ABSTRACT
Resveratrol (RSV) is a natural polyphenol present in grapes, the skin of peanuts, and several other plants with many health benefits. Autism spectrum disorder (ASD) is a neurodevelopmental disorder that may be linked to neural and synaptic development impairments. The present study aimed to analyze the preventive effects of RSV on the development of ASD-like behavior, using oxytocin receptor gene knockout (Oxtr-KO) and valproic acid-induced ASD (VPA-ASD) model mice. Genetic deficiencies in Oxtr are suggested to be involved in ASD etiology. Twenty-four hours after a single RSV injection to the Oxtr-KO mice, the social impairments caused by OXTR deficiency were ameliorated. RSV also improved social impairments in the VPA-ASD mice. Administration of RSV up-regulated silent information regulator 1 (Sirt1) gene and early growth response factor 3 (Egr3) gene expressions in the amygdala of the Oxtr-KO mice. Our data suggest that RSV may have therapeutic effects on ASD with multiple targets.
GRAPHICAL ABSTRACT
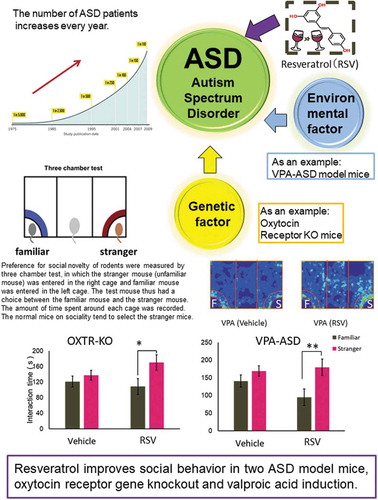
Resveratrol improves social behavior in two ASD model mice, oxytocin receptor gene knockout and valproic acid induction.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder, characterized by difficulties in communication, restricted interests, and repetitive behaviors. ASD symptoms start to appear in the first year of life and become apparent by the age of three [Citation1]. Various complicated risk factors, such as genetic mutations, environmental factors, or a combination of both, are suspected to cause ASD [Citation2]. A cohort study has reported the incidence of ASD to be 1.5% of the entire child population in the United States [Citation3]. Thus, the development of a promising therapeutic strategy for ASD is necessary. Resveratrol (RSV), a representative polyphenol [Citation4], is a beneficial bioactive substance, found in foods such as red wine and peanuts. RSV activates silent information regulator 1 (Sirt1) [Citation5], which is the nicotinamide adenine dinucleotide-dependent histone deacetylase. It has been reported that RSV has a wide range of health-promoting effects and pharmacological actions, including anti-oxidant [Citation6], anti-inflammatory [Citation7], anti-aging [Citation8], anti-cancer [Citation9], cardio-protective [Citation10], and neuroprotective [Citation11] properties. Moreover, RSV can improve changes in behavior caused by depression or ASD [Citation12,Citation13]. RSV exhibits poor water solubility and chemically instability, however, it has recently been enhanced as a therapeutic drug for neurological disease by encapsulating in many different types of nanoparticles [Citation14]. Oxytocin (OXT) peptide, a hormone consisting of nine amino acids, is required for lactation and contraction of the uterus. Recently, it was revealed that the OXT-OXT receptor (Oxtr) system has an important role in the regulation of social behavior, and Oxtr-KO mice display apparent impairments in social memory [Citation15]. Moreover, some patients with familial ASD showed single nucleotide polymorphisms in the Oxtr gene [Citation16]. A clinical study of OXT, intended to demonstrate its therapeutic effects for ASD, reported the potential for OXT to improve social deficits observed in ASD patients [Citation17]. In contrast, reports have also suggested insufficient therapeutic effects of OXT in some patients with ASD [Citation18]. Specific environmental factors, such as prenatal exposure to valproic acid (VPA), increase the risk of ASD. Previous studies have demonstrated that OXT exposure has therapeutic effects on VPA-induced ASD-like behavior [Citation19,Citation20]. While RSV has many potential health benefits, its potential to improve ASD-like behavior caused by many facts has not been explored. The aim of the current study was to examine the preventive effects of RSV on ASD-model mice caused by Oxtr-KO and exposure to VPA using.
