Abstract
Objective
Burosumab is an orphan medicinal product (OMP) approved in Europe for the treatment of X-linked hypophosphatemia (XLH). The aim of this study was to assess the value of burosumab versus conventional therapy for the treatment of paediatric XLH, through a multi-criteria decision analysis (MCDA) framework for health technology assessment (HTA) of OMPs in Portugal.
Methods
The MCDA framework considered 14 criteria related to disease burden, therapeutic value and economic burden. A multidisciplinary panel of national stakeholders participated in a two-phase exercise. In the first phase, relative weights and part-worth utilities for the criteria and their levels were elicited and a reimbursement likelihood function was calibrated through adaptive conjoint analysis. In the second phase, burosumab and conventional therapy were assessed against the criteria, providing a global value score (0-100) and reimbursement likelihood (0–100%) for both.
Results
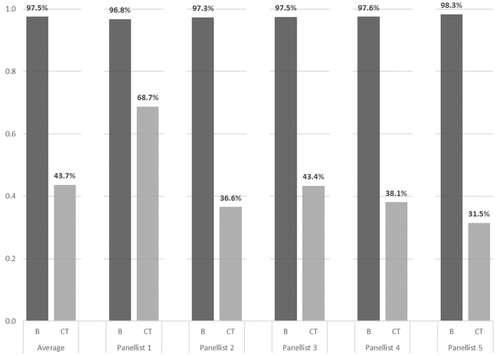
Of the 14 criteria, disease burden, therapeutic value and economic burden criteria represented 27.29%, 57.17% and 15.53% of the total weight in the decision, respectively. All disease burden and some therapeutic value criteria, typically not included in traditional HTA, represented 47.88% of the total weight. Burosumab was unanimously considered superior to conventional therapy, with an average (range) global value score of 84.96 (82.48–86.54) against 48.06 (43.37–57.68), and reimbursement likelihood of 97.50% (96.78%–98.32%) against 43.66% (31.48%–68.73%), respectively.
Conclusions
MCDA represents a powerful tool in HTA decision-making for OMPs. The results of this MCDA acknowledge burosumab as a disease-modifying drug, deemed superior to conventional therapy for the treatment of paediatric XLH.
Introduction
X-linked hypophosphatemia (XLH) is a rare genetic, progressive, lifelong disease characterized by a mutation in the PHEX gene, which causes the increase of fibroblast growth factor-23 (FGF-23) serum levels, leading to the inhibition of renal re-uptake of phosphate, calcium and vitamin D homeostasis abnormalities, and consequently poor bone mineralization leading to serious skeletal changes, namely rickets and osteomalaciaCitation1,Citation2.
Conventional therapy for children with XLH includes phosphate and vitamin D supplementation. This therapeutic approach aims to minimize the symptom burden but is not directed to the underlying cause of XLH. As such, conventional therapy presents only a limited effectiveness in the management of XLH, often rendering the skeletal deformities irreversible. Furthermore, conventional therapy is associated with safety and tolerability issues, and involves complex posology schemes requiring multiple daily intakes, which undermines the adherence to treatment and poses another barrier to treatment successCitation3–11.
Burosumab is a fully human monoclonal antibody (IgGI) directed to the underlying cause of XLH, binding to and inhibiting excess FGF-23 activity. By inhibiting FGF-23 activity, burosumab enables phosphate reabsorption in the kidney and promotes calcium and vitamin D homeostasis, leading to normal serum phosphorus, hence healing rickets and preventing skeletal deformities and growth decline. Burosumab was approved by the European Medicines Agency (EMA) in February 2018 as an Orphan Medicinal Product (OMP)Citation9,Citation12.
After regulatory authorization by EMA, timely and effective access to OMPs becomes a major issue. Granting patient access is crucially dependent on national healthcare systems across Europe and their financing and reimbursement policies, which in many countries rely on some type of health technology assessment (HTA)Citation13,Citation14. Besides important budget impact analyses, HTA agencies have historically considered comparative clinical benefit assessments and economic evaluations, with an emphasis on the cost per quality-adjusted life year (QALY) metric, to determine the value of new drugs and make recommendations with respect to their financing and reimbursement status.
A central aim of recent approaches to value measurement in HTA has been to incorporate additional parameters capturing other dimensions of value into the overall value assessment. It is not always clear, however, how these factors interact with one another, what their relative importance is, what trade-offs HTA agencies are willing to make between them and who’s preferences to consider when arriving at recommendationsCitation15–17. Indeed, for OMPs, HTA agencies frequently apply their own set of non-explicit rules for reimbursement, raising transparency and consistency concerns in the financing decision-making processes across European countries. For example, lower evidence requirements are in place in Germany, no full cost-effectiveness analysis is required in France, whereas others accept higher cost-effectiveness thresholds for orphan and ultra-orphan drugs (e.g. UK), raising inequality of access for patients with the same rare diseases living in different countries across EuropeCitation18.
