand break repair-inhibiting extracts against the wild type Escherichia coli strain.
Several fungal extracts with iDSB & DSBRi activities produce active fractions
The chemical constituents of fungal extracts are numerous and diverse, necessitating extensive fractionation procedures for isolating compounds exhibiting bioactivity of interest. As a result, partially purified fractions obtained from the fungal extracts were screened against the E. coli strains to ascertain whether the antibacterial activities exhibited by the extracts are also reproducibly shown by the fractions. A total of 51 1D-TLC fractions obtained from nine MEF extracts were screened against the E. coli strains; the nine MEF extracts comprised of four DSBRi (MEF167, MEF184, MEF185 and MEF232) and five general growth inhibitors (MEF198, MEF202, MEF206, MEF216 and MEF222). None of the 51 fractions exhibited selective antibacterial activity against the E. coli::DSB strain, while 26 fractions exhibited selective activity against the E. coli::recB strain (A). A total of 19 fractions also inhibited growth of the WT strain, even though ten of these fractions also showed activity against the E. coli::recB strain. This suggests that the fungal extracts previously identified as DSBRi may also contain compounds that exhibit iDSB activity. A total of 56 1D-TLC fractions obtained from one SBF and eight TEF extracts that previously showed iDSB or DSBRi activities were also screened against the E. coli strains (). The TEF285 and TEF293 extracts previously exhibited iDSB activity. Nevertheless, these two iDSB extracts generated TLC fractions that exhibited both iDSB and DSBRi activities. Thus, TEF285 and TEF293 extracts are composed of distinct chemical entities capable of DSB formation and inhibition of repair in E. coli.
(A) Antibacterial activities of 1D-TLC fractions from marine endophytic fungal extracts. (B) Antibacterial activities of 3D-TLC fractions from soil-borne and terrestrial endophytic fungal extracts. E. coli::recB represents an Escherichia coli strain containing a mutation in the recB gene, which renders the strain susceptible to DNA DSBs. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB, which is also the WT strain, represents the control Escherichia coli strain which is unable to induce the site-specific DNA DSB at the lacZ locus and has no mutation in the recB gene.
DSB: Double-strand break; SBF: Soil-borne fungi; TEF: Terrestrial endophytic fungi; WT: Wild type.
![Figure 2. Antibacterial activities of fractions from the fungal extracts.(A) Antibacterial activities of 1D-TLC fractions from marine endophytic fungal extracts. (B) Antibacterial activities of 3D-TLC fractions from soil-borne and terrestrial endophytic fungal extracts. E. coli::recB represents an Escherichia coli strain containing a mutation in the recB gene, which renders the strain susceptible to DNA DSBs. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB, which is also the WT strain, represents the control Escherichia coli strain which is unable to induce the site-specific DNA DSB at the lacZ locus and has no mutation in the recB gene.DSB: Double-strand break; SBF: Soil-borne fungi; TEF: Terrestrial endophytic fungi; WT: Wild type.](/cms/asset/a4d1d8a4-568c-4cb3-adb2-48dd3a3b0551/ifdd_a_12367015_f0003.jpg)
Table 2. Antibacterial activity of 1D-TLC fractions obtained from double-strand break-inducing and double-strand break repair inhibiting extracts.
iDSB & DSBRi active fractions from inactive total extracts
TEF249 and TEF287 extracts previously showed iDSB activity; however, none of their TLC fractions exhibited iDSB nor DSBRi activities, indicating a potential loss of genome stability-mediated antibacterial activity during fractionation (). This phenomenon could be due to the low abundance of chemical agents acting as synergistic combinations that have separated out below their limits of detection.
The screening of 54 1D-TLC fractions obtained from previously inactive SBF and TEF extracts also demonstrated that fractionation of previously inactive extracts generate fractions endowed with iDSB or DSBRi activities (). In addition, 144 extensively purified fractions (3D-TLC fractions) also obtained from previously inactive SBF extracts (SBF118 and SBF134) were also screened against the E. coli strains. These 3D-TLC fractionations were originally intended for a different study, but we took advantage of their availability to investigate the depth of distribution of iDSB and DSBRi chemical agents in extracts that did not show such activities at the total extract level. One of the 144 fractions showed iDSB activity while ten other fractions showed DSBRi activity, indicating that the extracts from SBF118 and SBF134 contain antibacterial compounds affecting genome stability in E. coli (B). The level of detection was not as high as would be anticipated for those extracts that exhibited iDSB or DSBRi activities at the total extract and 1D-TLC fraction levels. Nonetheless, these are interesting observations that suggest that iDSB or DSBRi activities are ubiquitous, notwithstanding the tedious work and great expense needed to obtain pure active iDSB or DSBRi compounds. Importantly, these observations also highlight a potential pitfall in bioactivity-guided fractionation, where inactive partially purified fractions are usually eliminated from downstream ultrafractionation procedures if they do not exhibit the desired antibacterial activity after fractionation.
