Abstract
Objective
Paracetamol has an established place in the management of mild-to-moderate pain, but has certain limitations, including varying bioavailability, and potential hepatotoxicity if taken in overdose. Effervescent formulations may help to overcome these limitations.
Methods
Pubmed searches, with no limits on date or language, were conducted in February 2020. Further references were identified from the reference lists of retrieved articles, and from the authors’ knowledge of the field.
Results
Effervescent formulations contain an organic acid (usually citric acid) and carbonate or bicarbonate salts (usually sodium bicarbonate). Upon contact with water, these react to form carbon dioxide, which facilitates the disintegration of the tablet and dissolution of the active drug. Moreover, sodium bicarbonate dose-dependently increases gastric emptying, which together with rapid dissolution facilitates drug absorption. In pharmacokinetic studies, effervescent formulations result in faster absorption of paracetamol than conventional oral formulations, and this translates into a faster onset of analgesia in clinical trials. Effervescent paracetamol has a favorable safety profile, with good tolerability. Importantly, the sodium content of some preparations does not appear to increase cardiovascular risk under real world conditions. Effervescent formulations may also offer advantages in terms of ease of administration and palatability.
Conclusions
Effervescent formulations of paracetamol result in faster drug absorption, and hence more rapid analgesia, than oral tablets, and offer a favorable tolerability and safety profile. The use of such formulations may therefore help to promote appropriate use of paracetamol.
Introduction
Paracetamol (acetaminophen) has an established place in the management of mild-to-moderate painCitation1–2, and numerous prescription-only and over-the counter formulations are available worldwide. Indeed, it is one of the most widely used analgesics in the World; in France, for example, the use of paracetamol has increased by 53% between 2006 and 2015, and consumption in France is currently the highest in Europe, with an annual consumption of approximately 10,000 tonsCitation3,Citation4. Paracetamol has a well-established benefit-risk profileCitation5 and is consistently recommended in most national and international guidelines as first-line therapy for common pain conditions such as dental pain, migraine, acute back pain, and postoperative painCitation6. Although the mechanism of action of paracetamol remains to be clearly defined, the analgesic effect appears to be centrally mediated, and involves direct and indirect inhibition of cyclooxygenase 1 and 2, in addition to activation of endogenous cannabinoids and spinal serotonergic pathwaysCitation7‒9.
However, although the beneficial analgesic and antipyretic effects of paracetamol are well documented, the drug does have certain limitations. For example, although it is rapidly and completely absorbed from the gastrointestinal tract following oral administration, its systemic bioavailability is reduced by dose-dependent first-pass metabolism in the liver, such that the oral bioavailability may range from only 40‒50% to 80‒90%, depending on the dose and formulationCitation2,Citation5,Citation10–14. Reduced bioavailability may compromise the attainment of therapeutic circulating drug concentrations. Furthermore, paracetamol overdose is associated with a significant risk of hepatotoxicityCitation15.
The pharmacokinetic limitations of conventional paracetamol formulations may be largely overcome by the use of effervescent formulations. According to the current European PharmacopoeiaCitation16, “Effervescent tablets are uncoated tablets generally containing acid substances and carbonates or hydrogen carbonates, which react rapidly in the presence of water to release carbon dioxide. They are intended to be dissolved or dispersed in water before administration”. According to the European criteria, the tablets should effervesce and disintegrate within 5 min when placed in 200 mL of water at 15–25 °C. Similarly, the United States Pharmacopoeia (USP) defines effervescent tablets as tablets prepared by compaction which contain, in addition to the drug substance(s), mixtures of acids (e.g. citric acid or tartaric acid) and carbonates, and/or sodium bicarbonateCitation17. Upon contact with water, these formulations release carbon dioxide, producing the characteristic effervescent action. They are considered as noncoated tablets, which should disintegrate within 15 min. However, there is no specific test for effervescent tablets in the USP.
Although there appear to be no published studies showing that effervescence alters the metabolism of paracetamol, effervescent paracetamol formulations do offer a number of potential biopharmaceutical and pharmacokinetic advantages over conventional formulations. Effervescence accelerates the disintegration of tablets, increases paracetamol dissolution, and renders the drug more hydrophilic;Citation18 in addition, effervescence increases gastric pH, thereby reducing drug contact time with the gastric mucosa and protecting the active drug from inactivation in the stomachCitation19,Citation20. Together, these properties result in a faster onset of action. However, the need to achieve rapid analgesia should be weighed against the need to facilitate appropriate use of the drug, avoiding the risk of overdose.
To data, no reviews of the use of paracetamol in various settings appear to have focused specifically on effervescent formulations. Hence, this paper aims to discuss the pharmaceutical, pharmacokinetic and clinical properties of effervescent paracetamol formulations, and the relative benefits and risks of these preparations.
Methods
Pubmed literature searches, with no limits on date or language, were conducted in February 2020, using various combinations of keywords including “effervescent paracetamol”, “effervescent acetaminophen”, “pharmacokinetics” and “safety”. Further references were identified by searching the reference lists of retrieved articles, and from the authors’ knowledge of the field. The final selection of references was the responsibility of the authors.
What are the biopharmaceutical characteristics of effervescent paracetamol formulations?
As described aboveCitation16,Citation17, effervescent paracetamol tablets contain as excipients an organic acid, typically citric or tartaric acid, and alkali metal carbonates or bicarbonates (typically sodium carbonate or bicarbonate)Citation18. In the presence of water, the acid reacts with the carbonate/bicarbonate to form carbon dioxide: this creates turbulence that reduces the thickness of the boundary diffusion layer at the surface of the tablet, thereby enhancing the disintegration of the tablet and dissolution of the active principleCitation21. Citric acid is the acid most widely used in effervescent formulations, partly due to its pleasant citrus taste; other acids, such as tartaric or fumaric acids, are typically used only in small amounts, due to their low solubility in waterCitation22. In in vitro experiments, sodium bicarbonate was shown to increase the dissolution of paracetamol from tablets at concentrations of 6300 or 31500 mg/LCitation23. As a result of this faster disintegration and dissolution, the drug is already in solution when it reaches the gastrointestinal tract, and hence, absorption is faster than with solid paracetamol tabletsCitation18. A significant correlation has been reported between the faster in vitro dissolution rate of an effervescent paracetamol formulation containing sodium bicarbonate, compared with standard oral tablets, and in vivo absorptionCitation24.