Multiple-criteria decision analysis (MCDA) methods are gaining increasing interest as an alternative methodological approach to overcome the limitations of traditional economic evaluation and the excessive emphasis on the cost per QALY metric in HTACitation19. MCDA is used to support decision makers according to their preferences, in cases where there are multiple and sometimes conflicting criteria. The main objective of MCDA is to synthesize the information relevant for decision-making. MCDA integrates objective measurements with value judgments and makes explicit subjectivity. Making a decision is not just a question of selecting a best alternative. Often the need is to prioritize all the alternatives for resource allocation, or to combine the strengths of preferences of individuals to form a collective preferenceCitation20.
The aim of this study was to assess, from a Portuguese societal HTA authority decision-making perspective, the socio-economic and therapeutic value of burosumab in comparison to conventional therapy for the treatment of XLH in childhood, through an MCDA framework for the assessment of OMPs.
Methods
MCDA framework
An OMP-specific MCDA framework was adopted, consisting of 14 criteria – with 3 to 4 levels each – divided over three domains related to disease burden, therapeutic value and economic burdenCitation21. Each of the 14 criteria, which were the result of a targeted literature review identifying criteria deemed important for OMP public financing approval, are described in more detail in and (Supplemental File 1).
Table 1. Description of the domains and criteria used in the multiple criteria decision analysis framework.
Adaptive conjoint analysis (ACA)Citation22,Citation23 was used to determine the value decision makers attach to the levels of each criterion and the resulting relative importance attributed to each of the criteria, in the considered decision-making context. Rooted in market research, in its simplest form, conjoint analysis considers that ‘consumers’ evaluate the overall value (or utility) of a complex ‘product’ based on the sum of the values of its separate yet conjoined parts (part-worth utilities).
In conjoint analysis, respondents evaluate alternative ‘profiles’ consisting of different combinations of criterion levels and indicate which profile they prefer. By systematically varying the criterion levels in each conjoint task and observing respondent preferences for the resulting profiles, conjoint analysis can estimate the part-worth utilities for the separate criterion levels that respondents are believed to subconsciously use to evaluate the presented profilesCitation22.
It has been argued that respondents cannot effectively process more than about six criteria at a time in a conjoint analysis exerciseCitation22. ACA moves beyond this limitation by adapting the exercise for each respondent, focusing on the criteria that are most relevant to the respondent and avoiding information overload by focusing on just two or three criteria at a time (partial profiles).
As part of the ACA, a set of profile combinations (hypothetical scenarios) is further considered, occupying the entire range from very unattractive to very attractive and the respondent is asked about the likelihood, if the decision would be his/hers, for each of the profile combinations to be granted public financing approval. The purpose of this section is to scale the part-worth utilities, so that the sums of the part-worth utilities for these profile combinations are approximately equal to logit transforms of the respondent's likelihood percentagesCitation22,Citation24.
As such, besides the significant value the part-worth utilities have in and of themselves, they also allow to predict the overall value (utility) and public financing likelihood for all possible profile combinations (given the set of criterion levels considered), including, in this particular case, the socio-economic and therapeutic value of both burosumab and conventional therapy for the treatment of XLH in childhood.
Interviews
A multidisciplinary panel of nine experts, involved in the management of XLH and/or healthcare decision-making in Portugal, took part in the study. The panel consisted of five physicians from different geographical regions (four pediatric nephrologists and one medical geneticist), selected for being key opinion leaders in the diagnosis and/or treatment of XLH, two patient representatives, from two national rare bone diseases associations, one health economist and one health policy maker, both selected for their outstanding contribution in their specific areas of expertise. The interviews were conducted in two phases.
In a first phase, as to account for the preferences in OMP reimbursement decision making of a vast range of stakeholders, face-to-face meetings with eight of the experts (three pediatric nephrologists, one medical geneticist, two patient representatives, one health economist and one health policy maker) were held to determine, through ACA, the value attached to the levels of each criterion (part-worth utilities) and the resulting relative importance attributed to each of the criteria. Interviews were computer assisted (Sawtooth Software – Lighthouse Studio)Citation25 and focused on the general broader decision-making context of OMP public financing.
Having finished the preference elicitation through ACA, the second phase of the interviews had the objective to determine the profiles of both burosumab and conventional therapy for the treatment of XLH in childhood, given the set of criterion levels considered. Through computer assisted interviews (Sawtooth Software – Lighthouse Studio)Citation25, respondents were to classify both burosumab and conventional therapy according to the criterion levels that best reflected their value, and taking into account an average XLH patient in childhood. Given the nature of these surveys, this phase of the interviews was completed only by four physician panellists (three pediatric nephrologists – two of which took part in the first phase of the interviews – and one medical geneticist) and one patient representative, with a clear understanding of XLH and its therapeutic alternatives.
A support document was sent to all panellists of the first phase prior to the face-to-face interviews to familiarize themselves with the objectives of the study and the criteria and levels of the MCDA framework. A second support document was provided to the panellists of the second phase prior to survey completion with the most up-to-date and available information on the socio-economic and clinical landscape of XLH in Portugal and the efficacy and safety of burosumab.
Data analysis
Part-worth utility estimation is an automatic part of the ACA interview within Sawtooth Software – Lighthouse Studio and does not require researcher involvementCitation25. Respondent part-worth utilities are calculated individually during each interview and can be used to predict the overall value and public financing likelihood for all possible profile combinations as soon as the interviews are completed.