Table 3. Antibacterial activity of 1D-TLC fractions obtained from inactive extracts.
Codeine as a regulator of iDSB or DSBRi activities
Natural antibacterial products of interest might usually be produced in very small quantities in fungal fermentation cultures, necessitating the utilization of suitable approaches for facilitating the identification and isolation of these compounds from the other myriad of compounds. We have previously reported that the E. coli::DSB strain is marginally sensitive to chloramphenicol, erythromycin, streptomycin and gentamycin in comparison to the WT strain [Citation6]. The plausibility of exacerbating the susceptibility of DSB repair-proficient E. coli to the site-specific DSB induced at the lacZ locus was investigated by screening a library of 56 compounds against the E. coli::DSB and WT strains using agar plates precoated with streptomycin. Eight of the 56 compounds exhibited marginal growth inhibition of the E. coli::DSB strain but caused no perturbation of growth of the WT strain, indicating that these eight compounds increased the sensitivity of E. coli to the DSB induced at the chromosomal lacZ locus in the presence of streptomycin (A). Five of these eight compounds were selected for assessment of their effect on the MIC of streptomycin against the E. coli::DSB and WT strains (B). Gliclazide showed no effect on the MIC of streptomycin against both the E. coli::DSB and WT strains in comparison to the control assay (MIC = 0.5 μg). Serine hydroxamate and N-acetylglucosamine caused an increase in the MIC of streptomycin against both the E. coli::DSB and WT strains (MIC = 1.0 μg). Codeine showed no effect on the MIC of streptomycin against the E. coli::DSB strain (MIC = 0.5 μg) but caused an increase in the MIC against the WT strain (MIC = 1.0 μg); the opposite effect was shown by hydrochlorothiazide against the E. coli strains. These results suggest that codeine induces a resistance mechanism to streptomycin in the WT strain; however, DSB induction in E. coli abrogates this resistance mechanism. The reversal of the resistance phenotype could also be deduced as representing a codeine-dependent increase in the sensitivity of E. coli to streptomycin in the presence of DSBs.
(A) The antibacterial profile of 56 existing compounds against Escherichia coli subjected to DSB induction in the presence of streptomycin. (B) Analysis of the effect of five (out of the 56) compounds on the minimum inhibitory concentration of streptomycin against E. coli subjected to DSB formation. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus.
DSB: Double-strand break.
![Figure 3. Screening for existing compounds that boosts the susceptibility of double-strand break repair-proficient Escherichia coli to site-specific double-strand breaks in the presence of streptomycin.(A) The antibacterial profile of 56 existing compounds against Escherichia coli subjected to DSB induction in the presence of streptomycin. (B) Analysis of the effect of five (out of the 56) compounds on the minimum inhibitory concentration of streptomycin against E. coli subjected to DSB formation. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus.DSB: Double-strand break.](/cms/asset/1f6cbd94-6e89-4bee-9f79-b9d0300fd981/ifdd_a_12367015_f0004.jpg)
It is expected that the increased susceptibility phenotype caused by codeine as a result of DSB induction in E. coli may be very useful for the screening and identification of novel antibacterial natural products that are usually produced in very small quantities in extracts from fungal fermentation. The insufficient production of these compounds leads to unnoticeable growth inhibition against test organisms using the routine disc diffusion or viability assays. We reckon that the antibacterial effect of these less abundant compounds can be manifested in E. coli cells exhibiting compromised fitness due to the phenotypic effect of codeine.
DSBRi super-regulation
A phenotypic interaction assay was used to explore the possibility of further compromising the fitness of E. coli by precoating agar plates with codeine and a sub-MIC of streptomycin, and testing for growth inhibition using paper discs infused with either hydrochlorothiazide, gliclazide, codeine, N-acetylglucosamine or serine hydroxamate. None of the five compounds inhibited growth of the WT strain, confirming their lack of antibacterial activity in the absence of DSB induction (). In contrast, serine hydroxamate was the only compound that did not exhibit inhibition of growth of the E. coli::DSB strain. Paper discs infused with either gliclazide or N-acetylglucosamine showed higher inhibition of growth of the E. coli::DSB strain in comparison to codeine-infused paper discs. These observations demonstrate that the fitness of E. coli was further compromised by the combination of codeine with either gliclazide or N-acetylglucosamine in the presence of DSB induction and sub-MIC of streptomycin. The data illustrate a model for combinatorial antibacterial chemotherapy that utilizes a set of nonbacteriostatic or nonbactericidal compounds to reduce the fitness of E. coli in the presence of DSBs. This would also be instrumental for screening and identification of novel antibacterial natural compounds that exhibit their antibacterial effects via iDSB and DSBRi mechanisms and are usually produced below the limits of detection of standard assays.