Materials and methods
Animals and experimental protocol
Wild-type C57BL6/J (WT) and Wild-type DBA/2 mice were purchased from CLEA Japan (Japan) and SLC Japan (Japan), respectively. Oxtr-KO mice obtained from Tohoku University [Citation15] were backcrossed with the C57BL6/J mice for more than 18 generations. At 2 to 4 months of age, WT and Oxtr-KO male mice were injected with RSV (Wako #180-02773, Japan) of 30 mg/kg (WT+RSV and Oxtr-KO+RSV) or corn oil of an equivalent volume (WT and Oxtr-KO) by intraperitoneal (i.p) administration 24 h before the three-chamber test. The WT pups on embryonic day 13.5 (E13.5) were exposed to VPA (Sigma, #P4543, USA) via their mother by intraperitoneal administration (600 mg/kg). The VPA-exposed male pups at 2 to 4 months of age were treated with RSV (VPA-ASD+RSV) or corn oil (VPA-ASD) as mentioned above. All animal procedures were approved by the Animal Experiment Committee of Tohoku University. Three to five mice were housed in each cage at a temperature of 23 ± 2°C under a 12-h light and dark cycle and were provided with food (Labo MR Stock, Nosan Co., Japan) and water ad libitum.
Three-chamber test
The test mice were socially isolated in cages (20 cm length × 12 cm width × 15 cm height) for 3 days prior to the experiment. Twenty-four hours before the three-chamber test, the test mice were administered with RSV or saline. To assess sociability and social novelty preference, we used a polyvinyl chloride box (63 cm length × 41 cm width × 30 cm height) equally divided into three compartments with two walls made of clear Plexiglas. The two walls had small openings (5 cm width × 3 cm height) allowing a test mouse access into each of the other compartments. A wire cage (10 cm diameter at the bottom, 18 cm height, vertical bars 1 cm apart) was placed in each side compartment. The wire cage allowed for auditory, visual, and olfactory interactions, as well as nose contact between the wire bars, but prevented fighting among animals. The test was performed as previously described [Citation21]. In brief, the test consisted of the following three 10-min sessions. During all sessions, the test mice could move freely in all compartments by passing through the openings. The first session was habituation, in which no stimuli were present. The second session was conducted to assess sociability, in which a stimulus mouse (Familiar) was in one wire cage and an inanimate object (a toy mouse) was present in the other wire cage. The third session measured social novelty preference, in which the toy mouse was replaced with a novel mouse (Stranger). The test mouse thus had a choice between the Familiar and the Stranger mice. The amount of interaction time the test mouse spent around each wire cage was recorded by a video camera fitted on top of the chamber. The Familiar and Stranger mice were two- to four-month-old male DBA/2 mice purchased from two different breeders. Data acquisition and analysis were performed automatically with the use of ANY-maze behavior tracking software (Muromachi, Japan).
Quantitative polymerase chain reaction
Following the three-chamber test, the test mice were euthanized by cervical dislocation. Total RNA was extracted from the amygdala of the test mice by homogenization in RNAiso (Takara #9108, Japan) according to the manufacturer’s instructions. RNA (1 µg) was reverse-transcribed to cDNA using a PrimeScript RT reagent kit with a genomic DNA eraser (Takara #RR047A, Japan) according to the manufacturer’s instructions. cDNA concentration was measured using a NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific, USA). SYBR Premix Ex Taq II (Takara #RR420A, Japan), and the Thermal Cycler Dice Real Time System (Takara #TP900, Japan) was used for quantitative real-time PCR (qRT-PCR) to detect gene expression. The primers used for target detection were 5ʹ- GACGATGACAGAACGTCACAC-3ʹ and 5ʹ-CGAGGATCGGTGCCAATCA-3ʹ for Sirt1 gene expression, 5ʹ-ATGTTGCCCCATTTGCCTTG-3ʹ and 5ʹ-TTAAAGCCCGGCTGATTGTC-3ʹ for Egr3 gene expression, 5ʹ-AACTTTGGCATTGTGGAAGG-3ʹ and 5ʹ-GGATGCAGGGATGATGTTCT-3ʹ for GAPDH gene expression.
