 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The anticancer effects of curcumin are based on the induction of apoptosis, but the specific mechanisms have not yet been fully elucidated. To address this issue, we investigated the effects of curcumin on the intrinsic apoptosis pathway using mitochondria from A549 cells. Curcumin decreased the levels of 14-3-3 proteins, key molecules that inhibit the activation of proapoptotic factors known as BH3-only proteins (e.g. Bad). Curcumin-induced suppression of 14-3-3 protein levels was associated with reduced cytosolic Bad and elevation of mitochondrial Bad, leading to a drop in the mitochondrial membrane potential. 14-3-3 proteins generally interact with Bad phosphorylated by AKT, thus preventing its translocation to the mitochondria where it can promote cell death. Curcumin not only decreased the expression of 14-3-3 proteins but also promoted Bad dephosphorylation in an AKT-dependent fashion. Our results provide novel evidence for the induction of apoptosis by curcumin at multiple stages of the mitochondrial cascade.
Graphical abstract
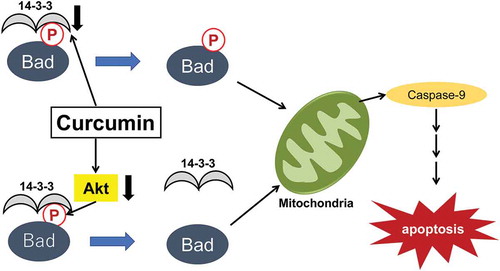
Curcumin induces apoptosis in lung cancer cells at multiple stages of the mitochondrial cascade.
KEYWORDS:
One of the main causes of cancer is an imbalance between cell proliferation and cell death. When cells avert death because of the absence of apoptotic signals, uncontrolled cell proliferation can occur, leading to various types of cancer [Citation1,Citation2]. Cancer cells often express several proteins that, when it is abnormally elevated, render the tumor cells resistant to apoptosis [Citation3]. 14-3-3 proteins, members of a highly conserved and ubiquitously expressed protein family involved in a wide range of cellular processes, including apoptosis, are often overexpressed in cancer cells, and the selective survival advantage that they confer may contribute to the process of tumor formation [Citation4–7]. Indeed, the expression of 14-3-3 proteins in certain cancer types has been correlated with poor prognosis and resistance to chemotherapy [Citation8–10]. Thus, reducing the levels of 14-3-3 proteins in cancer cells may be an effective means of preventing the progression of tumors.
We found that the downregulation of 14-3-3 proteins by curcumin in vitro inhibits proliferation and induces apoptosis in A549 cells. Curcumin, a naturally occurring polyphenol extract from the rhizome Curcuma longa, has been extensively studied for its anticancer properties associated with the inhibition of cell proliferation, cell cycle progression, angiogenesis, and the activation of apoptosis [Citation11]. Curcumin has been used as an adjuvant in chemotherapeutic treatments of cancer because of its low toxicity and limited side effects compared to cytotoxic drugs [Citation12,Citation13]. Accumulated data have shown a remarkable ability of curcumin and its derivatives to induce apoptosis in cancer cells through multiple target molecules and associated signaling pathways, such as transcription factors (NF-KB, AP-1, PPAR-γ, and p53), phosphatidyl-inositol 3-kinase/protein kinase B (PI3K/AKT), JNK, ROS, Bcl-2 family members, proteolytic enzymes, and death receptors [Citation11,Citation13–18]. The transduction of these curcumin-induced apoptotic signals eventually results in mitochondrial outer membrane permeability, which is a key step toward intrinsic apoptosis [Citation18,Citation19].