In addition to facilitating disintegration of the tablet, the use of sodium bicarbonate as excipient has been reported to increase the rate of gastric emptying, as measured by gamma scintigraphy, compared with conventional immediate-release solid formulationsCitation21. This increase appears to be dose-dependent up to isotonic bicarbonate concentrations of 12600 mg/LCitation1,Citation25. Since the rate of absorption of paracetamol is a function of gastric emptying rateCitation26, this effect of sodium bicarbonate might be expected to further accelerate the absorption of paracetamol from effervescent formulationsCitation1. This in turn may be expected to increase the bioavailability of paracetamol, expressed as the area under the concentration-time curve (AUC): this may be reported as the total AUC extrapolated to infinity (AUC0-∞) or the partial AUC at a specified time after dosing (AUC0-t). Evidence for such effects comes from a study in 12 healthy volunteers, in which effervescent paracetamol tablets were associated with both faster dissolution and more rapid gastric emptying, compared with conventional oral tablets. There were significant correlations between gastric emptying times and paracetamol bioavailability, as indicated by partial area under the curve (AUC), suggesting faster absorption of paracetamolCitation21. This faster absorption might in turn be expected to translate into a faster onset of analgesiaCitation27.
It should be noted that, while many effervescent paracetamol formulations are based on sodium bicarbonate, some also contain sodium or calcium carbonatesCitation28. Although each of these compounds produces carbon dioxide on exposure to gastric acid, the available evidence suggests that they may differ in their effects on paracetamol absorption. For example, in a pharmacokinetic study in healthy volunteers, the addition of calcium carbonate, 375 mg, to oral conventional immediate-release paracetamol 500 mg tablets had no significant effect on paracetamol absorption, whereas sodium bicarbonate, 400 or 630 mg, significantly increased the absorption rateCitation29. This would suggest that the enhanced paracetamol absorption seen with effervescent formulations is not due to an effect of carbonation per se on the permeability of the gastrointestinal epitheliumCitation24, as has been suggested by othersCitation30,Citation31.
Because effervescent paracetamol is administered as a solution, the volume of liquid in which the tablet is dissolved may also be relevant to the speed of absorptionCitation1. Dissolution of approximately 1.26 g of sodium bicarbonate in 100 mL water produces a solution of approximately 12600 mg/LCitation29, which as noted above is the concentration at which the effect of bicarbonate on gastric emptying is maximalCitation25. The volume of liquid administered might in theory be a consideration in certain patient groups in whom it may be desirable to encourage fluid intake, such as children or elderly patients, particularly when a febrile condition is presentCitation32.
The use of sodium bicarbonate as an excipient may also be a consideration in some patient groups, notably the elderly, hypertensive patients, or those with heart failure, in whom a low-sodium diet may be required. However, as will be discussed below, evidence for a link between sodium exposure through effervescent medications and an increased risk of hypertension or cardiovascular disease is equivocal.
How does the dosage form affect the pharmacokinetics of effervescent paracetamol formulations?
Paracetamol is categorized as a Biopharmaceutical Classification System (BCS) I active principleCitation33, indicating that it is highly water-soluble and well absorbed (≥ 85%) from the intestineCitation34,Citation35. Following administration of paracetamol in solution, peak plasma concentrations may be attained within 15‒30 min in fasting individuals, although absorption from conventional (non-effervescent) tablet formulations is slower and more variable; mean systemic bioavailability is approximately 75%Citation36. Paracetamol is predominately metabolized in the liver to form sulfate and glucuronide conjugates that are excreted in the urine, and only 2‒5% of a therapeutic dose is excreted unchangedCitation2,Citation36. Approximately 5‒10% of the dose if converted to a potentially hepatotoxic intermediate, N-acetyl-para-benzoquinone imine (NAPQI). This metabolite is normally converted via glutathione to form non-toxic mercapturic acid, which is then excreted in the urine. However, paracetamol overdose can lead to glutathione depletion and accumulation of toxic concentrations of NAPQI in the liverCitation2,Citation15.
The pharmacokinetics of effervescent paracetamol formulations have been evaluated in a number of studies in healthy volunteersCitation1,Citation10,Citation19,Citation29,Citation37. In general, these studies have shown that paracetamol is absorbed more quickly with effervescent formulations than with conventional immediate-release tablets, and that the bioavailability of paracetamol ‒ measured as the AUC ‒ is at least comparable with both modes of administration (). For example, in an early study, 20 healthy volunteers received 1000 mg paracetamol either as two oral conventional immediate-release tablets or as two effervescent tablets, with a three-week washout period between treatmentsCitation37. The mean time to maximum serum concentrations (Tmax) was significantly shorter with the effervescent formulation than with the conventional oral tablets (27 min [95% confidence interval 20‒34] versus 45 min [95% CI 34‒56], respectively, p = .004). In addition, although there was no significant difference in the maximum concentration attained (Cmax), the mean AUC measured at 3 h after dosing was significantly higher with the effervescent formulation (33.8 mg.h/L versus 30.0 mg.h/L, p = .003), indicating greater short-term bioavailability of paracetamol. However, the total AUC was not reported in this study. Similar results were obtained in a study comparing the pharmacokinetics of paracetamol delivered via two commercially available tablet formulations (one conventional formulation and one soluble formulation) and three developmental effervescent formulations containing various amounts of sodium bicarbonate (400 or 630 mg) or calcium carbonate (375 mg)Citation29. The addition of 630 mg sodium bicarbonate resulted in a significant reduction in Tmax, compared with both commercially available formulations (), a finding which the authors attributed to faster gastric emptying, and hence faster absorption from the small intestineCitation29.