Part-worth utilities, representing the value attached to the levels of each criterion, and the relative importance attributed to each of the criteria, resulting from the first phase of the interviews, will be presented graphically and through summary statistics. Results of the second phase of the interview, that is, the classification of burosumab and conventional therapy according to the criterion levels that best reflect their value will be presented individually and qualitatively for all five panellists involved.
The overall socio-economic and therapeutic value of burosumab and conventional therapy for the treatment of XLH in childhood will be calculated as the sum of the part-worth utilities over the separate criteria, after rescaling as to obtain an overall value between 0 and 100, for ease of interpretability. The overall value and public financing likelihood for both therapeutic alternatives will be presented graphically and through summary statistics.
Results
Relative importance and Part-Worth utilities
First phase individual interviews took place between August and December of 2018 and lasted an average 71 (range: 36–128) minutes. and S1 (Supplemental File 1) summarize the criteria importance and part-worth utility estimates, determined through ACA, from these interviews.
Figure 1. Average importance estimates for the criteria considered in the decision-making context of OMP public financing. Legend: Error bars represent 95% confidence intervals; Criteria related to the Disease Burden, Therapeutic Value and Economic Value are painted in green, blue and red, respectively.
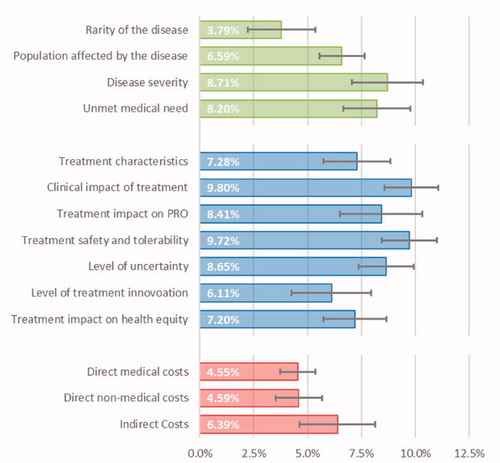
The multidisciplinary panel of experts considered that of the fourteen criteria elicited for OMP public financing approval in Portugal, the most important ones were ‘Clinical impact of treatment’ and ‘Treatment safety and tolerability’, represented by an average 9.80% (95%CI: 8.55–11.06%) and 9.72% (95%CI: 8.45%–11.00%) of the total weight in the decision, respectively. These criteria were closely followed by ‘Disease severity’ (8.71%; 95%CI: 7.06–10.35%), ‘Level of uncertainty’ (8.65%; 95% CI: 7.37–9.93%) and ‘Treatment impact on PRO’ (8.41%; 95%CI: 6.50%–10.33%).
Criteria considered least important for decision-making were ‘Rarity of disease’, ‘Direct medical costs’ and ‘Direct non-medical costs’, representing on average 3.79% (95%CI: 2.22–5.36%), 4.55% (95%CI: 3.73–5.37%) and 4.59% (95%CI: 3.52–5.66%) of the total weight in the decision, respectively. These three criteria can be understood to be less than half as important as the two most important criteria for decision-making, ‘Clinical impact of treatment’ and ‘Treatment safety and tolerability’.
Notwithstanding, disease burden related attributes like ‘Rarity of disease’, the type of ‘Population affected by the disease’, ‘Disease severity’ and ‘Unmet medical need’ together did account for over one quarter (27.29%) of the perceived total weight in the decision. Treatment related attributes like ‘Treatment characteristics’, ‘Level of treatment innovation’ and ‘Treatment impact on health equity’ also summed up to 20.59% of the total weight in the decision. Altogether, these attributes, typically not explicitly included in traditional clinical benefit assessment and economic evaluation based HTA, represented almost half (47.88%) of the total weight in the decision for OMP public financing approval.
From the part-worth utility estimates, it can be understood that the panel of experts attributed similar importance to rare diseases that predominantly affect infant and general populations, as well as to rare diseases that predominantly affect elderly and socio-economically disadvantaged/vulnerable populations, but with a clear preference for the former (Supplemental File 1: Figure S1). With respect to the criterion ‘Treatment characteristics’, there appeared to be some resistance, within the current decision-making context, in attributing substantially more importance to treatments targeted at treating the underlying cause of the disease, but without curative properties than to palliative/symptomatic treatments (Supplemental File 1: Figure S1).
A clear distinction in importance was however made between treatments that demonstrate moderate to high and substantial clinical benefits, over treatments that demonstrate marginal, and even, to some extent, reduced to moderate clinical benefits (Supplemental File 1: Figure S1). A similar pattern was observed for the criteria ‘Treatment impact on PRO’ and ‘Treatment safety and tolerability’ (Supplemental File 1: Figure S1).
With respect to ‘Direct medical costs’, no real difference in importance was given between situations in which direct medical cost are below 25,000€ per patient/year or between 25,000€ and 100,000€per patient/year, favoring both over higher annual per patient costs (Supplemental File 1: Figure S1). A clear preference was further given to situations with direct non-medical costs below 12,500€ per patient/year and all indirect cost below 13,776€ per patient/year (Supplemental File 1: Figure S1).