The intrinsic mitochondria-mediated apoptosis pathway is regulated by Bcl-2 family members, including antiapoptotic (Bcl-2, Bcl-XL) and proapoptotic (Bax, Bak, Bad, Bid, and Bim) molecules. Although several lines of evidence have indicated that curcumin modulates the levels of Bcl-2 and Bax in human cancer cell lines, leading to the disruption of the mitochondrial membrane and consequently leading to apoptosis, it is not clear how curcumin transmits apoptotic signals to the Bcl-2 family members [Citation12]. Targeting 14-3-3 proteins, which are critical regulators of Bcl-2 family members, and thereby disposing cancer cells toward apoptosis have been described previously in the literature [Citation20,Citation21]. The highly conserved family of 14-3-3 proteins consists of seven isoforms (β, ε, η, γ, σ, ζ, and τ/θ) and has been shown to exert antiapoptotic effects by interacting with various proapoptotic proteins, such as Bax, Bad, Bid, p53, and Ask-1 [Citation22–26]. In general, they recognize and interact with phosphoserine/threonine residues within a conserved motif on their target proteins [Citation27,Citation28]. Because of the phosphorylation-dependent nature of 14-3-3 interactions, they are tightly integrated into a phospho-relay signaling network consisting of PI3K/AKT and downstream molecules belonging to the Bcl-2 family [Citation29–31]. Bad (Bcl-2-associated death promoter), a proapoptotic molecule, is normally phosphorylated by survival-promoting kinases, such as AKT and PKA, and is sequestered in an inactive form in the cytosol through interaction with 14-3-3 proteins. In this process, 14-3-3 proteins also facilitate the access of such kinases to Bad and act as cofactors that participate in sequential protein phosphorylation events. In response to apoptotic stimuli, endogenous Bad is dephosphorylated, disrupting the association with 14-3-3 proteins and causing its translocation to the outer mitochondrial membrane where it promotes cell death [Citation25,Citation32]. Thus, understanding the role of 14-3-3 proteins in Bad-mediated apoptosis through the PI3K/AKT signaling pathway, in particular, may help reveal the mechanisms of curcumin-induced apoptosis in cancer cells.
In this study, we evaluated the effects of curcumin on the proliferation of A549 cells derived from lung adenocarcinoma and the mechanisms by which curcumin regulates cell proliferation. The data showed that curcumin inhibited the proliferation and induced the apoptosis of these cells. We also showed the downregulation of several 14-3-3 isoforms in response to the treatment of A549 cells with curcumin. The downregulation of 14-3-3 proteins by curcumin was associated not only with the decreased phosphorylation of Bad at Ser-136 and Bad translocation to the mitochondria, but also with the inactivation of AKT. These observations suggest that curcumin promotes apoptotic signaling at multiple steps of the mitochondrial apoptosis pathway, potentially contributing to the ability of curcumin to inhibit A549 cell proliferation. Our study highlights the importance of PI3K/AKT/Bad signaling for curcumin-induced apoptosis and demonstrates a novel mechanism for how 14-3-3 proteins play a critical role in the negative regulation of this apoptotic network in cancer cells.
Materials and methods
Materials
Rabbit polyclonal antibody against total 14-3-3 proteins (K-19) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Isoform-specific antibodies against 14-3-3 β, γ, ε, η, ξ, θ and σ were from IBL (Gunma, Japan). Rabbit monoclonal antibody (Mab) anti-Bad and rabbit polyclonal antibody anti-phospho-Bad (Ser-136) were purchased from Abcam (Cambridge, UK). Rabbit anti-caspase-9, anti-active-caspase-9, anti-pro-caspase-3, and anti-PARP (active-PARP) antibodies were obtained from Cell Signaling Technology Inc. (Danvers, MA, USA). Curcumin and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and viability assay
The A549 lung cancer cell line was obtained from NIBIOHN (Osaka, Japan) and cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum at 37°C. Curcumin was dissolved in dimethyl sulfoxide and added to the medium at the indicated concentrations. The activity of mitochondrial dehydrogenase 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure cell death/survival. The reaction product was measured by spectrophotometry at A570, and the relative viability of cells treated with curcumin versus untreated cells was calculated.
Immunoblotting and cell fractionation
A549 cells were lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM sodium orthovanadate, 1 mM EDTA, 0.1% NP-40, and 10 mM NaF) containing the Calbiochem Protease Inhibitor Cocktail Set III (Merck KGaA, Darmstadt, Germany). The cell lysates were resolved in Laemmli sample buffer. The samples then underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to a polyvinylidene difluoride membrane, reacted with the respective antibodies, and detected with an ECL chemiluminescence detection kit (GE Healthcare, Fairfield, CT, USA). The mitochondrial fraction was prepared as described previously [Citation7,Citation25]. A549 cells lysed in 20 mM HEPES-KOH (pH 7.5) buffer containing 0.25 M sucrose were homogenized in a Dounce homogenizer and centrifuged at 1,000 ×g to separate nuclei and unbroken cells. The supernatants were then centrifuged at 10,000 ×g for 15 min, and the pellets were collected as the heavy membrane/mitochondrial fraction.