Table 1. Pharmacokinetic studies with effervescent paracetamol formulations in healthy volunteers.
In a further study, 30 healthy volunteers received 500 mg paracetamol in conventional immediate-release tablets and capsules or effervescent formulationsCitation19. There was a statistically significant (p < .05) difference in serum paracetamol concentrations with the three formulations. The mean (SD) concentrations measured 60 min after dosing were 6.6 (2.4) mg/L with the tablet formulation, 11.3 (3.9) mg/L with the capsule formulation, and 15.3 (2.5) mg/L with the effervescent formulation.
The formulations evaluated in these studies used sodium salts, primarily sodium bicarbonate, as the carbonating agent.
A further studyCitation38 compared the pharmacokinetics of conventional and effervescent formulations of a fixed-dose combination of tramadol and paracetamol (75 mg/650 mg) in 26 patients who had undergone total gastric resection. Patients were randomized to receive a single dose of either formulation, given as two tablets (37.5 mg/325 mg), and plasma concentrations of each drug were measured over 10 h after dosing. Cmax of paracetamol was higher with the effervescent formulation than with the conventional formulation, as indicated by an effervescent/conventional Cmax ratio of 1.16 (90% CI 1.06‒1.27). The bioavailability of paracetamol from the two formulations was similar, as shown by a mean AUC0-t ratio of 0.99 (90% CI 0.88‒1.10).
Does food affect the pharmacokinetics of effervescent paracetamol?
Food has been reported to delay the absorption of paracetamol, possibly due to a prolongation of gastric emptying timeCitation1,Citation39,Citation40, and a number of studies have investigated the pharmacokinetics of effervescent paracetamol formulations in fed and fasted participantsCitation1,Citation41,Citation42. Rostami-Hodjegan et al.Citation42, for example, found that paracetamol was absorbed more quickly (i.e. shorter Tmax) from an effervescent formulation than from conventional tablets in both the fed and fasted states. With both formulations, the proportion of the dose released from the tablet was higher, and the mean time to release faster, in the fed state than in the fasted state. Similarly, both Di Girolamo et alCitation1 and Ibáñez et al.Citation41 have shown that the absorption of paracetamol is significantly faster with effervescent formulations than with conventional tablets in healthy volunteers fed a standard breakfast, with no significant differences in the extent of absorption, as measured by AUC, between effervescent and conventional formulations.
How efficacious are effervescent paracetamol formulations?
Early studies assessing the analgesic effect of different paracetamol formulations, have suggested that the onset of analgesia with conventional formulations of paracetamol may vary from 15 min to as long as 90 minCitation43‒45. An important question, therefore, is whether the faster absorption that can be achieved with effervescent paracetamol formulations translates into a faster onset of action.
This question was addressed in a randomized, double-blind, placebo-controlled trial involving 242 patients with moderate or severe pain following dental surgery, who were randomized to receive paracetamol 1000 mg given via either an effervescent formulation or a conventional tablet formulationCitation27. Using a double stopwatch technique, patients recorded both the time to first perceptible pain relief and the time to significant pain relief (defined as the earliest time when they were sure that the drug was working). In addition, pain intensity was assessed over 4 h after dosing, using both a 4-point categorical rating scale (0 = none; 1 = mild; 2 = moderate; 3 = severe) and a 100 mm visual analog scale (VAS). Pain relief was defined in terms of the Pain Intensity Difference (PID) from baseline in categorical and VAS scores, the Summed Pain Intensity Difference (SPID), defined as the sum of the PID scores weighted by the time interval between measurements, and Total Pain Relief (TOTPAR), defined as the sum of the pain relief values weighted by the time interval between measurements.
The median (95% CI) time to onset of analgesia was significantly shorter with effervescent paracetamol than with the conventional immediate-release tablet formulation (0.34 [0.33‒0.58] hours versus 0.75 (0.50‒0.75] hours p = .011) (), as was the median time to meaningful pain relief (0.75 [0.50‒1.00] versus 1.00 [1.00‒1.13] hours, respectively, p = .01). Overall, 70% of patients in each active treatment group experienced some degree of pain relief, compared with 19% of those receiving effervescent placebo and 15% of those receiving tablet placebo.
Figure 1. Kaplan–Meier plot showing time to onset of analgesia in patients with moderate-to-severe pain following dental surgery treated with 1000 mg effervescent paracetamol (solid squares), 1000 mg paracetamol tablets (solid triangles), effervescent placebo (open squares) or tablet placebo (open triangles). Reproduced with permission from Moller PL, et al. Citation27.
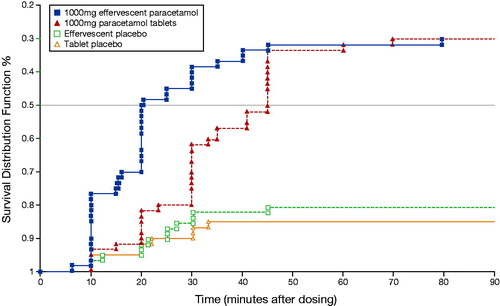
Effervescent paracetamol was significantly more effective than the tablet formulation at all time points to 45 min after dosing, when measured by both the VAS and the categorical rating scale. Mean SPID and TOTPAR values were similar in both active treatment groups, and were significantly higher than in the corresponding placebo groups.
What are the relative benefits and risks of effervescent paracetamol?
