ABSTRACT
This work aimed to assess the role of TLR8 in cerebral I/R injury and its in-depth pathogenesis. Bioinformatics analysis indicated that TLR8 was up-regulated in patients with ischemic stroke than that in healthy control, and miR-18a-5p was the upstream regulatory of TLR8. Then, the rat pheochromocytoma PC12 cells were exposed in oxygen-glucose-deprivation/reoxygenation (OGD/R) conditions to construct a model in vitro. The functional experiments indicated that OGD/R can decline the viability and elevate the apoptosis of PC12 cells, while up-regulation of miR-18a-5p can alleviate OGD/R-induced cell injury. Notably, overexpression of TLR8 reverses the miR-18a-5p-mediated protection on OGD/R-induced cells injury. Finally, we found that up-regulation of miR-18a-5p obviously declined the protein levels of TLR4 and TLR7 as well as the phosphorylation of NF-κB, while overexpression of TLR8 canceled the decrease caused by miR-18a-5p up-regulation. In summing, our results illustrated that miR-18a-5p/TLR8 axis can mitigate OGD/R-induced cells injury through TLRs and NF-κB pathway.
GRAPHICAL ABSTRACT
Overexpression of TLR8 reverses the miR-18a-5p-mediated protection on OGD/R-induced cells injury.
KEYWORDS:
Stroke is a serious threat to human life and health [Citation1]. The incidence rate increased year by year with higher mortality [Citation2]. Clinically, stroke is divided into ischemic stroke and hemorrhagic stroke, of which 70%-80% of patients are diagnosed as ischemic stroke [Citation3]. The pathophysiology of ischemic stroke is very complicated, and timely opening vascular is the most effective treatment [Citation4]. However, more and more studies have shown that when the cerebral ischemia area recovers again, a variety of protein factors and related signal pathways will be activated, resulting in reperfusion injury, which is characterized by increased apoptosis and partial neurological impairment [Citation5,Citation6]. To date, there is no effective treatment strategy for acute and chronic complications caused by cerebral ischemia-reperfusion (I/R) injury, and the therapeutic effect is still limited [Citation7]. Hence, it is urgent to find an effective treatment strategy to reduce the cerebral I/R injury.
More and more evidence showed that the aggravation of cerebral ischemic injury is caused by cerebral I/R-induced acute inflammatory response [Citation8,Citation9]. Toll-like receptors (TLRs) are the main receptors in the natural immune system to recognize pathogenic microorganisms and inflammation signals, which act as a significant role in inducing and regulating immune/inflammatory response [Citation10]. Importantly, it has been widely found that blocking or activating TLRs signaling pathway can effectively inhibit the occurrence and development of inflammatory cascade reaction in the acute stage of cerebral ischemia and reduce inflammatory damage [Citation11–13]. TLR8, an important member of the TLRs family, is also participated in the progression of ischemic cerebral injury. Brea D et al. considered that the abnormal expression of TLR8 was associated with the aggravation of inflammatory response and poor prognosis in acute ischemic stroke [Citation14]. Nevertheless, little work has been done on the research of TLR8 as a regulatory gene in cerebral I/R injury.
To date, more than 60% of the genes are considered to be regulated by microRNAs(miRNAs) [Citation15]. With the development of gene chip and gene sequencing technology, it has been found that noncoding RNA such as LNC RNA and miRNA play an important role in human diseases [Citation16,Citation17], including cerebral I/R injury [Citation18–20]. For instance, miR-375 was expressed at low levels in rat with cerebral I/R, which can mitigate the cerebral I/R injury by regulating Ctgf [Citation21]. Similarly, miR-34b/Keap1 axis contributed to protecting against cerebral I/R injury in rat [Citation22]. Herein, bioinformatics predictions revealed that miR-18a-5p was a regulatory miRNA on TLR8. Furthermore, miR-18a has been confirmed to be down-regulated in ischemic stroke [Citation23], but the specific mechanism has not been studied.
In this work, we established an in vitro cell model induced by oxygen glucose deprivation and reoxygenation (OGD/R). Based on literature and bioinformatics analysis, we hypothesized that miR-18a-5p could reduce the cerebral I/R injury through regulation of the TLRs pathway by targeting TLR8, hoping to open up a potential novel way for the therapy of cerebral I/R injury.