Quantification of apoptosis by flow cytometry
A549 cells were washed with Annexin V staining buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2) and incubated with CF488A-Annexin V and propidium iodide (Biotium, Inc., Hayward, CA, USA) in a staining buffer for 30 min at 37°C in the dark. Fluorescence was measured using a FACSCalibur (BD Biosciences, San Jose, CA, USA), and the data were analyzed using CellQuest software (BD Biosciences).
JC-1 staining and quantification
Mitochondrial permeability transition was determined by staining the cells with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) in the dark. Flow cytometry analysis was performed with the JC-1 Mitochondria Membrane Potential Kit (AAT Bioquest, Sunnyvale, CA, USA) according to the manufacturer’s instructions, using a FACSCalibur, and the results were analyzed using CellQuest software.
Statistical analyzes
The value was expressed as the mean ± standard deviation (S.D.) for three independent experiments. Two groups were compared using the Student’s t- test. The p-value less than 0.05 were considered as statistically significant. Statistical significance was also taken as *P < 0.05 and **P < 0.01.
Results
Curcumin inhibits proliferation and induces apoptosis in A549 cells
The survival of A549 cells treated with curcumin was measured using the MTT assay. After treatment with curcumin, the viability of the cells decreased in a concentration-dependent manner ()). In order to investigate whether the inhibitory effects of curcumin on A549 cell viability were induced via apoptotic cell death, we quantified the number of apoptotic cells in curcumin-treated cultures with CF488A-Annexin V(AV) using flow cytometry. AV-PI staining indicated that treatment with 50–100 μM curcumin markedly increased early and late apoptosis (), see also Supplementary Figure 1a). Several studies have shown that mitochondria might be a direct and important target of curcumin in sensitive cells [Citation18,Citation19]. We, therefore, studied the effects of curcumin on the depolarization of mitochondrial membranes. A549 cells were incubated in the presence or absence of curcumin and stained with JC-1, and curcumin treatment was found to lower the mitochondrial membrane potential (), see also Supplementary Figure 1b). These findings indicated that curcumin treatment led to mitochondria-dependent apoptosis in A549 cells.
Figure 1. Curcumin inhibits proliferation and induces apoptosis in A549 cells. (a) The viability of A549 cells treated with curcumin for 48 h was analyzed by the MTT assay. The value is represented as the percentage of cell viability without curcumin treatment, which was set at 100%. (b) Apoptotic cell death was measured by flow cytometry analysis with fluorescein-isothiocyanate-conjugated Annexin V/propidium iodide staining at 48 h to observe curcumin-induced apoptosis in A549 cells. Early and/or late apoptotic cells are represented by quantitative analysis. Columns show the mean values of three experiments (mean of total apoptotic cells ± standard deviation [SD]). . (c) A549 cells were treated for 48 h with various concentrations of curcumin and subjected to JC-1 staining to study changes in the mitochondrial membrane potential. The percentages indicate the fluorescence intensity of JC-1 measured with FACSCalibur. The data represent mean values of three separate experiments. Results are shown as means ± SD, **
(by Student’s t-test)
![Figure 1. Curcumin inhibits proliferation and induces apoptosis in A549 cells. (a) The viability of A549 cells treated with curcumin for 48 h was analyzed by the MTT assay. The value is represented as the percentage of cell viability without curcumin treatment, which was set at 100%. (b) Apoptotic cell death was measured by flow cytometry analysis with fluorescein-isothiocyanate-conjugated Annexin V/propidium iodide staining at 48 h to observe curcumin-induced apoptosis in A549 cells. Early and/or late apoptotic cells are represented by quantitative analysis. Columns show the mean values of three experiments (mean of total apoptotic cells ± standard deviation [SD]). P<0.01. (c) A549 cells were treated for 48 h with various concentrations of curcumin and subjected to JC-1 staining to study changes in the mitochondrial membrane potential. The percentages indicate the fluorescence intensity of JC-1 measured with FACSCalibur. The data represent mean values of three separate experiments. Results are shown as means ± SD, **P<0.01 (by Student’s t-test)](/cms/asset/04722515-893f-4396-aeeb-807842aa907c/tbbb_a_1808443_f0001_b.gif)
Involvement of 14-3-3 proteins and Bad in the apoptosis of A549 cells induced by curcumin treatment