Paracetamol is the most widely used analgesic and antipyretic agent worldwide, and has been available without prescription for more than 60 yearsCitation2. In general, it is considered to be a safe drug, and the first choice therapy for painCitation3,Citation6,Citation46. However, as noted above, the bioavailability of conventional paracetamol formulations is limited by first pass metabolism, possibly leading to delayed or suboptimal efficacy. Furthermore, in recent years, concerns about the adverse effects of accidental or deliberate overdosing have led to the introduction of restrictions on pack sizes and availability from non-pharmacy outlets in a number of European countriesCitation47. These concerns largely relate to hepatotoxicity: paracetamol overdosage is a principal cause of acute liver failure, and a major cause of liver transplantation in Europe and the United StatesCitation2,Citation15,Citation47. The relative benefits and risks of effervescent paracetamol should therefore be considered in the context of the need both to achieve rapid analgesia and to facilitate appropriate use of the drug, avoiding the risk of overdose.
Do effervescent formulations produce therapeutic drug concentrations?
Based on studies in children, it has been reported that circulating paracetamol concentrations of 10.6‒34.8 mg/L are needed to produce effective analgesiaCitation10,Citation37,Citation48, and it is generally assumed that this also applies to adultsCitation49,Citation50. However, the absorption kinetics of paracetamol following administration of conventional tablet formulations may vary markedlyCitation1,Citation29], and this might compromise a rapid attainment of adequate analgesic concentrations in many patients.
The pharmacokinetic studies reviewed aboveCitation1,Citation10,Citation19,Citation29,Citation37 show that these effective paracetamol concentrations are attained following administration of 500‒1000 mg effervescent paracetamol (). Furthermore, peak concentrations are achieved more quickly with effervescent paracetamol than with conventional immediate-release tablets, and this might be expected to translate into a faster onset of analgesia. For example, in the pharmacokinetic study by Rygnestad et al.Citation37, 85% of participants receiving effervescent paracetamol had attained a serum concentration of at least 10.6 mg/L (the lower limit of the therapeutic range) 15 min after dosing, compared with only 10% of those receiving conventional tablets. Evidence that this faster absorption does indeed result in more rapid analgesia comes from the clinical study in dental surgery patients by Møller et al.Citation27, in which both the median time to onset of analgesia, and the median time to meaningful pain relief, were significantly shorter with effervescent paracetamol than with conventional tablets. This is an important consideration because, in patients with acute pain, the speed of onset of analgesia is an important factor determining the likelihood of effective and sustained pain relief.Citation40,Citation51‒53.
The faster absorption of paracetamol from effervescent formulations, compared with conventional immediate-release tablets, is believed to be due to a combination of faster disintegration of the effervescent tablet leading to accelerate dissolution of the active principle, and a sodium bicarbonate-induced increase in the rate of gastric emptyingCitation1,Citation18,Citation21,Citation25. Because gastric emptying is slower after feeding than in the fasted state, it might be anticipated that administration of paracetamol with food would reduce absorption, resulting in a delayed or attenuated analgesic effect. A systematic review of 38 pharmacokinetic studies with immediate-release formulations of different analgesics found that food increased the Tmax of paracetamol by 32% and reduced Cmax by 42%Citation40. This reduction in peak drug concentrations when paracetamol is taken with food might be expected to reduce the analgesic efficacy. Reassuringly, pharmacokinetic studies have consistently found that coadministration with food does not appear to affect the faster absorption offered by effervescent paracetamol, compared with tabletsCitation1,Citation41,Citation42. This finding is clinically relevant because it may be assumed that coadministration with food reflects the conditions under which over-the-counter analgesics such as paracetamol are likely to be used in real-world settingsCitation1.
Safety of effervescent paracetamol
Adverse event profiles
The clinical experience with effervescent paracetamol products suggests that this form of administration is safe and well tolerated. In the study in dental surgery patients described aboveCitation27, 40% of patients in both the effervescent paracetamol and tablet paracetamol groups reported adverse effects, and the total number of adverse events was similar in both groups. The majority of adverse events (94‒97%) were mild or moderate in severity, and none were considered by the investigators to be related to study treatment. The most common adverse events in both groups were pain (effervescent paracetamol: n = 11, tablet paracetamol: n = 12), headache (n = 12 and n = 5, respectively) and a local site reaction referred to as ‘dry socket’ (n = 5 and n = 6, respectively)Citation27.
There appear to be no studies of the efficacy of effervescent paracetamol in specific patient populations, such as pregnant or lactating women, and cancer patients. As noted in the product labeling, extensive data have shown that paracetamol treatment at the lowest effective dose is not associated with significant safety concernsCitation28.
Risk of paracetamol overdose with effervescent paracetamol
Paracetamol overdosage is associated with significant hepatotoxicity, and accounts for 40‒70% of cases of acute liver failure in Europe and the United KingdomCitation15. Thus, although paracetamol is generally considered a safe drug, the risk of accidental or deliberate paracetamol overdose leading to hepatotoxicity represents a significant public health concernCitation47. In general, the risk of overdose with effervescent paracetamol might be considerably lower with effervescent formulations than with oral tablets, for a number of reasonsCitation54,Citation55. First, the sodium content of each tablet may have significant emetic effects, and the production of large amounts of carbon dioxide if multiple doses are taken could cause further nausea and vomiting. In addition, it is difficult to dissolve more than 6‒8 tablets in the same glass: a significant volume of water would be required to dissolve more than 1‒2 tablets.