Qualitative classification of X-linked hypophosphatemia treatment alternatives
The second phase individual interviews took place between December 2018 and April 2019 and took an average 31 (range: 19–52) minutes to complete. In this phase of the interviews, the panellists classified both burosumab and conventional therapy qualitatively on all criteria according to the levels that best reflected their value. The resulting profiles for both therapeutic alternatives and for all five panellists involved in this phase of the study are presented in Table S2 (Supplemental File 1).
Concerning the criteria on disease burden, no differences are observed between burosumab and conventional therapy, as is to be expected for therapeutic alternatives for the same therapeutic indication. For the pediatric population affected by XLH, the impact on morbidity and mortality is considered to be moderate to high, with almost no existing therapeutic alternatives.
With respect to therapeutic value, burosumab was unanimously classified better than conventional therapy on all criteria. The largest qualitative differences can be observed for ‘Level of treatment innovation’ (with all panellists considering burosumab to represent a high level of innovation and conventional therapy no innovation), and ‘Treatment impact on PRO’ (with some of the panellist considering burosumab to present substantial benefits on PRO, as opposed to only marginal benefits presented by conventional therapy). Burosumab was attributed the best ranking level on therapeutic value related criteria in 82.9% (29 out of 35) of cases.
For direct medical (not contemplating drug costs) as well as nonmedical costs, both therapeutic alternatives were classified mostly in the best ranking level, below 25,000€ per patient/year and 2,500€ per patient/year, respectively. More heterogeneity and larger qualitative differences were observed for indirect cost, mostly favoring burosumab with lower cost per patient/year over conventional therapy with costs up to and over 13,776€ per patient/year.
Overall value and financing likelihood of treatment alternatives
The overall socio-economic and therapeutic value of burosumab in comparison to conventional therapy, for the treatment of XLH in childhood, can be found in . These values represent the average sum of the part-worth utilities (Supplemental File 1: Figure S1) corresponding to the level classification of each profile over the considered criteria (Supplemental File 1: Table S2), rescaled to an overall value between 0 and 100. Results are presented individually for each panellist and as an aggregate panel average.
Figure 2. Overall socio-economic and therapeutic value for burosumab (B) and conventional therapy (CT). Legend: Superimposed are the portions of the overall value attributed to the Disease Burden (green), Therapeutic Value (blue) and Economic Burden (red).
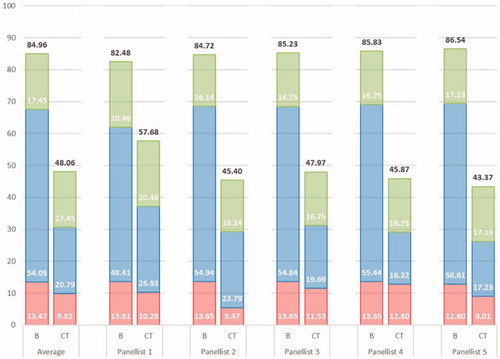
As can be understood from , the qualitative classification leads to an average overall value of burosumab of 84.96, with values attributed by individual panellists ranging between 82.48 and 86.54. For conventional therapy, value assessments ranged between 43.37 and 57.68, with an average overall value of 48.06. A slight negative correlation can be observed between the individual panellists’ values, with larger valuations of burosumab corresponding to lower valuations of conventional therapy.
As previously mentioned, both treatment alternatives were valued equally on disease burden related criteria. Burosumab was valued only slightly higher than conventional therapy on criteria related to the economic burden of the disease: with the exception of panellist 2, no differences larger than 5 points were observed. For criteria related to therapeutic value, however, for all but one panellist, burosumab was valued at least 30 points higher than conventional therapy. This reveals not only important differences in the treatment value of burosumab, but also the ability of the 14-criteria MCDA framework to clearly discriminate value according to domains and criteria.
The breakdown of the overall socio-economic and therapeutic value for burosumab (84.96) and conventional therapy (48.06) according to the decision-making criteria considered, can be found, superimposed over the average importance estimates, in . In general, the value of burosumab on all criteria related to the therapeutic value and economic burden were close to maximal, entailing that much of the remaining value for burosumab to obtain a perfect value of 100, is related to the disease burden represented by XLH in childhood.
Figure 3. Breakdown of the overall socio-economic and therapeutic value for burosumab and conventional therapy. Legend: Dark grey – Burosumab; Light grey – Conventional therapy; As a reference, the average importance estimates are presented for the criteria related to the Disease Burden (green), Therapeutic Value (blue) and Economic Burden (red).
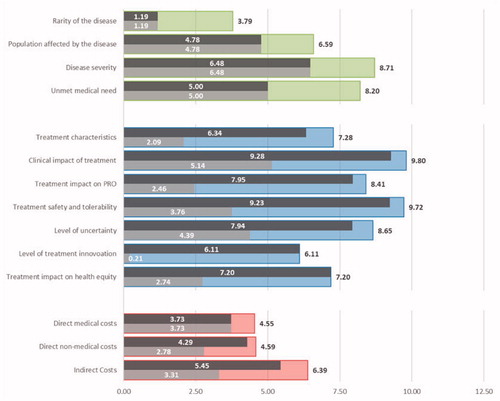
For the therapeutic value domain, burosumab obtained an average valuation of 54.05 (94.5%) out of a possible 57.18, while conventional therapy only 20.79 (Δ = 33.25). Besides substantial differences in typical clinical trial related criteria as ‘Treatment clinical impact’ (Δ = 4.13), ‘Treatment impact on patient reported outcomes’ (Δ = 5.49) and ‘Treatment safety and tolerability’ (Δ = 5.47), notable differences were also observed in criteria such as ‘Treatment characteristics’ (Δ = 4.25), ‘Impact on health equity’ (Δ = 4.45) and ‘Level of treatment innovation’ (Δ = 5.90) ().