Table 4. Super-regulation of the effect of codeine and sub-MIC of streptomycin in Escherichia coli subjected to constitutive double-strand break induction.
Codeine sensitizes E. coli to DSB through ribosome dysregulation
Since the sensitization effect of codeine and sub-MIC of streptomycin against the E. coli::DSB strain was detected using the disc diffusion assay, we also investigated whether this effect could be reproduced in liquid growth medium (nutrient broth). The growth assay for E. coli::DSB and WT strains in nutrient broth showed that the MIC of streptomycin for both strains was 0.125 μg (A). Codeine also affected the growth of the E. coli::DSB strain in the absence of streptomycin, indicating the sensitization effect of codeine is reproduced in liquid growth medium. Viability assays using alamar blue dye demonstrated that, generally, the E. coli::DSB strain exhibited lower viability compared with the WT strain in the presence of sub-MICs of streptomycin (B, A & B). Nonetheless, the viability of both strains of Escherichia coli was almost equivalent at 0.008 μg of streptomycin in the presence or absence of codeine. Hence, the equivalence of this amount of streptomycin was used for the subsequent molecular assays that investigated the effect of codeine and streptomycin on expression profiles of selected genes.
(A) Analysis of the effect of codeine, streptomycin and DSB induction on the growth of Escherichia coli. (B) Analysis of the effect of codeine, streptomycin and DSB induction on the viability of E. coli at 16 h after the addition of alamar blue dye. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.
DSB: Double-strand break.
![Figure 4. Effect of codeine and double-strand break induction on growth and viability of Escherichia coli at sub-minimum inhibitory concentrations of streptomycin.(A) Analysis of the effect of codeine, streptomycin and DSB induction on the growth of Escherichia coli. (B) Analysis of the effect of codeine, streptomycin and DSB induction on the viability of E. coli at 16 h after the addition of alamar blue dye. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.DSB: Double-strand break.](/cms/asset/520f3956-241f-4b48-bace-6f9dd4678a21/ifdd_a_12367015_f0005.jpg)
(A) Analysis of the effect of codeine, streptomycin and DSB induction on the viability of E. coli at 20 h after the addition of alamar blue dye. (B) Analysis of the effect of codeine, streptomycin and DSB induction on the viability of E. coli at 24 h after the addition of alamar blue dye. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA double-strand break at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.
DSB: Double-strand break.
![Figure 5. Effect of codeine and double-strand break induction on viability of Escherichia coli at sub-minimum inhibitory concentrations of streptomycin.(A) Analysis of the effect of codeine, streptomycin and DSB induction on the viability of E. coli at 20 h after the addition of alamar blue dye. (B) Analysis of the effect of codeine, streptomycin and DSB induction on the viability of E. coli at 24 h after the addition of alamar blue dye. E. coli::DSB represents a strain of E. coli that contains a genetic system for inducing a site-specific DNA double-strand break at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.DSB: Double-strand break.](/cms/asset/0da3f198-0dbd-46df-bd7f-f5a19511b9b1/ifdd_a_12367015_f0006.jpg)
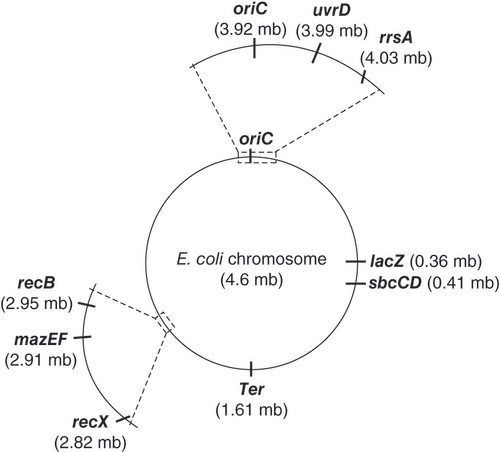
The recB gene (encoding a major DSB repair protein), uvrD and recX genes (encoding regulators of DSB repair), rrsA gene (encoding the RNA component of the 30S ribosomal subunit) and mazF gene (encoding the toxin component of a toxin–antitoxin system) were utilized for gene-expression analysis (). The uvrD and rrsA genes are located within close proximity to the origin of replication on the right arm of the E. coli chromosome. The recB, recX, mazE and mazF genes are found on the left arm of the chromosome ().
Table 5. Functions of the Escherichia coli genes selected for the RT-qPCR assay.
The site of double-strand breaks induction at the chromosomal lacZ locus is also shown.
Surprisingly, the rrsA gene, which was initially selected as a housekeeping gene for the purposes of data normalization, was found to be a key target gene for the molecular assay. This led us to consider types of normalizations with two completely different outcomes but similar overall inferences and conclusions ( & ).