Statistical methodology
Statistical analyses were performed using GraphPad Prism version 6.0. Data are expressed as mean ± standard error of the mean. Student t-tests were used for two-group comparisons, and differences with a P-value of <0.05 were considered statistically significant.
Results
RSV improved abnormal social novelty preference observed in Oxtr-KO mice and VPA-ASD mice
Normally, mice are interested in social novelty and prefer to spend more time around the cage of the Stranger mouse rather than that of the Familiar mouse in the three-chamber test. In order to evaluate the effects of RSV for normal mice on social novelty preference, the WT and the WT+RSV were tested (). The male WT and WT+RSV mice spent significantly more time around the Stranger than Familiar mouse (paired t test; WT: P = 0.0039; WT+RSV: P = 0.0098 ). Thus, social novelty preference was not affected by a single i.p. administration of RSV. Next, the male Oxtr-KO mice, as an ASD model, and the male Oxtr-KO+RSV mice were tested. Oxtr-KO mice showed no significant difference in interaction time with the Stranger and Familiar (paired t test; P = 0.32, ). The Oxtr-KO mice demonstrated reduced social novelty preference. However, the Oxtr-KO+RSV mice spent significantly more time around the Stranger than the Familiar mouse and showed normal social novelty preference (paired t test; P = 0.020, ). Thus, we showed that a single administration of RSV reversed the social impairments caused by Oxtr gene deficiency in ASD model mice (). Since RSV improved the impaired social novelty preference of the Oxtr-KO mice, it is likely that the administration of RSV may have a significant effect on ASD caused by Oxtr gene deficiency.
Figure 1. a. Schematic drawing of the experimental schedule RSV was administered i.p. (30 mg/kg) to male WT, Oxtr-KO, and VPA-ASD mice that were 2 to 4 months old. Three-chamber tests were performed 24 h after RSV administration. Immediately following the three-chamber test, the animals were euthanized and brain dissection was performed. b. Social novelty preference in WT mice. Values are provided as mean ± SEM (n = 10 per group). Values of P < 0.05 were considered statistically significant. (*P < 0.05, **P < 0.01).

Figure 2. a. Social novelty preference in Oxtr-KO mice without (Oxtr-KO) and with RSV administration (Oxtr-KO+RSV) Values are provided as mean ± SEM (n = 10 per group). Values of P < 0.05 were considered statistically significant. b. Social novelty preference in VPA-ASD mice without (VPA-ASD) and with RSV administration (VPA-ASD+RSV) Values are provided as mean ± SEM (n = 10 per group). Values of P < 0.05 were considered statistically significant. (*P < 0.05, **P < 0.01).

We also assessed another form of ASD using the VPA-ASD mice and VPA-ASD mice +RSV mice in the three-chamber test. VPA-ASD mice showed no significant differences regarding interaction time between the Stranger and Familiar mouse (paired t test; P = 0.13, ). However, the VPA-ASD mice+RSV mice spent significantly more time around the Stranger than the Familiar mouse and showed normal social novelty preference (paired t test; P = 0.0039, ). Therefore, we were also successful reversing the impairment in social novelty preference in adult VPA-ASD mice using a single administration of RSV.
RSV increased the expression of Sirt1 in amygdala of Oxtr-KO mice but not VPA-ASD mice
Previously we reported that microglial abnormality in the amygdala was a potential mechanism for the development of OXT/OXTR mediated ASD-like phenotypes [Citation22]. RSV has been shown to attenuate microglial activation via Sirt1 [Citation23]. Therefore, we collected the amygdala of the mice after the three-chamber test. RNA extraction was performed, and we examined the effect of Oxtr deletion or VPA treatment and RSV administration on the expression of the Sirt1 and Egr3 genes using qRT-PCR. Egr3 is a transcriptional activator of Sirt1 and one of the immediate-early genes that show increased expression following social stimulation [Citation24]. It has been reported that RSV induces the transcriptional up-regulation of EGR3 in human leukemia and cancer cells [Citation25]. Therefore, we examined the difference in Sirt1 and Egr3 mRNA expressions between Oxtr-KO mice with and without RSV treatment following social stimulation. We detected for the first time that expression levels of Sirt1 were significantly higher in the amygdala of the Oxtr-KO mice administered with RSV than those without (unpaired t-test; P = 0.021, ). Conversely, the expression levels of Sirt1 in the amygdala did not show any significant differences between the WT mice with and without RSV administration (unpaired test; P = 0.49, ). Regarding Egr3 in the amygdala, both the WT and Oxtr-KO mice treated with RSV had significantly higher expression levels than those without treatment (unpaired t test; P = 0.032 and P = 0.007, respectively, ). In contrast, no significant differences were observed in Sirt1 and Egr3 expressions between VPA-ASD mice and VPA-ASD mice +RSV (, b), suggesting that the improvement in social behavior induced by RSV in the VPA-ASD mice was via a pathway different from the Oxtr-KO mice.