Although potential differences in the economic burden related criteria between burosumab and conventional therapy are smaller due to lower average importance estimates, burosumab is valued reasonably high on all three criteria, with advantage over conventional therapy for direct non-medical (Δ = 1.51) and indirect costs (Δ = 2.14).
According to the panellists, burosumab should be considered for public financing 2.24 times more likely than CT, with an average (range) likelihood of 97.5% (96.8% − 98.3%) and 43.6% (31.5%−68.7%), respectively ().
Discussion
Our study was designed to evaluate the socio-economic and therapeutic value of burosumab for the treatment of XLH in childhood in comparison to conventional therapy, through an MCDA framework composed of three domains and fourteen criteria to evaluate the value of OMPsCitation21. This MCDA framework is suggested to be used alongside budget impact analyses as an alternative or complement to traditional economic evaluation, often emphasized on the cost per QALY metric, considered by HTA authorities. It includes criteria typically used by HTA authorities in Europe like costs (direct medical, direct non-medical and indirect) and effectiveness (treatment efficacy, safety, clinical value, impact on patient reported outcomes and evidence uncertainty). Furthermore, it also formally incorporates, in an explicit and systematic manner, other decision criteria frequently only considered in a non-explicit and ad hoc manner in other HTA frameworks for assessing innovative medicines, like disease related criteria such as disease rarity, severity, the type of population affected by the disease (children and elderly) and unmet clinical need, or treatment related attributes like the level of innovation and impact on health equity. Our research revealed that the inclusion of the latter criteria may be important in the assessment of the value of OMPs. The multidisciplinary panel of stakeholders involved in the management of XLH, including patients advocates, considered that almost half of OMPs value assessment should be based on these criteria. These findings are potentially relevant because HTA authorities across Europe tend to disregard these criteria while assessing new medicinesCitation26.
The three individual criteria considered as the most relevant to assess a new OMP according to Portuguese stakeholders were ‘Treatment clinical impact’, ‘Safety and tolerability’ and ‘Disease severity’. This reveals that treatment clinical aspects, including safety and tolerability are highly valued for HTA purposes, but also indicates that disease impact on patients should also be taken into consideration when assessing the value of a new drug. Other criteria that Portuguese stakeholders have identified with above average importance were ‘Level of uncertainty on the results of the research undertaken’, ‘Treatment impact on PRO’ and ‘Unmet medical need’. During interviews, participants have indeed stated that robust clinical evidence plays a crucial role in their trust in the value of a certain medicine. Most interviewees have also admitted that PRO are gaining importance in recent years and should be valued accordingly.
Although, on average, concerning the criterion ‘Population affected by the disease’ the panel of experts attributed the highest importance to rare diseases that predominantly affect infant populations, for some of the experts the estimated part-worth utilities pointed in the opposite direction. As such, despite all panellists in the second phase of the interviews having identified the disease as affecting predominantly an infant population, the heterogeneity in part-worth utility estimates for this criterion leads to a sub-optimal valuation.
The majority of the expert panel (four out of five) classified burosumab as a drug directed to the underlying cause of XLH and with curative properties (functional cure) and all five experts thought it was a highly innovative drug that would provide a substantial improvement on health equity for these patients, leading to notable difference in valuation between burosumab and conventional therapy. Additionally, all panellists classified burosumab’s ‘Level of uncertainty regarding the available evidence’ as minimal (n = 3, 60%) or reduced to moderate (n = 2, 40%), meaning they strongly believe results from phase II and III clinical trials will translate into clinical practice. Regarding economic burden related criteria, burosumab valued slightly higher on two of the three criteria as experts thought burosumab would result in an improvement of the patients’ condition and consequently in fewer hospital visits and hospitalizations, resulting in lower transportation costs (direct non-medical cost) and lower absenteeism (indirect cost).
There are several MCDA frameworks proposed in the literature for the assessment of OMPs. Schey et al. (2017) used a nine criteria framework, which includes: rarity, level of research undertaken, level of uncertainty of effectiveness, manufacturing complexity, follow-up measures, disease severity, available treatment alternatives, level of impact of disease, and unique indication or notCitation27. Sussex et al. (2013) identified eight nonmonetary attributes: four concerning the disease being treated (availability of effective treatment options/best supportive care in the absence of the new medicine; disease survival prognosis with current standard of care; disease morbidity and patient clinical disability with current standard of care; social impact of the disease on patients’ and carers’ daily lives with current standard of care) and four addressing the treatment itself (treatment innovation, evidence of treatment clinical efficacy and patient clinical outcome, treatment safety, social impact of the treatment on patients’ and carers’ daily lives)Citation28. Iskrov et al. (2016) organized a twelve criteria MCDA framework into three categories: health technology characteristics (five criteria), indicated disorder characteristics (two criteria), and public health aspects (five criteria)Citation29. More recently, Guarga et al. (2019) suggested a reflective MCDA to assess the value OMPs in the Catalan Health Service (CatSalut) composed by 10 quantitative and 4 qualitative criteria, respectively; disease severity, unmet needs, improvement of efficacy/effectiveness, improvement of safety/tolerability, improvement of patient perceived health/PRO, type of therapeutic benefit, annual patient cost of treatment, other medical costs, quality of evidence, expert consensus/clinical practice guidelines; population priorities and access (principle of equity), common goal and specific interests, system capacity and appropriate use of OMPs, opportunity costs and affordability (budget impact)Citation30.