The E. coli::No DSB-untreated cultures were used for normalization of the E. coli::No DSB-treated cultures, the E. coli::DSB-untreated cultures and the E. coli::DSB-treated cultures. E. coli::DSB represents a strain of Escherichia coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.
DSB: Double-strand break.
![Figure 7. Effect of codeine and sub-minimum inhibitory concentration of streptomycin on the expression profile of selected genes in Escherichia coli subjected to double-strand break formation.The E. coli::No DSB-untreated cultures were used for normalization of the E. coli::No DSB-treated cultures, the E. coli::DSB-untreated cultures and the E. coli::DSB-treated cultures. E. coli::DSB represents a strain of Escherichia coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.DSB: Double-strand break.](/cms/asset/86e5e90b-1463-4782-9bf9-5e0a528452f8/ifdd_a_12367015_f0008.jpg)
E. coli::DSB-treated cultures were normalized to E. coli::DSB-untreated cultures and E. coli::No DSB-treated cultures were normalized to E. coli::No DSB-untreated cultures. E. coli::DSB represents a strain of Escherichia coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.
DSB: Double-strand break.
![Figure 8. Effect of codeine and sub-minimum inhibitory concentrations of streptomycin on the expression profile of selected genes in Escherichia coli subjected to double-strand break formation.E. coli::DSB-treated cultures were normalized to E. coli::DSB-untreated cultures and E. coli::No DSB-treated cultures were normalized to E. coli::No DSB-untreated cultures. E. coli::DSB represents a strain of Escherichia coli that contains a genetic system for inducing a site-specific DNA DSB at the lacZ locus of the chromosome [Citation11]. E. coli::No DSB represents the control E. coli strain that is unable to induce the site-specific DNA DSB at the lacZ locus. Error bars represent the standard error of the mean of three independent experiments.DSB: Double-strand break.](/cms/asset/119c3968-90f8-4e25-82e8-509abc9b7747/ifdd_a_12367015_f0009.jpg)
Despite the crucial role of DSB induction in compromising the fitness of E. coli, the expression profiles of the recB, recX or uvrD genes in the E. coli::DSB strain were only marginally affected by codeine, streptomycin or the combination of these compounds, relative to the WT strain ( & ). Similarly, these treatment conditions did not perturb the expression of the mazF gene in the E. coli::DSB strain in comparison to the WT strain. The untreated E. coli::DSB strain showed higher expression levels for the four genes (recB, recX, uvrD and mazF) compared with the untreated WT strain, confirming that DSB elicits a subtle increase in gene expression levels in the stress response genes. Furthermore, the expression levels of these four genes in treated and untreated E.coli::DSB strain were similar, which might possibly indicate a more predominant post-transcriptional regulatory DSB response mechanisms.
Streptomycin caused a 7.54-fold increase in expression of the rrsA gene in the E. coli::DSB strain in comparison to the untreated WT strain and 3.43-fold increase when compared to the corresponding streptomycin-treated WT strain (). This observation might suggest that increased perturbation in ribosome function could be a cause of the increased susceptibility of E. coli to the ribosome-targeted antibiotic in the presence of DSBs, as we have previously reported [Citation6], leading to a robust increase in expression of the rrsA gene. Codeine also caused 4.13- and 3.87-fold increases in expression of the rrsA gene in the WT and E. coli::DSB strains, respectively, in comparison to the untreated WT strain. This indicates that the compromised fitness of the E. coli::DSB strain in response to treatment with codeine might be mediated via a potential reduction in global protein synthesis and not only DSB repair proteins (). Moreover, the combination of codeine and streptomycin caused only 2.96- and 2.10-fold increases in the expression of the rrsA gene in the E. coli::DSB and WT strains, respectively, in comparison to the untreated WT strain. This indicates a net reduction by 0.91- and 4.58-fold for the individual treatments with codeine and streptomycin, respectively. Moreover, the expression levels of the rrsA gene in the WT strain treated with streptomycin alone and codeine-streptomycin was nearly the same at fold changes of 2.2 and 2.1, respectively, in comparison to the untreated WT strain. This might suggest a form of contention between two distinct ribosome dysregulation mechanisms, resulting in higher loss of fitness in a DSB-dependent manner.
The strain-specific normalization approach did not demonstrate any significant treatment-dependent changes in the expression of the four stress-response genes, although the expression levels of the recX and maxF genes were moderately higher in the E. coli::DSB strain compared with WT across all the three treatment conditions. Conversely, the expression levels of the uvrD gene were higher in the WT compared with the E. coli::DSB strain. In relation to the rrsA gene, streptomycin caused an opposite effect; a fold change of 3.11 was obtained for the E. coli::DSB strain in comparison to the 2.2-fold change obtained for the WT strain (). Consequently, we infer that the molecular mechanism underlying the compromised fitness of the E. coli::DSB strain in response to treatment with codeine only or codeine-streptomycin is most likely different from the known mechanism of action of streptomycin. Moreover, the dysregulation of ribosome function caused by codeine in the WT strain, leading to higher expression of the rrsA gene, is counteracted in the presence of DSB or streptomycin. Overall, the effect of codeine might be occurring at both the upstream of ribosome biogenesis and downstream of ribosome function, with dysregulation of the upstream biogenesis phase being more dominant-negative. Furthermore, this effect is dependent on the presence of DSB as suppression of the rrsA gene occurs in the E. coli::DSB strain and in combination with streptomycin.