A single case reportCitation54 has described a patient with potentially hepatotoxic serum levels of paracetamol (142 mg/L) after the ingestion of 16 effervescent tablets, apparently over a period of 3 h. The patient was successfully treated with intravenous N-acetylcysteine. The authors emphasized that intoxication with effervescent paracetamol is much less common than with oral tablets, which limits the risk of a single massive overdose leading to life-threatening hepatotoxicity. They therefore concluded that ‘this case report does not modify the important assertion that effervescent paracetamol use could limit the risk of life-threatening intoxication by a single massive overdose,’ and that ‘there is therefore no need to discourage the use of effervescent paracetamol’Citation54. It is noteworthy that in Sweden conventional paracetamol tablets were withdrawn from non-pharmacy outlets in 2015, and hence effervescent formulations are the only formulations available on an over-the-counter basisCitation47.
Clinical implications of sodium exposure through effervescent products
The total sodium exposure for a patient taking effervescent paracetamol will depend on both the daily dose and the sodium content of the formulation. The sodium content of effervescent paracetamol products varies markedly. It has been reportedCitation56 that some effervescent formulations of paracetamol 500 mg can contain up to 16.9 mmol of sodium per tablet, and hence the maximum recommended dose of eight tablets per day would result in an intake of 135.2 mmol sodium, which exceeds the maximum daily intake recommended by the World Health Organization (WHO)Citation57. However, a number of low-sodium effervescent formulations are available.
Because effervescent paracetamol formulations typically contain sodium bicarbonate, concerns have been expressed that high levels of sodium in regular users of such preparations might trigger or exacerbate cardiovascular disorders, such as hypertension or heart failure, if taken regularly or in high dosesCitation56,Citation58. Observational studies in small numbers of patients have suggested a link between exposure to sodium via soluble or effervescent drug formulations and poorly controlled blood pressureCitation59,Citation60. In addition, case–control studies in primary care patients in the United Kingdom found significant associations between the use of sodium-containing medications and increased risks of hypertension, stroke and all-cause mortality, compared with users of standard formulations of the same drugsCitation56,Citation61. More recently, a randomized crossover trial in 46 patients reported that mean 24-hour systolic blood pressure was significantly higher in hypertensive patients taking effervescent paracetamol 1 g three times daily for 3 weeks, compared with a non-effervescent formulation (mean treatment difference 4.0 mm Hg, 95% confidence interval 1.4‒6.6 mm Hg, p = .004)Citation62. It should be noted that approximately 31% of patients were receiving antihypertensive therapy on enrolment into the study. Furthermore, patients received paracetamol daily for 7 weeks, whereas such prolonged exposure would not normally be expected in real world conditionsCitation63.
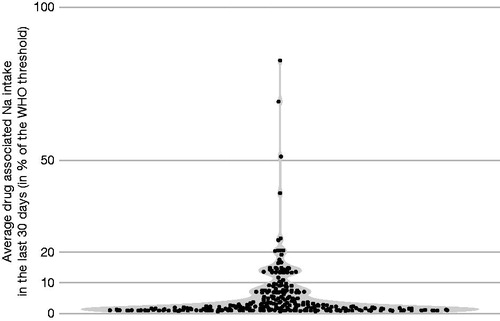
The available real world evidence suggests that the majority of people taking effervescent medications are not exposed to excessively high amounts of sodium. In an observational study involving 1043 French participants undergoing routine health checks, 26.9% had been exposed to effervescent medications during the previous 30 days, and 7.3% had used these medications at least twice per week during this periodCitation63. Participants with conditions associated with a low sodium requirement, such as hypertension or diuretic treatment, were as likely to be using effervescent tablets as those for whom sodium intake was not a concern. Paracetamol was the most widely used effervescent drug, accounting for approximately 40‒45% of cases at all levels of exposure. Calculation of the daily drug-related sodium intake in each participant exposed to effervescent medications showed that the majority of participants had a drug-related sodium intake that was less than 10% of the WHO recommended daily intake (). There appears to be only a single case report of hypernatremia associated with effervescent paracetamol useCitation64.
Figure 2. Smoothed estimation of daily drug-related sodium exposure in French participants (n = 281) who reported using effervescent medications within the previous 30 days. Data are presented as a percentage of the World Health Organization (WHO) recommended daily sodium intake of 2 g. Each dot represents a single participant. Reproduced with permission from Perrin et al. Citation63.
Moreover, several experimental studies in different animal models or in humansCitation65‒67 show that the type of sodium salt must be taken into consideration when assessing the physiological effects of sodium exposure. Indeed, these studies show that the intake of sodium bicarbonate or of sodium chloride does not have the same effects on several biological or hemodynamic parameters such as blood pressureCitation68‒70. By contrast, the preparation used in the crossover trial described above, which showed a significant increase in blood pressure with effervescent paracetamol, contained sodium citrateCitation62. These findings suggest that while the amount of sodium in effervescent formulations should be taken into consideration in specific patient populations, such as the elderly, hypertensive patients, and patients following a low-salt diet, this is unlikely to be a concern for most users. Although there appear to be no published data to support this suggestion, it is generally recommended that effervescent paracetamol should only be used for short periods (≤7 days), as there may be a potential transient increase in cardiovascular risk during this periodCitation1.
Patients’ perspectives on effervescent formulations
The faster onset of action offered by effervescent formulations is an important consideration for patients. There is evidence that consumers may regard an over-the-counter product as having a fast action if pain relief begins within 30 min of dosingCitation71,Citation72. In addition to the potential for a faster onset of action, patients may opt for an effervescent paracetamol formulation for a number of reasons. These include ease of administration (most oral formulations of paracetamol are often relatively large, and therefore difficult to swallow) and greater palatability compared with some other liquid formulations or uncoated tablets (which often have an unpleasant bitter taste [Citation18]. In the French observational study described aboveCitation63, users of effervescent medications were asked to rate their reasons for preferring such formulations on 10-point Likert scales (where 1=‘not at all important’ and 10=‘most important to me’). The two most important factors were a perceived rapidity of action and ease of swallowing, with mean Likert scores of 4.7 and 4.6, respectively. For both of these, there were no significant differences in Likert scores between occasional users (defined as participants using effervescent medications no more than once per week) and frequent users (defined as those using effervescent medications at least twice per week). However, compared with occasional users, frequent users expressed significantly higher preferences due to taste and pleasurable aspects of taking effervescent medications.