Other MCDA frameworks have been suggested including EVIDEM, an open-source collaboratively MCDA framework designed to appraise the holistic value of healthcare interventions with an adaptation to rare diseases issues and policies and the Transparent Value Framework (TVF) supported by the European Commission throughout the Platform on Access to Medicines in Europe and the Working Group on Mechanism of Coordinated Access to Orphan Medicinal ProductsCitation31,Citation32. The TVF includes four main criteria: unmet need, relative effectiveness, response rate and degree of certainty, and three complementary criteria: number of patients, burden of disease, added value of the TVFCitation32. Despite the initial merits of TVF until now there has been little consensus on the most appropriate assessment criteria, perspective or appraisal process, leading to considerable variation in the degree and speed of adoption of OMPs across European countriesCitation13,Citation33.
Results from the application of these different MCDA frameworks including ours are not comparable because they involve different sets of value criteria and different valuation techniques to estimate criteria weights. Schey et al. (2017) did not use any formal weight estimation method. Rather these authors have worked with alternative scenarios using non preference anchored, fixed weightsCitation27. Sussex et al. (2013) and Iskrov et al. (2016) selected the value measurement model to weighting of their value attributesCitation28,Citation29. Guarga et al. (2019) followed a simpler approach based on a direct rating scale (five-point weighting technique), while the adapted EVIDEM MCDA framework by Wagner et al. (2016) used hierarchical point allocation. Both fall under the structured-weighting methodsCitation30,Citation31,Citation34.
Our research is focused on MCDA weighting estimation by ACA, a choice based stated preference methodCitation35. The pros and cons of each weighting method have been extensively discussed elsewhere and should be judged not only on their level of precision but also on their theoretical foundationsCitation31,Citation36,Citation37. Although not comparable, the criteria used in our research have some resemblances with the research by Guarga et al. (2019)Citation30. Our approach allows for more granularity at criteria hierarchy and the results we obtained showed substantial differences in the ranking of the criteria relative to Guarga et al. (2019)Citation30. Our most preferred attributes were clinical impact of the intervention under assessment and its safety/tolerability profile, both treatment related. On the contrary, in Guarga et al. (2019) the two highest ranked criteria were disease related: disease severity and unmet needCitation30. These two criteria also present in our MCDA framework were ordered third and sixth, respectively, with substantial lower weight than in Guarga et al. (2019)Citation30. Nonetheless, attributes included in our three domains (disease related = 27,3%; treatment related = 57,2%; costs related = 15,5%) were given similar aggregate weights as for the same domains in Guarga et al. (2019) (disease related = 24,8%; treatment related = 57,0%; costs related = 18,2%)Citation30.
Our MCDA framework applied to the treatment of XLH provided a clear and straightforward assessment of the value of burosumab. There is no other study in the literature reporting MCDA results for burosumab. So, no benchmark exists for comparison. However, burosumab was assessed by NICE in England under the revised highly specialised technology (HST) evaluation and recommended for utilization in NHS EnglandCitation10. The decision made by NICE was supported by the magnitude of the incremental therapeutic improvement of burosumab, as revealed through the number of additional QALYs gained, with the incremental cost-effectiveness ratio threshold being determined by the QALY gainCitation38. As such, burosumab was considered to provide value for money and recommended for use in the NHS within the context of a HSTCitation38. HST has the broadest latitude of any NICE appraisal processes and is able to consider other non-quantified criteria beyond direct health benefits of burosumab to support its decision, such as productivity impact and the nature of the population (children), having led to burosumab being considered an innovative treatment for people with XLHCitation38. The NICE assessment and appraisal of burosumab and our MCDA framework applied to burosumab both provide the same outcome, that is, burosumab is highly innovative and provides good value in XLH treatment, albeit with totally different methods. The NICE decision was granted on explicit cost-effectiveness assessment plus other supplementary non-quantifiable criteria, while our approach was totally based on quantifiable preference-based criteria, including disease attributes and equity judgments.
The majority of MCDA frameworks applied to OMPs presented in the literature tend to report results for the overall value that are bounded on a 0 to 1 scale (equivalent to 0 to 100). As such, cardinality and ordinality properties are accounted for and this is important for comparative reasons.