Discussion
The global antimicrobial resistance menace necessitates the utilization of unorthodox approaches, in addition to paradigm methodologies, for the discovery of novel antibiotics that would be useful for preventing the spread of bacterial pathogens [Citation24–27]. In this study, a bacterial genomics-guided approach was utilized to identify fungal secondary metabolites that are capable of instigating genome instability in E. coli. We observed that 8.44% of the total fungal extracts consist of natural products that disrupt genome stability via formation of DSBs or inhibition of DSB repair, which occurs mainly via homologous recombination in E. coli. The number of extracts containing natural products affecting bacterial genome stability was only twofold less than the general growth inhibitors identified in this study, indicating that these classes of compounds are not produced rarely by fungi. Moreover, the synergistic antibacterial activities of these two categories of natural products (iDSB and DSBRi) against the WT E. coli strain shows that these compounds are suitable candidates for the development of combination antibiotic chemotherapy, especially against the diverse infections caused by Gram-negative bacteria. The data in this study provide additional evidence for the vital role of fungal biodiversity as sources of antibacterial natural products that could be explored for novel antibiotic drug discovery [Citation28–30].
Bioactive natural products of interest are usually produced together with several other compounds, thereby necessitating extensive fractionation techniques for the isolation and characterization of these compounds from their native sources [Citation31]. Bioactivity-guided fractionation has previously been utilized for the discovery of natural products exhibiting anticancer and antibacterial activities [Citation32,Citation33]. The isolation and purification of bioactive natural products from the initial crude extract represents one of the strenuous tasks associated with exploring natural products for the discovery and development of novel drugs [Citation34]. Nonetheless, recent advancements in available technologies facilitates timely isolation of novel bioactive compounds [Citation35]. Fractionation of the iDSB and DSBRi extracts showed that natural products affecting bacterial genome stability were present in some of the eluted fractions, even though the fractionation also led to some loss of iDSB or DSBRi activities previously detected in these extracts. Conversely, we also demonstrated that previously inactive extracts generated fractions showing iDSB and DSBRi activities. These observations highlight potential challenges that are usually associated with bioactivity-guided fractionation procedures [Citation36]. Nevertheless, the consistent detection of natural products exhibiting iDSB and DSBRi activities in the extensively purified 3D-TLC fractions shows that these compounds have the potential to be isolated in a pure state from extracts for downstream elucidation of their chemical structure and validation of their antibacterial activities.
Fluoroquinolones are the most notable antibiotics currently used in clinics that have been shown to elicit bactericidal activity via DSB formation [Citation9]. Cystobactamids are also natural antibacterial products isolated from myxobacteria that share the same cellular targets as the fluoroquinolones. Interestingly, it has been reported that despite the increasing emergence of drug-resistant bacterial pathogens against fluoroquinolones, there is no crossresistance of cystobactamids to fluoroquinolones [Citation37]. It is possible that the natural products displaying iDSB activity in this study may also affect bacterial topoisomerase II enzymes (DNA gyrase or topoisomerase IV inhibitors). Nevertheless, the isolation of natural products exhibiting iDSB activities from several morphologically diverse fungi in this study could suggest that iDSB activities could also be a concomitant effect of inhibition of other bacterial cellular targets, that is, nongyrase or nontopoisomerase IV inhibitors.
A chemical biology-based approach for drug repurposing was also utilized in this study for two main reasons. First, to identify already existing compounds that could exacerbate the antibacterial effect of sub-MIC streptomycin in E. coli subjected to constitutive DSB formation. Second, we were also interested in the identification of cellular targets underlying the chemically mediated increase in susceptibility of E. coli to sub-MIC streptomycin in the presence of DSB formation. Interestingly, the compounds that exacerbated this desired phenotype in E. coli were not notable for boosting the antibacterial activity of streptomycin or other antibiotics used in clinics. We have previously demonstrated that codeine, a weak opioid used for pain management in clinics [Citation38], caused an increase in the MIC of streptomycin against E. coli, and this phenotype was reversed in the presence of DSB induction [Citation6]. This DSB-dependent susceptibility caused by codeine is hypothesized to represent a booster of the antibacterial effect of sub-MIC of streptomycin against E.coli. We are optimistic that the chemically mediated susceptibility phenotype in E. coli, in addition to the available DSB-modified/mutant strains, would be useful for screening and identification of antibacterial natural products. Moreover, these natural products would be relevant, especially when the active agents are produced in very small quantities by fungal species, and hence are mostly undetected by paradigm viability assays [Citation35]. The targeted isolation of these originally undetectable natural products could lead to the availability of a plethora of antibacterial drug candidates that have not been exposed to existing bacterial pathogens. As these potential novel antibacterial compounds are derived from natural sources, they are also more likely to exhibit desirable pharmacological properties in comparison to synthetic compounds [Citation39]. The combinatorial nature of the strategy for targeting bacterial genome stability will also create diverse antibacterial regimens that can be selected to be specific to different species of bacterial pathogens.