Materials and methods
Data collection
The ischemic stroke samples were downloaded from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) (access number: GSE 16,561) to analyze the differential expression of TLR8, which contains 39 ischemic stroke patients and 24 healthy control. The upstream regulatory miRNAs of TLR8 were predicted using the miRNA gene prediction site miRTarBase (http://mirtarbase.mbc.edu.tw/php/index.php) and TargetScan (http://www.targetscan.org). The data of 20 ischemic stroke patients and 20 healthy control were also downloaded from the GEO database (access number: GSE 110,993) to analyze differential expression of miR-18a-5p.
Cells treatment
Rat pheochromocytoma PC12 cells (American Type Culture Collection, American) were routinely cultured in Dulbecco’s modified eagle’s medium (DMEM; Carlsbad, CA, USA) supplement with 10% fetal bovine serum (FBS; Sigma-Aldrich, MO, USA), 100 U/mL penicillin and 0.1 mg/mL streptomycin. The OGDR model was established following the previous description with minor modifications [Citation24–27]. Briefly, cells were cultured in an anoxic environment (95% N2 and 5% CO2 at 37°C) contains glucose-free DMEM (Gibco, USA) medium for 2 h, and then replaced with normal glucose level medium to terminate the OGD and incubated the PC12 cells for 12 h at room temperature for reoxygenation (95% air, 5% CO2). Simultaneously, control groups were treated without OGD exposure.
Si-TLR8 (5′-TCGACAATCTGACAAATTTGAAG-3′), si-con (5ʹ- GAACTAGAACATCAAGATGACATG-3ʹ), pcDNA3.1-TLR8 and corresponding control vector were synthesized from Shanghai GenePharma Co., Ltd. (Shanghai, China), which were used for overexpression/knockdown of TLR8. Similarly, for up-regulation/inhibition of miR-18a-5p, the miR-18a-5p mimic/inhibitor, and negative control obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China) were used. Transfection was performed using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA), as directed by manufacturer. The follow-up experiments were performed after 48 h of transfection.
qRT-PCR assay
TRIzol reagent (Invitrogen) was utilized to extract total DNA from cell lines, and then PrimeScript RT Master Mix (Takara, Dalian, China) was used to reverse transcription the extracted RNA to the total cDNA based on the manufacturer’s agreement. Subsequently, qRT-PCR was employed to detect the expression level of TLR8 using the SYBR Premix Ex Taq II (TaKaRa, Japan). The procedure was conducted at 95°C for 5 min, followed by 40 cycles of 95°C for 30 s and then at 65°C for 45 s. The primer sequences were:
TLR8:
F: 5ʹ- TGGAAACTGCCCAAGGTGTT-3ʹ,
R: 5ʹ- AACACCTTGGGCAGTTTCCA-3ʹ;
GAPDH:
F: 5ʹ- TGATGGGTGTGAACCACGAG-3ʹ,
R: 5ʹ- TGATGGCATGGACTGTGGTC-3ʹ
Western blotting assay
Proteins were detached from cells by lysis buffer containing protein inhibitor, and then the protein concentration was measured by the BCA kit (Pierce, Appleton, WI). The extracted proteins were separated by SDS-PAGE and then transferred to the PVDF membrane. The membranes were sealed with 5% skimmed milk powder at room temperature for 1 h, which then incubated with primary antibody at 4°C overnight. Then, the membranes were incubated with the second antibody at room temperature for 1 h. After that, the blots were developed using an EasyBlot ECL Kit (Sangon Biotech, Shanghai, China). The intensity of the blot was calculated using QUANTITY ONE software after imaging, and GAPDH was used as a loading control.
Cell counting kit 8 (CCK-8) assay
The transfected cells were inoculated in a 96-well plate at the density of 1*103/well, followed by undergoing OGD/R. Then, cells were traditional cultured in a carbon dioxide incubator with 10 μL of CCK8 reagent (Dojindo, Kumamoto, Japan) for 2 h before detection. The Microplate reader (Bio-Rad, CA, USA) was used to detect the OD values at 0 h, 24 h, 48 h and 72 h.