Clinical trials with other effervescent medications have also shown that taste and ease of administration are important considerations for users of effervescent medicationsCitation73,Citation74. In a study in healthy children, 71% of participants preferred the taste of an effervescent ranitidine formulation to that of a syrup formulation, and the same proportion of adults indicated that they would prefer to administer the effervescent formulation for this reasonCitation73. This is an important consideration, given that paracetamol has an established place in the symptomatic management of febrile illness in childrenCitation75. Similarly, in a study in patients aged 65 years and older, participants reported that effervescent and orally disintegrating formulations were the most acceptable means of administering medications, and this preference was particularly marked among participants with dysphagiaCitation74. A strong preference for effervescent medication, rather than a viscous suspension, has also been reported in a clinical trial in patients receiving budesonide for the treatment of eosinophilic esophagitisCitation76.
The need to take effervescent preparations with water may in theory be regarded as an inconvenience by some users. However, there appear to be no published data showing that this is the case.
Limitations of this review
A limitation of this review is its narrative format. However, extensive literature searches (albeit not systematic) were performed to inform the discussion, and given the sparseness of the literature on effervescent paracetamol it seems unlikely that significant publications have been missed.
Conclusions
In addition to paracetamol, effervescent formulations are available for a variety of medications, including vitamin C, aspirin, antacids, betaine, glucosamine, and calcium supplementsCitation19,Citation63. The diversity of such preparations reflects the potential advantages of this route of administration, notably a rapid onset of action and convenient, palatable, dosingCitation63.
The studies reviewed in this paper clearly show that effervescent formulations of paracetamol result in faster absorption of the drug than oral tablets, and that this translates into more rapid analgesia. Furthermore, effervescent paracetamol formulations offer a favorable tolerability and safety profile; in addition, they might offer a low risk of overdosage lower, and thus of hepatotoxicity compared with oral tabletsCitation54. The use of such formulations may therefore help to promote appropriate use of paracetamol, thereby reducing the substantial public health burden associated with paracetamol overdosesCitation2.
Transparency
Declaration of funding
Claude Dubray and Philippe Maincent received honoraria from UPSA for the development of this paper.
Declaration of interest
Jean Yves Milon is an employee of UPSA. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed equally to the conceptualization, scope and content of the review. All authors critically reviewed each draft of the paper, and approved the final version for publication. All authors agree to be accountable for all aspects of the work.
Acknowledgements
Medical writing and editorial support in the preparation of this paper was provided by Content Ed Net, France, and funded by UPSA.
References
- Di Girolamo G, Opezzo JA, Lopez MI, et al. Relative bioavailability of new formulation of paracetamol effervescent powder containing sodium bicarbonate versus paracetamol tablets: a comparative pharmacokinetic study in fed subjects. Exp Opin Pharmacother. 2007;8(15):2449–2457.
- Tittarelli R, Pellegrini M, Scarpellini MG, et al. Hepatotoxicity of paracetamol and related fatalities. Eur Rev Med Pharmacol Sci. 2017;21(1 Suppl):95–101.
- Hider-Mlynarz K, Cavalié P, Maison P. Trends in analgesic consumption in France over the last 10 years and comparison of patterns across Europe. Br J Clin Pharmacol. 2018;84(6):1324–1334.
- Moore RA, Moore N. Paracetamol and pain: the kiloton problem. Eur J Hosp Pharm. 2016;23(4):187–188.
- Jibril F, Sharaby S, Mohamed A, et al. Intravenous versus oral acetaminophen for pain: systematic review of current evidence to support clinical decision-making. Can J Hosp Pharm. 2015;68(3):238–247.
- Hagen M, Alchin J. Nonprescription drugs recommended in guidelines for common pain conditions. Pain Manag. 2020;10(2):117–129.
- Graham GG, Davies MJ, Day RO, et al. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21(3):201–232.
- Jozwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharmaceut. 2014;71:11–23.
- Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263–275.
- Sevilla-Tirado FJ, Gonzalez-Vallejo EB, Leary AC, et al. Bioavailability of two new formulations of paracetamol, compared with three marketed formulations, in healthy volunteers. Methods Find Exp Clin Pharmacol. 2003;25(7):531–535.
- Ameer B, Divoll M, Abernethy DR, et al. Absolute and relative bioavailability of oral acetaminophen preparations. J Pharm Sci. 1983;72(8):955–958.
- Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokin. 1982;7:93–107.
- Rawlins MD, Henderson DB, Hijab AR. Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol. 1977;11(4):283–286.
- Singla NK, Parulan C, Samson R, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12(7):523–532.
- Lee WM. Acetaminophen (APAP) hepatotoxicity-Isn't it time for APAP to go away? J Hepatol. 2017;67(6):1324–1331.
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (Ph. Eur.), 10th Edition; 2019. Available from: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition..
- USP 43-NF 38 (United States Pharmacopeia-National Formulary): United States Pharmacopeial Convention; 2019. Available from: https://www.uspnf.com/.
- Srinath KR, Chowdary CP, Palanisamy P, et al. Formulation and evaluation of effervescent tablets of paracetamol. Int J Pharmaceut Res Dev (IJPRD). 2011;3:76–104.
- Fathi M, Kazemi S, Zahedi F, et al. Comparison of oral bioavailability of acetaminophen tablets, capsules and effervescent dosage forms in healthy volunteers. Curr Issues Pharmacy Med Sci. 2018;31(1):5–9.
- Moes AJ. Gastroretentive dosage forms. Crit Rev Therap Drug Carrier Syst. 1993;10(2):143–195.