Preliminary gene-expression analysis has eliminated the potential role of a DSB repair gene (recB) [Citation40] or regulators of DSB repair (recX and uvrD) [Citation41,Citation42] in the molecular mechanism underlying the chemically mediated susceptibility phenotype in E. coli. In contrast, 16S rRNA, which is a target for streptomycin, was affected by codeine treatment, albeit via a mechanism that is opposite to the effect of streptomycin treatment. The effect of the combination of codeine and streptomycin on expression of the 16S rRNA gene was also identical to the effect exhibited by codeine only, but not streptomycin only. Thus, we hypothesize that the molecular mechanism underlying the chemically mediated susceptibility phenotype in E. coli is different from the known mode of action of streptomycin. A previous study that utilized x-ray crystallography demonstrated that helices 1, 18, 27 and 44 of 16S rRNA and ribosomal protein S12 constitute the binding site for streptomycin [Citation43]. A subsequent study also showed that streptomycin causes misreading of the genetic code by inducing a lateral shift or distortion of helix 44 of 16S rRNA [Citation44]. We propose a mechanism of ribosomal destabilization by streptomycin and codeine when utilized individually, and the dysregulation of ribosome biogenesis in a DSB-dependent manner by codeine. Moreover, codeine may exhibit dominant-negative effects in the presence of DSBs, thereby overriding suppression of the mechanism driving increased synthesis of 16S rRNA when utilized alone and in combination with streptomycin.
Future studies using whole-transcriptome sequencing and high-resolution imaging would reveal holistic bacterial gene-expression profiles and mechanisms of chromatin dynamics that are disturbed during the increased susceptibility of E. coli to streptomycin in the presence of DSBs and codeine. Final isolation of the iDSB and DSBRi metabolites in their pure state would also be followed by validation of their antibacterial activities and mechanisms of action. Ultimately, it is anticipated that these approaches would lead to the identification of new bacterial cellular targets and novel antibacterial compounds that could be explored for antibiotic drug discovery.
Conclusion
Fungal secondary metabolites affecting bacterial genome stability represent new classes of antibacterial compounds that are being explored for the development of antibiotic combination chemotherapy. Codeine exacerbates the antibacterial effect of sub-MIC streptomycin in E. coli subjected to constitutive DSB formation. This effect of codeine could be useful for screening and identification of antibacterial natural products that are usually produced in very small quantities and are mostly undetected by the paradigm viability assays. Finally, this study demonstrates that even though codeine disrupts the expression of the 16S rRNA gene, its molecular mechanism is different from the known mechanism of action of streptomycin.
The increasing prevalence of drug resistant bacterial strains in clinics necessitates continual screening of underexplored natural products for the identification of novel antibacterial compounds. This menace also underscores the need to utilize unorthodox approaches, in addition to the paradigm methodologies, for the discovery of novel antibiotics that affect new cellular targets in bacteria.
A bacterial genetics-guided approach was used in this study for screening of extracts and fractions obtained from fungal fermentation cultures for the identification of candidates that exhibit antibacterial activities via either the formation of DNA double-strand breaks (DSBs) or inhibition of the concomitant repair event in Escherichia coli. A chemical biology-based approach was also used to identify already existing compounds that exacerbate the antibacterial effect of sub-MIC streptomycin in E. coli subjected to constitutive DSB formation.
A total of 8.44% of the extracts exhibited antibacterial activity via either formation of DSBs (iDSB) or inhibition of DSB repair (DSBRi) in E. coli. Pairing of iDSB extracts with DSBRi extracts demonstrated that these two categories of antibacterial extracts exhibit synergistic activity against E. coli.
Fractionation of DSBRi extracts led to the identification of fractions that exhibit iDSB activity; fractionation of iDSB extracts generated fractions that exhibits both iDSB and DSBRi activities; and fractionation of inactive extracts generated fractions endowed with iDSB or DSBRi activities.
Codeine induces a resistance mechanism to streptomycin in E. coli, which is abrogated in response to DSB induction.
Reversal of the resistance phenotype in the presence of DSBs represents a codeine-dependent increase in the sensitivity of E. coli to streptomycin.