Apoptosis assay
The cells were stained and quantified with Annexin V-FITC/PI kit (4A Biotech, Beijing, China) following the manufacturer’s instructions. The transfected cells were incubated in 200 μL binding buffer supplement with 10 μL of Annexin V-FITC and 5 μL of PI for 1 h at room temperature in the dark. Then, flow cytometry was used for apoptotic analysis. Finally, the flowing results were analyzed by Flowjo software. Briefly, there are necrotic cells in Q1, late apoptotic cells in Q2, early apoptotic cells in Q4 and living cells in Q3. We mainly calculated apoptotic cells, so we use the sum of Q2 + Q4 as the statistical result.
Dual-luciferase reporter assay
So as to further confirm our bioinformatics prediction that miR-18a-5p is an upstream regulatory miRNA of TLR8, dual-Luciferase reporter assay was performed. The wild and mutant TLR8-3ʹURT were cloned into pmirGLO luciferase vector to construct TLR8-WT and TLR8-MUT vectors. Wherein, the TLR8-WT contains the binding sites of 3ʹUTR with miR-18a-5p. Then, cells were co-transfected with miR-18a-5p mimic/inhibitor/NC and TLR8-WT or TLR8-MUT by Lipofectamine 2000, following the standard protocol. After transfection for 48 h, the luciferase activity was measured by Dual-Luciferase TM system (Promega) as directed by manufacturer.
Statistical analysis
The experimental data in this work were analyzed by statistical analysis software SPSS22.0. Students’t-test (two groups) or one-way ANOVA variance analysis with posttest of Dunnett (multiple groups) were used for comparison between groups. Gene sets enrichment analysis was used to screening pathway. P < 0.05 indicated that the difference has statistical significance.
Results
TLR8 was up-regulated in ischemic stroke patients and PC12 cells under OGD/R treatment
Firstly, based on the gene sets enrichment analysis, we found that the highly expressed genes in ischemic stroke are mostly enriched in TLRs signaling pathway, and TLRs pathway is positively correlated with ischemic stroke ()). So we selected TLRs signaling pathway for research. Then, we sought to elucidate the differential expression and function of TLRs pathway-related genes in patients with ischemic stroke. The data including ischemic stroke patients (n = 39) and healthy control (n = 24) were analyzed by GEO Online Analysis Tool GEO2R. As demonstrated in ), TLR8 expression in ischemic stroke group was clearly elevated compared with the control group (P < 0.01).
Figure 1. The expression level of TRL8 in OGD/R-treated PC12 cells. (a) Toll-like receptor (TLR) signaling was positively correlated with ischemic stroke. (b) The expression level of TRL8 in ischemic stroke patients was clearly enhanced compared with control group, P < 0.01. (c–e) OGD/R-induced PC12 cells injury is accompanied by TLR8 overexpression. **P < 0.01 vs. sham group

Additionally, qRT-PCR and western blotting assays were conducted to confirm the expression of TLR8 in OGD/R-treated PC12 cells. As expected, OGD/R treatment resulted in the up-regulation of TLR8 in PC12 cells (). These results demonstrated that the overexpression of TLR8 may contribute to the OGD/R-induced cell injury.
Knockdown of TLR8 enhanced the viability while overexpression of TLR8 declined the viability of OGD/R-treated PC12 cells
To study the function of TLR8, gain-of-function, and loss-of-function assays were carried out using pcDNA3.1-TLR8 and si-TLR8. PC12 cells were separately transfected with si-TLR8 and pcDNA3.1-TLR8. After transfection for 24 h, we carried out qRT-PCR and western blotting assay to detect the TLR8 expression in transfected cells. As presented in , transfection of pcDNA3.1-TLR8 can markedly increase the mRNA and protein levels of TLR8 compared with the vector group. Contrarily, the mRNA and protein expression level of TLR8 in cells transfected with si-TLR8 was markedly decreased than that in si-con group (). These results provided a basis for our subsequent experiments.
Figure 2. Effects of TLR8 on cell viability in OGD/R-treated PC12 cells. (a–c) The mRNA and protein levels of TLR8 in cells transfected with pcDNA3.1-TLR8 or vector compared with control. **P < 0.01. (d–f) The mRNA and protein expression of TLR8 in cells transfected with si-TLR8 or si-con compared with control. **P < 0.01. (g) Cells were respectively transfected with pcDNA3.1-TLR8, vector, si-TLR8 and si-con, followed by treating with OGD/R. Then, cell viability was detected using CCK-8 assay. **P < 0.01 vs. sham group, ## P < 0.01 vs. OGD/R group

To assess the contribution of TLR8 in OGD/R-treated cells viability, CCK-8 assay was performed. As illustrated in , OGD/R treatment can obviously decline the cell viability, and this influence was markedly enhanced by overexpression of TLR8 and reversed by knockdown of TLR8 (P < 0.01). From these results we can conclude that down-regulation of TLR8 can block the OGD/R-induced reduction of cell viability, which possibly alleviate the OGD/R-induced cells injury.