Previous studies demonstrated not only that 14-3-3 proteins are cytoprotective [Citation7,Citation33], but also that they interfere with cell death induced by a wide variety of stimuli [Citation22–26]. Based on the hypothesis that a higher level of 14-3-3 proteins protects against cell death and increases the survival of cancer cells, we examined the effects of curcumin on 14-3-3 protein family members. The expression levels of 14-3-3 proteins in A549 cells after treatment with various concentrations of curcumin were analyzed by immunoblotting. Curcumin significantly decreased the expression levels of total 14-3-3 proteins in A549 cells, specifically the β, γ, and η isoforms, in a dose-dependent manner ()), though the expression of 14-3-3 ζ, τ, σ, and ε did not change (data not shown). In addition, 14-3-3 proteins have been implicated in apoptotic signaling through the negative regulation of Bad activity. They generally interact with and sequester phosphorylated Bad, preventing its translocation to the mitochondria and, thereby, inhibiting cell death [Citation25,Citation32]. Because curcumin suppresses 14-3-3 protein expression in A549 cells, it is proposed that curcumin reduces cytosolic Bad sequestration and reciprocally enhances Bad localization to the mitochondria. To confirm this, we prepared mitochondrial fractions from A549 cells incubated with or without curcumin, and immunoblotted for Bad in these fractions. The results showed that, in the presence of curcumin, Bad was localized to the mitochondria in a dose-dependent manner ()). These results suggested that curcumin induces Bad translocation to the mitochondria as a consequence of 14-3-3 protein family suppression. On the other hand, apoptotic signal transmission to the mitochondria leads to the activation of caspase-9, which then converts procaspase-3 into active caspase-3, resulting in PARP cleavage and apoptosis. In order to examine whether curcumin influences downstream mitochondrial-related apoptotic events, we measured the activation of caspase-9 and cleavage of PARP in A549 cells treated with curcumin. As shown in ), fully processed caspase-9 was predominantly identified with an anti-active caspase-9 antibody in curcumin-treated A549 cells. Curcumin also induced the cleavage of pro-caspae-3 and poly(ADP-ribose) polymerase (PARP) in a concentration-dependent manner ()).
Figure 2. Involvement of 14-3-3 proteins and Bad in the apoptosis of A549 cells induced by curcumin. (a) Downregulation of the expression of a 14-3-3 protein by curcumin. Extracts prepared from A549 cells treated with curcumin at the specified concentrations for 48 h were separated by SDS-PAGE and immunoblotted with antibodies recognizing each specific isoform of 14-3-3. Pan indicates the anti-14-3-3 antibody that recognizes all isoforms of 14-3-3 proteins. The quantity of each protein was estimated by densitometric analysis using ImageJ. The 14-3-3/β-actin ratios are shown in the right panel. The results shown as means±S.D. The data represent mean values of three separate experiments. Significances were determined by Student’s t-test (*P < 0.05; **P < 0.01). (b) A549 cells, treated as described earlier, were lysed and fractionated by differential centrifugation to separate the mitochondria from the cytosol. The translocation of Bad to the mitochondria was visualized by immunoblotting of the mitochondrial fraction using an anti-Bad antibody. Grp75 was used as a loading control to ensure the use of equal amounts of mitochondria in each lane. The quantity of Bad was estimated by densitometric analysis. The results shown as means±S.D. Statistically significant differences with P < 0.05 were considered significant (*P < 0.05; **P < 0.01). (c) A549 cells were treated with curcumin, and cell extracts were immunoblotted with antibodies recognizing procaspase-9, native PARP, cleaved caspase-9, and cleaved PARP. The results shown are representative of three separate experiments

Curcumin inhibits the phosphorylation of Bad through a PI3K/AKT-dependent mechanism
Survival factors, acting through antiapoptotic kinases, can induce endogenous Bad phosphorylation at two evolutionarily conserved sites, Ser-112 and Ser-136, which inhibits Bad-dependent apoptosis [Citation31]. Phosphorylation at both sites is critical for inactivating and sequestering Bad via its interaction with 14-3-3 proteins [Citation27,Citation28]. In order to further examine Bad phosphorylation in cells undergoing apoptosis induced by curcumin, the phosphorylation state of Bad after exposure of A549 cells to various concentrations of curcumin was assayed by immunoblot analysis with an antibody that specifically recognizes phosphorylation at Ser-136. We observed a prominent decrease in Bad phosphorylation (at serine-136) after treatment with curcumin ()). It was previously reported that Bad phosphorylation at Ser-136 is promoted by the PI3K/AKT pathway. Furthermore, previous studies have shown that 14-3-3 proteins act as molecular regulators and potential anticancer agents in PI3K/AKT-activated cancer by binding to and inhibiting AKT [Citation29,Citation34–36]. In order to examine whether curcumin suppresses PI3K/AKT signaling by regulating the protein phosphorylation levels, we assessed the phosphorylation status of AKT. As shown in ), curcumin reduced the expression of AKT in A549 cells, which occurred in concert with Bad dephosphorylation. On the other hand, suppressing AKT activation by the addition of a PI3K inhibitor, LY294002, decreased the viability of A549 cells to the level of curcumin treatment ( and )). These observations confirmed that curcumin induces apoptosis through Bad dephosphorylation and that its mechanism of action is mediated by the inhibition of PI3K/AKT signaling.