Figure 3. Sirt1 and Egr3 mRNA expression analysis by qRT-PCR All samples were collected immediately after the three-chamber test. a. All data represent the relative value of Sirt1 mRNA against Gapdh mRNA in Amygdala. Left graph: Sirt1 mRNA expression in the WT mice (n = 4), and WT mice administered with RSV (WT+RSV) (n = 3). Middle graph: Oxtr-KO mice (n = 7), and Oxtr-KO mice administered with RSV (Oxtr-KO+RSV) (n = 7). Right graph: VPA-ASD mice (n = 3) and VPA-ASD mice administered with RSV (VPA+RSV) (n = 4). Data are represented as mean ± SEM. (*P < 0.05, **P < 0.01,) b. All data represent the relative value of Egr3 mRNA against Gapdh mRNA in Amygdala. Left graph: Egr3 mRNA expression in the WT mice (n = 4), and WT mice administered with RSV (WT+RSV) (n = 3). Middle graph: Oxtr-KO mice (n = 7), and Oxtr-KO mice administered with RSV (Oxtr-KO+RSV) (n = 7). Right graph: VPA-ASD mice (n = 3) and VPA-ASD mice administered with RSV (VPA+RSV) (n = 4). Data are represented as mean ± SEM. (*P < 0.05, **P < 0.01,).

Discussion
To our knowledge, our study reports, for the first time, that a single administration of RSV (30 mg/kg, i.p.) in male Oxtr-KO mice and VPA-ASD mice can improve their social impairments. The levels of Sirt1 mRNA in the amygdala of the Oxtr-KO mice treated with RSV were significantly higher than those in the mice not treated with RSV. However, this was not the case in the WT or VPA-ASD mice. Many studies have reported that RSV is a potent neuroprotective agent [Citation12,Citation26,Citation27], which stimulates Sirt1 expression. Sirt1 is an NAD+-dependent class III histone deacetylase that transfers acetyl groups from ε-N-acetyl-lysine residues to histones in order to regulate transcription. Also, Sirt1 plays an essential role in neurodegenerative disorders, including Alzheimer’s, Parkinson’s, and Huntington’s diseases. Michan et al. reported that Sirt1 is essential for normal cognitive function and synaptic plasticity [Citation28]. An increase in Sirt1 expression, as a result of RSV administration in the Oxtr-KO mice, might have induced normal neural activity in their amygdala. The brains of Sirt1-KO mice reportedly display decreased dendritic branching and phosphorylation of extracellular signal-regulated kinase 1/2 (Erk1/2) [Citation28]. We previously reported the abnormal activation of microglial cells and the reduction of a postsynaptic density protein PSD95 expression in the MeA and LS regions of the Oxtr-KO mouse brain [Citation22]. Abnormal activation of microglial cells leads to the damage to the neuron but not the protective properties, and a decrease in the density of synapses occurs. We also reported that the excessive activation of microglia is one of the causes of the social impairments of Oxtr-KO mice [Citation22]. In this report, we showed that the administration of RSV upregulates Sirt1 expression in the amygdala of Oxtr-KO mice. It was possible that the anti-oxidative activity of RSV through the regulation of Sirt1 expression prevented the abnormal activation of microglia and ameliorated the ASD-like behavior of Oxtr-KO mice. In contrast, RSV acts as a Calcium signaling modulator, cyclo-oxygenase inhibitor, PPAR-α activator, and an anti-inflammatory agent through the regulation of eNOS or microRNA production [Citation29]. In addition to Sirt1 expression, it was also possible that RSV caused amelioration of ASD-like phenotypes in Oxtr-KO mice. Recently, it was reported that anxiety caused by obesity is improved by RSV, which increases the expression of Sirt1. Xu et al. reported that exposure to a short-term high-fat diet (HFD) induces anxiety in mice, and decreases Sirt1 expression in the prefrontal cortex and amygdala [Citation30]. Moreover, RSV improves the anxious behavior of HFD-fed mice [Citation30]. We previously reported that Oxtr-KO mice develop late-onset obesity [Citation31]. Assuming that obesity caused by the loss of Oxtr has the same influence as that of HFD, the improvement of ASD-like