Targeted Regulation of TLR8 by miR-18a-5p
Inspired by the results of TLR8 regulating OGD/R-induced cell injury, we conjectured that TLR8 might be targeted by upstream regulatory molecules to regulate this process. In order to clarify the molecular basis of TLR8, the miRNA target gene prediction sites miRTarBase and TargetScan were used to find the important regulatory molecules of TLR8. The predicted data prompted that miR-18a-5p might combine with the 3′-UTR of TLR8 ()). Next, dual-luciferase reporter assay was conducted to determine this forecasting. As presented in ), up-regulation of miR‑18a-5p caused a noticeable reduction in luciferase activity of the WT-TLR8 3ʹ‑UTR reporter, but not in the MUT-TLR8 group. Similarly, inhibition of miR-18a-5p obviously enhanced the luciferase activity of the WT-TLR8 3ʹ‑UTR reporter, but this effect was hindered in mutations.
Figure 3. MiR-18a-5p directly targets TLR8 in OGD/R-treated PC12 cells. (a) Interplay between miR-18a-5p and the 3ʹ‑UTR of TLR8 was predicted by miRTarBase and TargetScan. (b) After transfection with miR-18a-5p mimic/inhibitor/NC, the luciferase activity of reporters containing the WT or MUT TLR8 3ʹ‑UTR. **P < 0.01 vs. NC group. (c) MiR-18a-5p was expressed at low levels in ischemic stroke patients than that in control. P < 0.05. (d–f) The mRNA and protein levels of TLR8 in control group, miR-18a-5p mimic group, pcDNA3.1-TLR8 group and miR-18a-5p mimic + pcDNA3.1-TLR8 group. **P < 0.01 vs. the control group, &&P < 0.01 vs. the miR-18a-5p mimic group, ##P < 0.01 vs. the pcDNA3.1-TLR8 group. (g–i) The mRNA and protein levels of TLR8 in control group, miR-18a-5p inhibitor group, si-TLR8 group and miR-18a-5p inhibitor + si-TLR8 group. **P < 0.01 vs. the control group, &&P < 0.01 vs. the miR-18a-5p inhibitor group, ##P < 0.01 vs. the si-TLR8 group

Then, we analyzed the differential expression of miR-18a-5p in ischemic stroke patients (n = 20) and healthy control (n = 20) based on GEO database (GSE110993). The results presented in indicated that miR-18a-5p was lowly expressed in ischemic stroke patients. Moreover, we further studied whether the expression of TLR8 was regulated by miR-18a-5p using qRT-PCR and western blotting assay. It can be seen from , transfection of miR-18a-5p mimic can obviously decline the mRNA and protein levels of TLR8, while overexpression of TLR8 can block the miR-18a-5p mimic-mediated decrease. In contrast, inhibition of miR-18a-5p markedly increased the mRNA and protein expression levels of TLR8, but TLR8 expression was markedly declined in cells co-transfected with si-TLR8 and miR-18a-5p inhibitor than that in miR-18a-5p inhibitor group (). From the above results, we can easily obverse that miR-18a-5p directly targets TLR8 and negatively regulates its expression.