- Kelly K, O'Mahony B, Lindsay B, et al. Comparison of the rates of disintegration, gastric emptying, and drug absorption following administration of a new and a conventional paracetamol formulation, using gamma scintigraphy. Pharmaceut Res. 2003;20(10):1668–1673.
- Ipci K, Öktemer T, Birdane L, et al. Effervescent tablets: a safe and practical delivery system for drug administration. ENT Updates. 2016;6:46–50.
- Shaw LR, Irwin WJ, Grattan TJ, et al. The effect of selected water-soluble excipients on the dissolution of paracetamol and Ibuprofen. Drug Dev Ind Pharm. 2005;31(6):515–525.
- Rostami-Hodjegan A, Shiran MR, Tucker GT, et al. A new rapidly absorbed paracetamol tablet containing sodium bicarbonate. II. Dissolution studies and in vitro/in vivo correlation. Drug Dev Indust Pharm. 2002;28(5):533–543.
- Hunt JN, Pathak JD. The osmotic effects of some simple molecules and ions on gastric emptying. J Physiol. 1960;154(2):254–269.
- Heading RC, Nimmo J, Prescott LF, et al. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol. 1973;47(2):415–421.
- Moller PL, Norholt SE, Ganry HE, et al. Time to onset of analgesia and analgesic efficacy of effervescent acetaminophen 1000 mg compared to tablet acetaminophen 1000 mg in postoperative dental pain: a single-dose, double-blind, randomized, placebo-controlled study. J Clin Pharmacol. 2000;40(4):370–378.
- Paracetamol 500 mg Effervescent Tablets. Summary of Product Characteristics; 2020. [Cited 2020 Sept 1]. Available from: https://www.medicines.org.uk/emc/product/6064/smpc. Accessed 1 September 2020.
- Grattan T, Hickman R, Darby-Dowman A, et al. A five way crossover human volunteer study to compare the pharmacokinetics of paracetamol following oral administration of two commercially available paracetamol tablets and three development tablets containing paracetamol in combination with sodium bicarbonate or calcium carbonate. Eur J Pharm Biopharm. 2000;49(3):225–259.
- Eichman JD, Robinson JR. Mechanistic studies on effervescent-induced permeability enhancement. Pharmaceut Res. 1998;15(6):925–930.
- Eichman JD, Yassin AEB, Robinson JR. The influence of in-vivo carbonation on GI physiological processes and drug permeability. Eur J Pharmaceut Biopharmaceut. 1997;44(1):33–38.
- Patel SG, Siddaiah M. Formulation and evaluation of effervescent tablets: a review. J Drug Delivery Ther. 2018;8(6):296–303.
- Rathnayake AD, Mannapperuma U, Thambawita D, et al. Determination of in-vitro equivalence of paracetamol tablets. Int J Multidisc Stud. 2014;1:1–7.
- Amidon GL, Lennernas H, Shah VP, et al. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharmaceut Res. 1995;12(3):413–420.
- Shah VP, Amidon GGL, Amidon H, et al. G.L. Amidon, H. Lennernas, V.P. Shah, and J.R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability, Pharm Res 12, 413-420, 1995-backstory of BCS. Aaps J. 2014;16(5):894–898.
- Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl(S2):291S–298S.
- Rygnestad T, Zahlsen K, Samdal FA. Absorption of effervescent paracetamol tablets relative to ordinary paracetamol tablets in healthy volunteers. Eur J Clin Pharmacol. 2000;56(2):141–143.
- Szałek E, Karbownik A, Murawa D, et al. The pharmacokinetics of the effervescent vs. conventional tramadol/paracetamol fixed-dose combination tablet in patients after total gastric resection. Pharmacol Rep. 2014;66(1):159–164.
- Divoll M, Greenblatt DJ, Ameer B, et al. Effect of food on acetaminophen absorption in young and elderly subjects. J Clin Pharmacol. 1982;22(11-12):571–576.
- Moore RA, Derry S, Wiffen PJ, et al. Effects of food on pharmacokinetics of immediate release oral formulations of aspirin, dipyrone, paracetamol and NSAIDs - a systematic review. Br J Clin Pharmacol. 2015;80(3):381–388.
- Ibanez Y, Rodriguez JM, Lujan M, et al. A pharmacokinetic study investigating the rate of absorption of a 500 mg dose of a rapidly absorbed paracetamol tablet and a standard paracetamol tablet. Curr Med Res Opinion. 2006;22:1893–1897.
- Rostami-Hodjegan A, Shiran MR, Ayesh R, et al. A new rapidly absorbed paracetamol tablet containing sodium bicarbonate. I. A four-way crossover study to compare the concentration-time profile of paracetamol from the new paracetamol/sodium bicarbonate tablet and a conventional paracetamol tablet in fed and fasted volunteers. Drug Dev Ind Pharm. 2002;28(5):523–531.
- Ganry H, Insuasty JH, Schmidely N. Comparison of onset of analgesia of effervescent and non-effervescent paracetamol based on calculation of rates of pain reduction abstract. J Clin Pharmacol. 1996;36:855.
- Mehlisch DR, Jasper RD, Brown P, et al. Comparative study of ibuprofen lysine and acetaminophen in patients with postoperative dental pain. Clin Ther. 1995;17(5):852–860.
- Seymour RA, Kelly PJ, Hawkesford JE. The efficacy of ketoprofen and paracetamol (acetaminophen) in postoperative pain after third molar surgery. Br J Clin Pharmacol. 1996;41(6):581–585.
- Varrassi G, Muller-Schwefe G, Pergolizzi J, et al. Pharmacological treatment of chronic pain - the need for CHANGE. Curr Med Res Opin. 2010;26(5):1231–1245.