Our study presents some limitations that need to be addressed in order to adequately contextualise the results we report. The development of the current MCDA framework originally took place in the beginning of 2015 and predates best practice guidelines on the design of MCDA frameworks, such as those published by the International Society for Pharmacoeconomics and Outcomes ResearchCitation35. As a result, the original development of the framework did not follow a meanwhile recommended deliberative process with several stakeholders involved. Rather it was based on a literature review for relevant domains and criteria identification from previous research in the literature. The final set of domains, criteria and criterion levels were defined by consensus of a reduced panel composed of a health economist, a mathematician and an outcomes researcher. Nonetheless, the decisions of this panel were based on desirable completeness, non-redundancy and nonoverlap criteria propertiesCitation35. There is no rule as to how many criteria should be included in an analysis. Previous research found criteria ranging from 3 to 19Citation19. Good practice guidelines advocate for as few criteria as consistent to allow well-founded decisions, however there is a trade-off between a more comprehensive set of criteria and increased validity, and a more parsimonious set of criteria enhancing time and cognitive efforts associated with weight estimationCitation35. We felt comfortable with a set of 14 criteria because we used ACA for preferences elicitation which reduces substantially the cognitive burden to stakeholders within a manageable response time effort (average 71 min).
Our weighting for MCDA criteria and value of burosumab estimation was based on a reduced number of experts. This should be taken into account when interpreting our results. Still, we tried to have a fair representation of stakeholders including clinicians with experience in treating XLH, patient representatives, health policy makers and health economists. Despite the differences in stakeholder background their preferences showed lower dispersion on the overall burosumab value (83.48 to 86.54) and on the likelihood of granting public financing to burosumab (96.8% to 98.3%). Direct medical cost criteria excluded XLH-related treatment (burosumab and conventional therapy). This may bias MCDA results in favor of the most expensive treatment option. Yet, it should be noted that the weighting elicitation exercise was independent of any particular treatment option and included direct medical costs trade-offs ranging from lower than 25,000€ per patient/year to above 250,000€ per patient/year. The low weight attached to direct medical costs by the expert panel of only 4.6% of the overall value tends to minimize the possible bias towards the most expensive options.
Conclusions
The MCDA framework for OMPs represents a powerful tool for decision-making in the context of HTA for rare diseases, providing a systematic, transparent and consistent approach for the complex and multi-dimensional evaluation of health technologies. The results of this MCDA acknowledge burosumab as a disease-modifying drug, being deemed considerably superior to conventional therapy for the treatment of children and adolescents with XLH by a representative set of Portuguese stakeholders.
Transparency
Declaration of funding
The study was funded by Kyowa Kirin International. The authors had no restrictions or limitations to the definition of study design, data collection, analysis and interpretation or preparation of the manuscript.
Declaration of financial/other relationships
IA, TF, HP and SS declare to have received a fee, by the funding institution, for participation in the two round MCDA interviews. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics approval and consent to participate
Not applicable.
Supplemental Material
Download MS Word (122 KB)Acknowledgements
We acknowledge the contribution of Pedro Ferreira, Maria do Céu Barreiros, Conceição Mota and Margarida Abranches to the study as participants in one of the phases of the MCDA interviews and to Jorge Félix and João Almeida who were involved in the original MCDA framework conception.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- Fuente R, Gil-Peña H, Claramunt-Taberner D, et al. X-linked hypophosphatemia and growth. Rev Endocr Metab Disord. 2017;18(1):107–115.
- Pavone V, Testa G, Gioitta Iachino S, et al. Hypophosphatemic rickets: etiology, clinical features and treatment. Eur J Orthop Surg Traumatol. 2015;25(2):221–226.
- Carpenter TO, Imel EA, Holm IA, et al. A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381–1388.
- Ruppe MD. X-linked hypophosphatemia, Gene Reviews. Seattle (WA): University of Washington; 2017.
- Petersen DJ, Boniface AM, Schranck FW, et al. X-linked hypophosphatemic rickets: a study (with literature review) of linear growth response to calcitriol and phosphate therapy. J Bone Miner Res. 1992;7(6):583–597.
- Goodyer PR, Kronick JB, Jequier S, et al. Nephrocalcinosis and its relationship to treatment of hereditary rickets. J Pediatr. 1987;111(5):700–704.
- Reusz GS, Hoyer PF, Lucas M, et al. X linked hypophosphataemia: treatment, height gain, and nephrocalcinosis. Arch Dis Child. 1990;65(10):1125–1128.
- Taylor A, Sherman NH, Norman ME. Nephrocalcinosis in X-linked hypophosphatemia: effect of treatment versus disease. Pediatr Nephrol. 1995;9(2):173–175.
- Verge CF, Lam A, Simpson JM, et al. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med. 1991;325(26):1843–1848.
- The National Institute for Health and Care Excellence (NICE). Highly specialised technologies guidance - Burosumab for treating X-linked hypophosphataemia in children and young people. [cited August 11, 2019]. Available from: www.nice.org.uk/guidance/hst8.
- Imel EA, White KE. Pharmacological management of X-linked hypophosphataemia. Br J Clin Pharmacol. 2019;85(6):1188–1198.
- Kyowa Kirin. Crysvita (burosumab), Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/crysvita-epar-product-information_en.pdf
- Zamora B, Maignen F, O'Neill P, et al. Comparing access to orphan medicinal products in Europe. Orphanet J Rare Dis. 2019;14(1):95.