Overexpression of TLR8 reverses the miR-18a-5p-mediated protection on OGD/R-induced cells injury
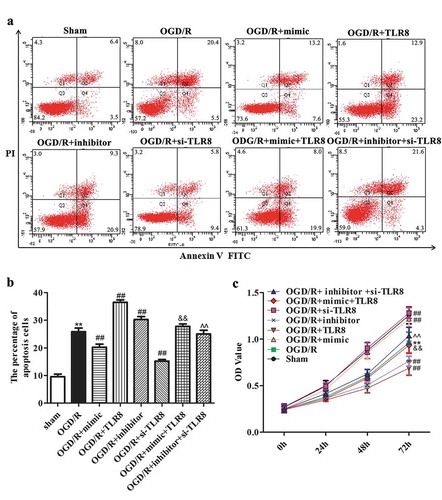
To evaluate whether miR-18a-5p/TLR8 axis contributes to OGD/R-induced cells injury, CCK-8 and flow cytometry were carried out to detect cell viability and cell apoptosis, respectively. As presented in , OGD/R treatment resulted in a noticeable increase in the number of apoptotic cells compared to the sham group. Simultaneously, transfection of miR-18a-5p mimic can markedly reduce the cell apoptotic than that in OGD/R group, whereas overexpression of TLR8 showed an opposite effect. Importantly, the number of apoptosis cells was clearly enhanced in OGD/R-treated PC12 cells co-transfected with miR-18a-5p mimic and pcDNA3.1-TLR8 compared with the miR-18a-5p mimic group. Analogously, inhibition of miR-18a-5p clearly enhanced the number of apoptosis cells, while knockdown of TLR8 reversed this effect.
Figure 4. The influence of miR-18a-5p/TLR8 axis on biological phenotype of OGD/R-treated PC12 cells. Cells were respectively transfected with miR-18a-5p mimic, pcDNA3.1-TLR8, miR-18a-5p mimic + pcDNA3.1-TLR8, miR-18a-5p inhibitor, si-TLR8 and miR-18a-5p inhibitor + si-TLR8, and then treated with OGD/R. (a–b) Cell apoptosis and (c) cell viability were respectively detected using flow cytometry and CCK-8 assay. **P < 0.01 vs. sham group, ## P < 0.01 vs. OGD/R group. &&P < 0.01 vs. OGD/R + mimic group, ^^P < 0.01 vs. OGD/R + inhibitor group

Simultaneously, the results of CCK-8 were presented in that up-regulation of TLR8 resulted in the loss of the promoting influence of miR-18a-5p on OGD/R-treated cell viability. Conversely, knockdown of TLR8 reversed the miR-18a-5p inhibitor-mediated reduction on OGD/R-treated cell viability. These results in this chapter revealed that miR-18a-5p functions in alleviating OGD/R-induced injury through targeting TLR8.
TLRs and Nuclear factor kinase B (NF-κB) pathways mediated the regulation of miR-18a-5p/TLR8 on OGD/R-induced injury in PC12 cells
To in-depth study the protective mechanism of miR-18a-5p/TLR8 axis on OGD-R-induced injury, the protein levels of pathway-related factors were assessed using western blotting assay. From we can see that after OGD/R-treated PC12 cells transfected with miR-18a-5p mimic, the protein levels of TLR4, TLR7, and the phosphorylation of NF-κB were markedly decreased than that in OGD/R-treated PC12 cells, while overexpression of TLR8 hindered the decrease caused by up-regulation of miR-18-5p. Concurrently, the protein level of total NF-κB was almost unchanged. In contrast, inhibition of miR-18a-5p caused a significant increase in the protein levels of TLR4, TLR7, and the phosphorylation of NF-κB in OGD/R-treated PC12 cells, while after the OGD/R-treated PC12 cells co-transfected with miR-18a-5p inhibitor and si-TLR8, the protein levels of these factors were markedly decreased than that in miR-18a-5p inhibitor group. Consistently, the protein level of total NF-κB was also almost unchanged (). Overall, our data illustrated that miR-18a-5p/TLR8 axis can protect OGD/R-induced injury through TLRs and NF-κB pathway, hoping to provide a new scheme for protecting cerebral I/R injury in clinical therapeutics.
Figure 5. The influence of miR-18a-5p/TLR8 axis on TLRs and NF-κB pathways in OGD/R-treated PC12 cells. (a–d) The protein levels of TLR4, TLR7 as well as the phosphorylation of NF-κB and NF-κB in different transfections. **P < 0.01 vs. OGD/R group, ##P < 0.01 vs. OGD/R+ mimic group, && P < 0.01 vs. OGD/R+ TLR8 group. @@P < 0.01 vs. OGD/R+ inhibitor group, ^^ P < 0.01 vs. OGD/R+ si-TLR8 group

Discussion
The main ascertainment of this work is that miR-18a-5p can alleviate the OGD/R-induced injury through targeting TLR8 and inhibiting the TLRs and NF-κB signaling pathway in vitro. In this paper, we discovered that TLR8 is an OGD/R-responsive gene that participated in regulating OGD/R-induced injury. TLR8 was up-regulated in ischemic stroke patients and OGD/R-treated PC12 cells, and depletion of TLR8 enhanced the cell viability. Then, we identified that miR-18a-5p directly targets TLR8, which was lowly expressed in ischemic stroke patients. Further data indicated that miR-18a-5p/TLR8 axis can protect PC12 cells against OGD/R-induced injury, while this protection was achieved by regulating TLRs and NF-κB pathway. Besides, the mechanism diagram of miR-18a-5p was presented in the supplementary file 1.