The fitness of E. coli was further compromised by the combination of codeine with either gliclazide or N-acetylglucosamine in the presence of DSB induction and sub-minimum inhibitory concentrations (MICs) of streptomycin.
Molecular analysis demonstrated that expression of a DSBR gene and its regulators was only marginally affected by codeine, streptomycin or the combination of these compounds, in response to DSB induction. Similarly, these treatment conditions did not perturb expression of the mazF gene.
Streptomycin caused a disruption in expression of the rrsA gene, which was exacerbated in response to DSB induction. Codeine caused a significant disruption in expression of the rrsA gene, but was opposite to the effect exhibited by streptomycin. Codeine exhibited a dominant effect in the expression of the rrsA gene in the presence of streptomycin.
This study corroborates the vital role of fungal biodiversity as a source of natural antibacterial products that could be explored for novel antibiotic drug discovery.
Fungal metabolites exhibiting iDSB and DSBRi antibacterial activities have great potential for the development of new antibiotic combination chemotherapy regimens.
The DSB-dependent increase in the activity of sub-MIC streptomycin caused by codeine might be useful for the screening and identification of natural antibacterial products that are usually produced in very small quantities and are mostly undetected by paradigm viability assays.
Disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of African Academy of Sciences, NEPAD Agency, Wellcome Trust or the UK government.
Supplemental Text 1
Download MS Word (15.7 KB)Acknowledgments
V Amarh and PK Arthur express their heartfelt gratitude to Felix Selasi Dewornu, Leonard Asare and Elikem Kisser for various forms technical assistance offered to this project during their internship in our laboratory.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.4155/fdd-2023-0005
Financial disclosure
V Amarh and PK Arthur were supported by funds from a World Bank African Centres of Excellence grant (ACE02-WACCBIP: Awandare) and a DELTAS Africa grant (DEL-15-007: Awandare). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences’s Alliance for Accelerating Excellence in Science in Africa and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107755/Z/15/Z: Awandare) and the UK government. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
- Tacconelli E, Carrara E, Savoldi A et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18(3), 318–327 (2018).
- Rello J, Kalwaje Eshwara V, Lagunes L et al. A global priority list of the TOp TEn resistant Microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur. J. Clin. Microbiol. Infect. Dis. 38(2), 319–323 (2019).
- Hetzler L, Kollef MH, Yuenger V, Micek ST, Betthauser KD. New antimicrobial treatment options for severe Gram-negative infections. Curr. Opin. Crit. Care. 28(5), 522–533 (2022).
- Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 25(6), 1340 (2020).
- Jayalakshmi J, Priyadharshini MS. Restricting high-end antibiotics usage – challenge accepted!. J. Family Med. Prim. Care. 8(10), 3292–3296 (2019).
- Amarh V, Arthur PK. DNA double-strand break formation and repair as targets for novel antibiotic combination chemotherapy. Future Sci. OA 5(8), FSO411 (2019).
- Carson C, Naber KG. Role of fluoroquinolones in the treatment of serious bacterial urinary tract infections. Drugs 64(12), 1359–1373 (2004).
- Emmerson AM, Jones AM. The quinolones: decades of development and use. J. Antimicrob. Chemother. 51(Suppl. 1), 13–20 (2003).
- Wohlkonig A, Chan PF, Fosberry AP et al. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct. Mol. Biol. 17(9), 1152–1153 (2010).
- Drlica K, Malik M, Kerns RJ, Zhao X. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 52(2), 385–392 (2008).
- Eykelenboom JK, Blackwood JK, Okely E, Leach DR. SbcCD causes a double-strand break at a DNA palindrome in the Escherichia coli chromosome. Mol. Cell 29(5), 644–651 (2008).
- Avalos J, Limón MC. Fungal secondary metabolism. Encyclopedia 2, 1–13 (2022).
- Keller NP, Turner G, Bennett JW. Fungal secondary metabolism – from biochemistry to genomics. Nat. Rev. Microbiol. 3(12), 937–947 (2005).
- Schmitt EK, Hoff B, Kück U. Regulation of cephalosporin biosynthesis. Adv. Biochem. Eng. Biotechnol. 88, 1–43 (2004).
- Petersen AB, Rønnest MH, Larsen TO, Clausen MH. The chemistry of griseofulvin. Chem. Rev. 114(24), 12088–12107 (2014).
- Limón MC, Rodríguez-Ortiz R, Avalos J. Bikaverin production and applications. Appl. Microbiol. Biotechnol. 87(1), 21–29 (2010).
- Survase SA, Kagliwal LD, Annapure US, Singhal RS. Cyclosporin A – a review on fermentative production, downstream processing and pharmacological applications. Biotechnol. Adv. 29(4), 418–435 (2011).