behavior in the Oxtr-KO mice after RSV administration in the current study may have resulted from an increase in Sirt1 expression. We demonstrated that the Egr3 mRNA levels were significantly higher in the amygdala of the Oxtr-KO mice + RSV and WT mice + RSV than in that of the “without treatment” group, though this effect was not shown in the VPA-ASD mice. We further demonstrated that Egr3 mRNA levels were increased in the amygdala of Oxtr-KO mice and WT male mice treated with RSV compared to those without treatment, while this was not observed in the VPA-ASD mice. Egr3 is one of the immediate-early genes and is a transcription factor. A previous study demonstrated that Egr3 binds to Sirt1 promoters and regulates its expression [Citation24]. Our findings emphasize the importance of the effect of RSV not only in the ASD model mice but also in WT mice.
We found that a single administration of RSV in adult male VPA-ASD mice improved their social impairments. VPA is known to be antiepileptic, associated with a high-risk of ASD in humans [Citation32]. As an animal model of ASD, prenatal VPA administration in rodents was well studied. Pups exposed to prenatal VPA showed social defects, anatomical and functional alterations in the cerebral cortex and cerebellum [Citation33]. Fontes-Dutra reported the localization of Parvalbumin positive GABAergic neurons altered in the primary sensory cortex and amygdala in the VPA-ASD mice [Citation34]. We did not detect a difference in the expression levels of Gad1 and Gad2 in the amygdala of VPA-ASD mice and VPA-ASD mice +RSV (data not shown). Considering VPA is known to be an HDAC inhibitor, while RSV increases the expression of Sirt1, which is an HDAC, we expected that RSV administration in VPA-ASD mice induces that the expression of Sirt1, but we did not detect a change in Sirt1 expression. It was reported that the administration of astaxanthin or piperine, which are potent antioxidants, to VPA-ASD mice improved their ASD-like behavior and reduced the brain oxidative stress [Citation35,Citation36]. As observed in the reports mentioned above, the anti-oxidant function of RSV possibly improved the social defect in the VPA-ASD mice.
A previous study reported that VPA-ASD rats exhibit lower levels of Oxt mRNA, fewer Oxt-positive cells in the supraoptic nucleus, and lower levels of Oxt concentration in the cerebrospinal fluid when compared to control mice [Citation18]. Additionally, an acute intranasal administration of exogenous Oxt restores the social preference and repetitive behavior of VPA-ASD rats [Citation19]. Another study reported that intranasal administration of Oxt to VPA-ASD mice improves the VPA-induced social interaction deficit, and increases c-Fos expression in the paraventricular nucleus, prefrontal cortex, and somatosensory cortex [Citation20]. These findings indicate that VPA-induced ASD model mechanisms are affected by the OXT-OXTR system. Administration of RSV or OXT improved the behavior of the VPA-ASD mice, and RSV ameliorated the social defect of Oxtr-KO mice. If RSV has common function(s) in the Oxtr-KO mice and VPA-ASD mice, leading to amelioration of ASD-like behaviors, RSV would have target(s) at the downstream of the OXT-OXTR signaling pathway. OXTR belongs to the type A G-protein-coupled receptor (GPCR) family, and Oxt binds Oxtr to finally exert various physiological functions, which are mediated by second messengers and crosstalks with multiple cell signaling pathways. For instance, Oxytocin increases the activity of Erk1/2 through the mediation of G-protein [Citation37]. Some studies indicated that RSV activated Erk1/2 in vivo and in vitro [Citation38,Citation39]. Administration of RSV alleviated the depression-like behavior via Erk1/2 activation in the PFC and hippocampus of depression-model rat [Citation39]. It is possible that RSV induces Erk1/2 activation in the PFC and hippocampus, and ameliorates ASD-like behavior, commonly in Oxtr-KO mice and VPA-ASD mice.