To our knowledge, I/R injury is associated with excessive inflammation [Citation28]. TLRs is one of the main participants in the process of injurious inflammation [Citation28], and the contribution of their family members to the injury of I/R has been widely reported. TLR4 was highly expressed after cerebral I/R injury [Citation11], and in mice with TLR4-deletion, cerebral I/R injury was mitigated [Citation29]. Sun W et al. found that the inhibition of TLR2 reduced the expression of pro-inflammatory factors during cerebral I/R-injury [Citation30]. Additionally, in acute ischemic stroke, the abnormal expression of TLR7 and TLR8 aggravates the inflammatory response and leads to poor prognosis [Citation14]. TLR8, as a member of TLRs receptor, is located in the cytoplasm. It mainly recognizes single-stranded RNA and participates in the progression of virus infection [Citation31]. Some studies have suggested that TLR8 can also participate in the modulation of cell proliferation, cell transformation, and apoptosis, which is closely related to many diseases such as inflammation and tumor [Citation32]. Up to now, there are few studies on TLR8 in cerebral I/R injury. Our data showed that TLR8 was up-regulated in ischemic stroke patients and OGD/R-treated PC12 cells, and inhibition of TLR8 could reverse the decline of cell viability induced by OGD/R. These findings indicated that TLR8 may be participated in the development of cerebral I/R injury.
Many molecules have been identified as regulators of TLR signaling pathway, including phosphatase, protein kinase, ubiquitin-related protein, etc [Citation33]. Wherein, microRNA (miRNA) has been identified as a new regulatory subfamily involved in the regulation of TLR signaling pathways [Citation34,Citation35]. In this paper, our prediction indicated that TLR8 is a direct target of miR-18a-5p. MiR-18a-5p has been reported to function as an oncogene in a variety of cancers, such as osteosarcoma [Citation36], renal cell carcinoma [Citation37], nasopharyngeal carcinoma [Citation38],etc. Importantly, miR-18a has been proved to have the function of regulating human brain endothelial cells [Citation39,Citation40]. MiR-18a-5p, as a branch of miR-18a, has not been studied in ischemic brain disease, including cerebral I/R injury. Our results confirmed that miR-18a-5p directly targeted TLR8, which was highly expressed in patients with ischemic stroke. Then, the cellular functional experiment revealed that miR-18a-5p can reduce the injury caused by OGD/R via targeting TLR8.
Previously, studies have found that activation of multiple signaling pathways was participated in the process of cerebral I/R injury [Citation41–43], which affects the expression of downstream apoptosis-related target genes and then affects neuronal apoptosis [Citation44]. Numerous studies have proved that TLRs contributed to regulating the cerebral I/R injury [Citation28,Citation45]. Interestingly, NF-κB has been shown to be activated by TLRs, which is considered as a central regulator of inflammatory response [Citation46]. It has been widely reported that TLR4/NF-κB signaling pathway was participated in the inflammatory process of cerebral I/R injury [Citation47–49]. In our work, we revealed that up-regulation of miR-18a-5p decreased the levels of TLRs/NF-κB-related factors, while overexpression of TLR8 can reverse this reduction, suggesting that TLRs and NF-κB pathway mediates the protective influence of miR-18a-5p/TLR8 axis on cerebral I/R injury.
In summing, our results provide a substantial evidence for the initial hypothesis that miR-18a-5p/TLR8 axis act as a potential regulator to mitigate ODG/R-induced injury through TLRs and NF-κB pathway in vitro. It supplied novel targets and a reliable scientific basis for clinical treatment of cerebral I/R injury. Nevertheless, the findings of this work are restricted to OGD/R models in vitro, the functions of miR-18a-5p/TLR8 axis in cerebral I/R injury in vivo need further verification.