- Malinowski KP, Kawalec P, Trąbka W, et al. Reimbursement legislations and decision making for orphan drugs in Central and Eastern European Countries. Front Pharmacol. 2019;10:487–487.
- Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Health Econ. 2018;19(1):123–152.
- Nicod E, Kanavos P. Scientific and social value judgments for orphan drugs in health technology assessment. Int J Technol Assess Health Care. 2016;32(4):218–232.
- Sussex J, Towse A, Devlin N. Operationalizing value-based pricing of medicines: a taxonomy of approaches. Pharmacoeconomics. 2013;31(1):1–10.
- Baran-Kooiker A, Czech M, Kooiker C. Multi-criteria decision analysis (MCDA) models in health technology assessment of orphan drugs-a systematic literature review. next steps in methodology development? Front Public Health. Front Public Health, 2018;6:287.
- Marsh K, Lanitis T, Neasham D, et al. Assessing the value of healthcare interventions using multi-criteria decision analysis: a review of the literature. Pharmacoeconomics. 2014;32(4):345–365.
- Pavan M, Todeschini R. Multicriteria decision-making methods. In: Comprehensive chemometrics [Recurso electrónico]: chemical and biochemical data analysis. Vol. 1. Elsevier; 2020.
- Santos M, Oliveira A, Vandewalle B, et al. A multi-criteria decision analysis assessment of teduglutide in the treatment of adults with short bowel syndrome and intestinal failure in Portugal. In: XXXVIII Congresso da Sociedade Portuguesa de Cirurgia. Lisbon; 2018.
- Orme B. Getting started with conjoint analysis: strategies for product design and pricing research. Vol. 3. Manhattan Beach (CA): Research Publishers; 2014.
- Johnson RM. Adaptive Conjoint Analysis. in Sawtooth Software Conference on Perceptual Mapping, Conjoint Analysis, and Computer Interviewing. 1987.
- The ACA/Web v6.0 Technical Paper. Technical Paper Series 2007;Available from: https://www.sawtoothsoftware.com/support/technical-papers/aca-related-papers/aca-technical-paper-2007.
- Vaisbich MH, Koch VH. Hypophosphatemic rickets: results of a long-term follow-up. Pediatr Nephrol. 2006;21(2):230–234.
- Angelis A, Kanavos P. Multiple Criteria Decision Analysis (MCDA) for evaluating new medicines in Health Technology Assessment and beyond: The Advance Value Framework. Soc Sci Med. 2017;188:137–156.
- Schey C, Krabbe PFM, Postma MJ, et al. Multi-criteria decision analysis (MCDA): testing a proposed MCDA framework for orphan drugs. Orphanet J Rare Dis. 2017;12(1):10–10.
- Sussex J, Rollet P, Garau M, et al. A pilot study of multicriteria decision analysis for valuing orphan medicines. Value Health. 2013;16(8):1163–1169.
- Iskrov G, Miteva-Katrandzhieva T, Stefanov R. Multi-criteria decision analysis for assessment and appraisal of orphan drugs. Front Public Health. 2016;4:214–214.
- Guarga L, Badia X, Obach M, et al. Implementing reflective multicriteria decision analysis (MCDA) to assess orphan drugs value in the Catalan Health Service (CatSalut). Orphanet J Rare Dis. 2019;14(1):157.
- Wagner M, Khoury H, Willet J, et al. Can the EVIDEM framework tackle issues raised by evaluating treatments for rare diseases: analysis of issues and policies, and context-specific adaptation. Pharmacoeconomics. 2016;34(3):285–301.
- Working Group on Mechanism of Coordinated Access to Orphan Medicinal Products (MoCA-OMP). Transparent Value Framework. 2014. [cited August 11, 2019]. Available from: http://download2.eurordis.org.s3.amazonaws.com/moca/history/WG%20MoCA-OMP%20Transparent%20Value%20Framework.pdf.
- Annemans L, Aymé S, Le Cam Y, et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL). Orphanet J Rare Dis. 2017;12(1):50.
- Ho M, Saha A, McCleary KK, Medical Device Innovation Consortium’s Patient Centered Benefit-Risk Steering Committee, et al. A framework for incorporating patient preferences regarding benefits and risks into regulatory assessment of medical technologies. Value Health. 2016;19(6):746–750.,
- Marsh K, IJzerman M, Thokala P, ISPOR Task Force, et al. Multiple criteria decision analysis for health care decision making-emerging good practices: report 2 of the ISPOR MCDA emerging good practices task force. Value Health. 2016;19(2):125–137.,
- Nikou S, Mezei J, Sarlin P. A process view to evaluate and understand preference elicitation. J Multi-Crit Decis Anal. 2015;22(5-6):305–329.
- van Til J, Groothuis-Oudshoorn C, Lieferink M, et al. Does technique matter;a pilot study exploring weighting techniques for a multi-criteria decision support framework. Cost Eff Resour Alloc. 2014;12(1):22.,
- National Institute for Health and Care Excellence (NICE). Final Evaluation Document: Burosumab for treating X-linked hypophosphataemia in children and young people. 2018. [cited August 11, 2019]; Available from: https://www.nice.org.uk/guidance/hst8/evidence.