These results demonstrated that a single RSV i.p. administration is effective for several types of ASD. In the current study, we demonstrated, for the first time, that a single i.p. administration of RSV to adult Oxtr-KO mice and VPA-ASD mice improved their social impairment. Bambini-Junior et al. reported that prenatal sequential co-administration of RSV with VPA prevents VPA-induced social impairments [Citation13]. Previous results, taken together with our study, suggest that RSV might be valuable, not only for the prevention of ASD but also as a therapeutic option. Some studies have demonstrated that injection of an OXTR antagonist into the medial amygdala of WT mice prevents normal social recognition and that OXTR activation in the medial amygdala is essential for the acquisition of social memories [Citation40,Citation41]. It has been suggested that the OXT-OXTR system in the amygdala regulates social behavior. Here, we reported the increase in Sirt1 mRNA in the amygdala of Oxtr-KO mice by RSV administration. Increases in Sirt1 might be one of the possible mechanisms for social novelty preference impairments. Further studies are needed to determine the molecular targets of RSV in ASD mice, as well as changes in their expression or other modifications.
Author contribution
S.H., S.K., and K.N. designed the research; S.K. and S.H. performed the research; S.H. wrote the paper; R.T., T.Y., and K.H. contributed to the collection and interpretation of data; K.S. and K.N. assisted in the preparation of the manuscript; all authors critically reviewed the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Park HR, Lee JM, Moon HE, et al. A short review on the current understanding of autism spectrum disorders. Exp Neurobiol. 2016;1:1–13.
- Tordjman S, Somogyi E, Coulon N, et al. Gene × environment interactions in autism spectrum disorders: role of epigenetic mechanisms. Front Psychiatry. 2014;5:53.
- Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67:1–23.
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506.
- Ghosh S, Liu B, Zhou Z. Resveratrol activates SIRT1 in a Lamin A-dependent manner. Cell Cycle. 2013;12:872–876.
- Olas B, Wachowicz B, Majsterek I, et al. Resveratrol may reduce oxidative stress induced by platinum compounds in human plasma, blood platelets and lymphocytes. Anticancer Drugs. 2005;16:659–665.
- de Sá Coutinho D, Pacheco MT, Frozza RL, et al. Anti-inflammatory effects of resveratrol: mechanistic insights. Int J Mol Sci. 2018;19(6):E1812.
- Li J, Zhang CX, Liu YM, et al. A comparative study of anti-aging properties and mechanism: resveratrol and caloric restriction. Oncotarget. 2017;8:65717–65729.
- Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209–225.
- Wu JM, Hsieh TC, Wang Z. Cardioprotection by resveratrol: a review of effects/targets in cultured cells and animal tissues. Am J Cardiovasc Dis. 2011;1:38–47.
- Guo Z, Liu Y, Cheng M. Resveratrol protects bupivacaine-induced neuro-apoptosis in dorsal root ganglion neurons via activation on tropomyosin receptor kinase A. Biomed Pharmacother. 2018;103:1545–1551.
- Li YC, Liu YM, Shen JD, et al. Resveratrol ameliorates the depressive-like behaviors and metabolic abnormalities induced by chronic corticosterone injection. Molecules. 2016;21:10. DOI:10.3390/molecules21101341
- Bambini-Junior V, Zanatta G, Della Flora Nunes G, et al. Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci Lett. 2014;583:176–181.
- Andrade S, Ramalho MJ, Pereira MDC, et al. Resveratrol brain delivery for neurological disorders prevention and treatment. Front Pharmacol. 2018. DOI:10.3389/fphar.2018.01261
- Takayanagi Y, Yoshida M, Bielsky IF, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101.
- Slane MM, Lusk LG, Boomer KB, et al. Social cognition, face processing, and oxytocin receptor single nucleotide polymorphisms in typically developing children. Dev Cogn Neurosci. 2014;9:160–171.
- Yatawara CJ, Einfeld SL, Hickie IB, et al. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2016;21:1225–1231.