Authors’ contributions
LYY and MXJ performed the experiments. LYY, MXJ, and YNN analyzed the data. LYY, MXJ, and YNN wrote the manuscript. LYY and MXJ reviewed and edited the manuscript. All authors read and approved the final manuscript.
Supplementary_file_1.pdf
Download PDF (113.1 KB)Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability statement
All data generated or analysed during this study are included in this published article. https://www.85ncbi.nlm.nih.gov/geo/.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Perez-Alvarez MJ, Wandosell F. Stroke and neuroinflamation: role of sexual hormones. Curr Pharm Des. 2016;22:1334–1349.
- Alam MM, Mohammad AA, Shuaib U, et al. Homocysteine reduces endothelial progenitor cells in stroke patients through apoptosis. J Cereb Blood Flow Metab. 2009;29:157–165.
- Liao LY, Lau BW, Sanchez-Vidana DI, et al. Exogenous neural stem cell transplantation for cerebral ischemia. Neural Regen Res. 2019;14:1129–1137.
- Yin X, Yang T, Gong Y, et al. Determinants of emergency medical services utilization among acute ischemic stroke patients in Hubei Province in China. Stroke. 2016;47:891–894.
- Yu G, Liang Y, Huang Z, et al. Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J Neuroinflammation. 2016;13:119.
- Chen H, Yoshioka H, Kim GS, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517.
- Xiao J, Kong R, Hu J. Inhibition of microRNA-429 attenuates oxygen-glucose deprivation/reoxygenation-induced neuronal injury by promoting expression of GATA-binding protein 4. Neuroreport. 2018;29:723–730.
- Morioka T, Kalehua AN, Streit WJ. Progressive expression of immunomolecules on microglial cells in rat dorsal hippocampus following transient forebrain ischemia. Acta Neuropathol. 1992;83:149–157.
- Stoll G. Inflammatory cytokines in the nervous system: multifunctional mediators in autoimmunity and cerebral ischemia. Rev Neurol (Paris). 2002;158:887–891.
- Hua F, Tang H, Wang J, et al. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab. 2015;35:536–542.
- Caso JR, Pradillo JM, Hurtado O, et al. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608.
- Lehnardt S, Lehmann S, Kaul D, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33.
- Brea D, Blanco M, Ramos-Cabrer P, et al. Toll-like receptors 2 and 4 in ischemic stroke: outcome and therapeutic values. J Cereb Blood Flow Metab. 2011;31:1424–1431.
- Brea D, Sobrino T, Rodriguez-Yanez M, et al. Toll-like receptors 7 and 8 expression is associated with poor outcome and greater inflammatory response in acute ischemic stroke. Clin Immunol. 2011;139:193–198.
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379.
- Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017;1509:1–10.
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222.
- Mirzaei H, Momeni F, Saadatpour L, et al. MicroRNA: relevance to stroke diagnosis, prognosis, and therapy. J Cell Physiol. 2018;233:856–865.
- Jolana L, Kamil D. The role of microRNA in ischemic and hemorrhagic stroke. Curr Drug Deliv. 2017;14:816–831.
- Di Y, Lei Y, Yu F, et al. MicroRNAs expression and function in cerebral ischemia reperfusion injury. J Mol Neurosci. 2014;53:242–250.
- Ou J, Kou L, Liang L, et al. MiR-375 attenuates injury of cerebral ischemia/reperfusion via targetting Ctgf. Biosci Rep. 2017;37. DOI:10.1042/BSR20171242
- Huang R, Ma J, Niu B, et al. MiR-34b protects against focal cerebral ischemia-reperfusion (I/R) injury in rat by targeting Keap1. J Stroke Cerebrovasc Dis. 2019;28:1–9.
- Zhang L, Luo X, Chen F, et al. LncRNA SNHG1 regulates cerebrovascular pathologies as a competing endogenous RNA through HIF-1alpha/VEGF signaling in ischemic stroke. J Cell Biochem. 2018;119:5460–5472.
- Huang XP, Ding H, Yang XQ, et al. Synergism and mechanism of astragaloside IV combined with ginsenoside Rg1 against autophagic injury of PC12 cells induced by oxygen glucose deprivation/reoxygenation. Biomed Pharmacother. 2017;89:124–134.