- Mulder KC, Mulinari F, Franco OL, Soares MS, Magalhães BS, Parachin NS. Lovastatin production: from molecular basis to industrial process optimization. Biotechnol. Adv. 33(6 Pt 1), 648–665 (2015).
- Amna T, Amina M, Sharma PR et al. Effect of precursors feeding and media manipulation on production of novel anticancer pro-drug camptothecin from endophytic fungus. Brazilian journal of microbiology. Braz. Soc. Microbiol. 43(4), 1476–1490 (2012).
- Aboagye SY, Amarh V, Lartey PA, Arthur PK. Wood-decaying fungi found in southern Ghana: a potential source of new anti-infective compounds. AAS Open Res. 2, 20 (2019).
- Blessie EJ, Wruck W, Abbey BA et al. Transcriptomic analysis of marine endophytic fungi extract identifies highly enriched anti-fungal fractions targeting cancer pathways in HepG2 cell lines. BMC Genom. 21(1), 265 (2020).
- Greco C, Keller NP, Rokas A. Unearthing fungal chemodiversity and prospects for drug discovery. Curr. Opin. Microbiol. 51, 22–29 (2019).
- Amarh V, White MA, Leach DRF. Dynamics of RecA-mediated repair of replication-dependent DNA breaks. J. Cell. Biol. 217(7), 2299–2307 (2018).
- Hegemann JD, Birkelbach J, Walesch S, Müller R. Current developments in antibiotic discovery: global microbial diversity as a source for evolutionary optimized anti-bacterials. EMBO Rep. 24(1), e56184 (2023).
- Walesch S, Birkelbach J, Jézéquel G et al. Fighting antibiotic resistance-strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 24(1), e56033 (2023).
- Miethke M, Pieroni M, Weber T et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 5(10), 726–749 (2021).
- Baranova AA, Alferova VA, Korshun VA, Tyurin AP. Modern trends in natural antibiotic discovery. Life (Basel). 13(5), 1073 (2023).
- Niu G, Annamalai T, Wang X et al. A diverse global fungal library for drug discovery. PeerJ. 8, e10392 (2020).
- Karwehl S, Stadler M. Exploitation of fungal biodiversity for discovery of novel antibiotics. Curr. Top. Microbiol. Immunol. 398, 303–338 (2016).
- Conrado R, Gomes TC, Roque GSC, De Souza AO. Overview of bioactive fungal secondary metabolites: cytotoxic and antimicrobial compounds. Antibiotics (Basel) 11(11), 1604 (2022).
- Abdelmohsen UR, Sayed AM, Elmaidomy AH. Natural products’ extraction and isolation-between conventional and modern techniques. Front. Nat. Prod. 1, 1 (2022).
- Afsar T, Razak S, Almajwal A et al. Bioassay-guided isolation and characterization of lead antimicrobial compounds from Acacia hydaspica plant extract. AMB Express 12(1), 156 (2022).
- Najmi A, Javed SA, Al Bratty M, Alhazmi HA. Modern approaches in the discovery and development of plant-based natural products and their analogues as potential therapeutic agents. Molecules 27(2), 349 (2022).
- Rosén J, Gottfries J, Muresan S, Backlund A, Oprea TI. Novel chemical space exploration via natural products. J. Med. Chem. 52(7), 1953–1962 (2009).
- Atanasov AG, Zotchev SB, Dirsch VM; International Natural Product Sciences Taskforce; Supuran CT. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20(3), 200–216 (2021).
- Weller MG. A unifying review of bioassay-guided fractionation, effect-directed analysis and related techniques. Sensors (Basel) 12(7), 9181–9209 (2012).
- Baumann S, Herrmann J, Raju R et al. Cystobactamids: myxobacterial topoisomerase inhibitors exhibiting potent antibacterial activity. Angew. Chem. Int. Ed. Engl. 53(52), 14605–14609 (2014).
- Owusu Obeng A, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy 37(9), 1105–1121 (2017).
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6(1), 29–40 (2007).
- Sinha AK, Possoz C, Leach DRF. The roles of bacterial DNA double-strand break repair proteins in chromosomal DNA replication. FEMS Microbiol. Rev. 44(3), 351–368 (2020).
- Alekseev A, Pobegalov G, Morozova N et al. A new insight into RecA filament regulation by RecX from the analysis of conformation-specific interactions. Elife 11, e78409 (2022).
- Petrova V, Chen SH, Molzberger ET et al. Active displacement of RecA filaments by UvrD translocase activity. Nucleic Acids Res. 43(8), 4133–4149 (2015).
- Carter AP, Clemons WM, Brodersen DE, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407(6802), 340–348 (2000).
- Demirci H, Murphy F 4th, Murphy E, Gregory ST, Dahlberg AE, Jogl G. A structural basis for streptomycin-induced misreading of the genetic code. Nat. Commun. 4, 1355 (2013).