- Guastella AJ, Gray KM, Rinehart NJ, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol. 2015;56:444–452.
- Dai YC, Zhang HF, Schön M, et al. Neonatal oxytocin treatment ameliorates autistic-like behaviors and oxytocin deficiency in valproic acid-induced rat model of autism. Front Cell Neurosci. 2018;12:355.
- Hara Y, Ago Y, Higuchi M, et al. Oxytocin attenuates deficits in social interaction but not recognition memory in a prenatal valproic acid-induced mouse model of autism. Horm Behav. 2017;96:130–136.
- Nadler JJ, Moy SS, Dold G, et al. Automated apparatus for quantitation of social approach behaviours in mice. Genes Brain Behav. 2004;3(5):303–314.
- Miyazaki S, Hiraoka Y, Hidema S, et al. Prenatal minocycline treatment alters synaptic protein expression, and rescues reduced mother call rate in oxytocin receptor-knockout mice. Biochem Biophys Res Commun. 2016;472:319–323.
- Zhang S, Gao L, Liu X, et al. Resveratrol attenuates microglial activation via SIRT1-SOCS1 pathway. Evid Based Complement Alternat Med. 2017;2017:1–10.
- Chandra R, Francis TC, Konkalmatt P, et al. Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci. 2015;35:7927–7937.
- Mizutani N, Omori Y, Kawamoto Y, et al. Resveratrol-induced transcriptional up-regulation of ASMase (SMPD1) of human leukemia and cancer cells. Biochem Biophys Res Commun. 2016;470:851–856.
- Xie YK, Zhou X, Yuan HT, et al. Resveratrol reduces brain injury after subarachnoid hemorrhage by inhibiting oxidative stress and endoplasmic reticulum stress. Neural Regen Res. 2019;14:1734–1742.
- Dou Z, Rong X, Zhao E, et al. Neuroprotection of resveratrol against focal cerebral ischemia/reperfusion injury in mice through a mechanism targeting gut-brain axis. Cell Mol Neurobiol. 2019;39:883–898.
- Michan S, Li Y, Chou MM-H, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707.
- Bhandari R, Paliwal JK, Kuhad A. Dietary phytochemicals as neurotherapeutics for autism spectrum disorder: plausible mechanism and evidence. Adv Neurobiol. 2020;24:615–646.
- Xu L, Xu S, Lin L, et al. High-fat diet mediates anxiolytic-like behaviors in a time-dependent manner through the regulation of SIRT1 in the brain. Neuroscience. 2018;21:237–245.
- Takayanagi Y, Kasahara Y, Onaka T, et al. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955.
- Ornoy A, Weinstein-Fudim L, Ergaz Z. Prevention or amelioration of autism-like symptoms in animal models: will it bring us closer to treating human ASD? Int J Mol Sci. 2019;20(5):1074.
- Ingram JL, Peckham SM, Tisdale B, et al. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol. 2000;22(3):319–324.
- Fontes-Dutra M, Santos-Terra J, Deckmann I, et al. Resveratrol prevents cellular and behavioral sensory alterations in the animal model of autism induced by valproic acid. Front Synaptic Neurosci. 2018;10:9.
- Al-Amin MM, Rahman MM, Khan FR, et al. Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav Brain Res. 2015;286:112–121.
- Pragnya B, Kameshwari JS, Veeresh B. Ameliorating effect of piperine on behavioral abnormalities and oxidative markers in sodium valproate induced autism in BALB/C mice. Behav Brain Res. 2014;270:86–94.
- Blume A, Bosch OJ, Miklos S, et al. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27(8):1947–1956.
- Mustafi SB, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS One. 2010;5(1):e8719.
- Wang X, Xie Y, Zhang T, et al. Resveratrol reverses chronic restraint stress-induced depression-like behaviour: involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in rats. Brain Res Bull. 2016;125:134–143.
- Ferguson JN, Aldag JM, Insel TR, et al. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285.
- Bertelsen F, Folloni D, Møller A, et al. Suppressed play behaviour and decreased oxytocin receptor binding in the amygdala after prenatal exposure to low-dose valproic acid. Behav Pharmacol. 2017;28:450–457.