- Li C, Liu Y, Tang P, et al. Hydrogen sulfide prevents OGD/R-induced apoptosis by suppressing the phosphorylation of p38 and secretion of IL-6 in PC12 cells. Neuroreport. 2016;27:230–234.
- Zhong R, Chen Q, Zhang X, et al. L-3-n-butylphthalide soft capsules in the treatment of Parkinson disease dementia: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98:e16082.
- Huang W, Liu X, Cao J, et al. miR-134 regulates ischemia/reperfusion injury-induced neuronal cell death by regulating CREB signaling. J Mol Neurosci. 2015;55:821–829.
- Arumugam TV, Okun E, Tang S-C, et al. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32(1):4–16.
- Cao CX, Yang QW, Lv FL, et al. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353:509–514.
- Sun W, Ding Z, Xu S, et al. Crosstalk between TLR2 and Sphk1 in microglia in the cerebral ischemia/reperfusion-induced inflammatory response. Int J Mol Med. 2017;40:1750–1758.
- Lee J, Chuang TH, Redecke V, et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–6651.
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825.
- Virtue A, Wang H, Yang XF. MicroRNAs and toll-like receptor/interleukin-1 receptor signaling. J Hematol Oncol. 2012;5:66.
- He X, Jing Z, Cheng G. MicroRNAs: new regulators of Toll-like receptor signalling pathways. Biomed Res Int. 2014;2014:945169.
- Li Y, Shi X. MicroRNAs in the regulation of TLR and RIG-I pathways. Cell Mol Immunol. 2013;10:65–71.
- Lu C, Peng K, Guo H, et al. miR-18a-5p promotes cell invasion and migration of osteosarcoma by directly targeting IRF2. Oncol Lett. 2018;16:3150–3156.
- Zhou L, Li Z, Pan X, et al. Identification of miR-18a-5p as an oncogene and prognostic biomarker in RCC. Am J Transl Res. 2018;10:1874–1886.
- Wang H, Wei X, Wu B, et al. Tumor-educated platelet miR-34c-3p and miR-18a-5p as potential liquid biopsy biomarkers for nasopharyngeal carcinoma diagnosis. Cancer Manag Res. 2019;11:3351–3360.
- Ferreira R, Santos T, Amar A, et al. Argonaute-2 promotes miR-18a entry in human brain endothelial cells. J Am Heart Assoc. 2014;3:e000968.
- Han F, Wu Y, Jiang W. MicroRNA-18a decreases choroidal endothelial cell proliferation and migration by inhibiting HIF1A expression. Med Sci Monit. 2015;21:1642–1647.
- Zhang X, Du Q, Yang Y, et al. Salidroside alleviates ischemic brain injury in mice with ischemic stroke through regulating BDNK mediated PI3K/Akt pathway. Biochem Pharmacol. 2018;156:99–108.
- Yu J, Li C, Ding Q, et al. Netrin-1 ameliorates blood-brain barrier impairment secondary to ischemic stroke via the activation of PI3K pathway. Front Neurosci. 2017;11:700.
- Hou Y, Wang K, Wan W, et al. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018;5:245–255.
- Lu Y, Chu C, Liang-Fen W, et al. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. Plos One. 2013;8:e55839.
- Hua F, Ma J, Ha T, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–108.
- Shi CX, Ding YB, Jin FYJ, et al. Effects of sevoflurane post-conditioning in cerebral ischemia-reperfusion injury via TLR4/NF-κB pathway in rats. Eur Rev Med Pharmacol Sci. 2018;22:1770–1775.
- Liu J, Chen Q, Jian Z, et al. Daphnetin protects against cerebral ischemia/reperfusion injury in mice via inhibition of TLR4/NF-kappaB signaling pathway. Biomed Res Int. 2016;2016:2816056.
- Zhang FB, Wang JP, Zhang HX, et al. Effect of beta-patchoulene on cerebral ischemia-reperfusion injury and the TLR4/NF-kappaB signaling pathway. Exp Ther Med. 2019;17:3335–3342.
- Wang X, An F, Wang S, et al. Orientin attenuates cerebral ischemia/reperfusion injury in rat model through the AQP-4 and TLR4/NF-κB/TNF-α signaling pathway. J Stroke Cerebrovasc Dis. 2017;26(10):2199–2214.