- Morthorst BR, Erlangsen A, Nordentoft M, et al. Availability of paracetamol sold over the counter in Europe: a descriptive cross-sectional international survey of pack size restriction. Basic Clin Pharmacol Toxicol. 2018;122(6):643–649.
- Anderson BJ, Holford NH, Woollard GA, et al. Perioperative pharmacodynamics of acetaminophen analgesia in children. Anesthesiology. 1999;90(2):411–421.
- Gibb IA, Anderson BJ. Paracetamol (acetaminophen) pharmacodynamics: interpreting the plasma concentration. Arch Dis Child. 2008;93(3):241–247.
- Mian P, van Esdonk MJ, Olkkola KT, et al. Population pharmacokinetic modelling of intravenous paracetamol in fit older people displays extensive unexplained variability. Br J Clin Pharmacol. 2019;85(1):126–135.
- Moore RA, Derry S, Straube S, et al. Faster, higher, stronger? Evidence for formulation and efficacy for ibuprofen in acute pain. Pain. 2014;155(1):14–21.
- Moore RA, Derry S, Straube S, et al. Validating speed of onset as a key component of good analgesic response in acute pain. Eur J Pain. 2015;19(2):187–192.
- Laska EM, Siegel C, Sunshine A. Onset and duration: measurement and analysis. Clin Pharmacol Ther. 1991;49(1):1–5.
- Verschuren F, Thys F, Wittebole X, et al. Effervescent paracetamol poisoning: a case report. Eur J Emerg Med. 2002;9(4):339–341.
- Garnier R, Riboulet-Delmas G, Efthymiou ML. [Acute paracetamol poisoning. A retrospective study of data from the Paris Antipoison Center. 1974-1981]. Sem Hop Ther. 1982;58:435–439.
- George J, Majeed W, Mackenzie IS, et al. Association between cardiovascular events and sodium-containing effervescent, dispersible, and soluble drugs: nested case-control study. BMJ. 2013;347:f6954.
- Global status report on noncommunicable diseases 2014. Geneva: World Health Organization; 2014.
- Perrin G, Korb-Savoldelli V, Karras A, et al. Cardiovascular risk associated with high sodium-containing drugs: a systematic review. PLoS One. 2017;12(7):e0180634.
- Benitez-Camps M, Vinyoles-Bargallo E, Rebagliato-Nadal O, et al. Evaluation of the relationship between effervescent paracetamol and blood pressure: clinical trial. BMC Cardiovasc Dis. 2015;15:167.
- Ubeda A, Llopico J, Sanchez MT. Blood pressure reduction in hypertensive patients after withdrawal of effervescent medication. Pharmacoepidem Drug Safe. 2009;18(5):417–419.
- Wei L, Mackenzie IS, MacDonald TM, et al. Cardiovascular risk associated with sodium-containing medicines. Expert Opin Drug Saf. 2014;13(11):1515–1523.
- Benitez-Camps M, Morros Padros R, Pera-Pujadas H, in representation of Paracetamol Investigators, et al. Effect of effervescent paracetamol on blood pressure: a crossover randomized clinical trial. J Hypertens. 2018;36(8):1656–1662.
- Perrin G, Berdot S, Thomas F, et al. Evaluation of exposure to effervescent drugs in a large health check-up population in France: a cross-sectional study. BMJ Open. 2018;8(7):e022368.
- Siau K, Khanna A. Hypernatremia secondary to soluble paracetamol use in an elderly man: a case report. Cases J. 2009;2:6707.
- Kurtz TW, Morris RC. Jr. Dietary chloride and bicarbonate as determinants of desoxycorticosterone hypertension. J Hyperten. 1984;2:S371–S373.
- Luft FC, Steinberg H, Ganten U, et al. Effect of sodium chloride and sodium bicarbonate on blood pressure in stroke-prone spontaneously hypertensive rats. Clin Sci (Lond)). 1988;74(6):577–585.
- Zicha J, Kunes J. Haemodynamic changes induced by short- and long-term sodium chloride or sodium bicarbonate intake in deoxycorticosterone-treated rats. Acta Physiol Scand. 1994;151(2):217–223.
- Luft FC, Zemel MB, Sowers JA, et al. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens. 1990;8(7):663–670.
- Ganry O, Boudet J, Wargon C, et al. Effect of sodium bicarbonate and sodium chloride on arterial blood pressure, plasma renin activity and urinary prostaglandins in healthy volunteers. J Hypertens (Suppl). 1993;11:S202–S203.
- Schorr U, Distler A, Sharma AM. Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: a randomized double-blind crossover trial. J Hypertens. 1996;14:131–135.
- Burnett I, Schachtel B, Sanner K, et al. Onset of analgesia of a paracetamol tablet containing sodium bicarbonate: A double-blind, placebo-controlled study in adult patients with acute sore throat. Clin Ther. 2006;28(9):1273–1278.
- Schachtel BP, Meskin NA, Sanner KM. How 'fast' is over-the-counter (OTC) pain relief? [abstract. Clin Pharmacol Ther. 2005;77(2):P52–P52.
- Ameen VZ, Pobiner BF, Giguere GC, et al. Ranitidine (Zantac) syrup versus Ranitidine effervescent tablets (Zantac) EFFERdose) in children: a single-center taste preference study. Paediatr Drugs. 2006;8(4):265–270.
- Liu F, Ghaffur A, Bains J, et al. Acceptability of oral solid medicines in older adults with and without dysphagia: A nested pilot validation questionnaire based observational study. Int J Pharm. 2016;512(2):374–381.
- Trippella G, Ciarcia M, de Martino M, et al. Prescribing controversies: an updated review and meta-analysis on combined/alternating use of ibuprofen and paracetamol in febrile children. Front Pediatr. 2019;7:217.
- Miehlke S, Hruz P, Vieth M, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut. 2016;65(3):390–399.