Figure 3. Curcumin induces the dephosphorylation of Bad through the inhibition of PI3K/AKT signaling. (a) A549 cells were treated with the indicated concentration of curcumin for 48 h. The degrees of phosphorylated Bad (Ser-136) and total Bad were assessed by immunoblotting using the indicated antibodies. The phosphorylation status of Bad was quantified by densitometric analysis using ImageJ. The results are shown as means±S.D. from triplicated experiments. Statistical significance was counted for three independent experiments with Student t-test (*P < 0.05; **P < 0.01). (b) Effects of curcumin on the expression of AKT and its phosphorylation at Ser-473 in A549 cells. A549 cells were exposed to curcumin for 48 h. The degrees of phosphorylated AKT and total AKT were visualized by immunoblotting with an anti-phospho-AKT (Ser-473) or AKT antibody. Data are representative of three separate experiments. The relative intensities of each band were compared with densitometric analysis. The data represent mean values of three separate experiments. The results are shown as means ± SD, ** (by Student’s t-test). (c) Effect of a PI3K inhibitor (LY294002) on AKT phosphorylation (left panel) and cell viability (right panel) in A549 cells. Cells were incubated with the indicated concentration of LY294002 for 48 h, and cell extracts were immunoblotted with each specific antibody. The value is represented as the percentage of cell viability without LY294002 treatment, which was set at 100%. Results are shown as means ± SD, **
(by Student’s t-test). The quantities of phosphorylated and total AKT were estimated using densitometric analysis. The data are representative of three separate experiments and represent mean values of three separate experiments

Discussion
Curcumin has been used in traditional Chinese and Indian medicine for centuries to treat a variety of illnesses. The beneficial properties of curcumin include its antioxidant, anti-inflammatory, antimicrobial, and antiproliferative activities against cancer cells [Citation37]. Recently, more attention has been paid to the anticancer effects of curcumin. Curcumin has been found to inhibit carcinogenesis in preclinical experiments using various cancer cell lines, including breast, lung, pancreatic, gastric, colon, hepatic, and prostate cancer cells [Citation11]. The most attractive feature of curcumin is that it is toxic to cancer cells, but not to normal cells, as compared with the currently available cytotoxic drugs [Citation12,Citation13,Citation38]. One widely known anticancer effect of curcumin on cancer cells is the activation of the apoptosis pathway [Citation11]. Although the induction of apoptosis by curcumin is well established, the detailed mechanisms and pathways by which this occurs remain unknown. In the present study, we showed that curcumin significantly decreased the expression of 14-3-3 proteins in lung adenocarcinoma cell lines and induced the apoptosis of A549 cells by increasing the dephosphorylation at Ser-136 of the proapoptotic molecule Bad and, thus, promoting its translocation to the mitochondria. We also demonstrated that the inactivation of PI3K/AKT signaling upstream of Bad is a critical event in apoptosis induced by curcumin.
To the best of our knowledge, this is the first study demonstrating the involvement of Bad, as well as a protective role for 14-3-3 proteins, in curcumin-induced cell death. This is an important observation because targeting the expression or function of a 14-3-3 protein has been suggested as a potential treatment strategy for several types of cancer, on the basis of the hypothesis that a higher level of 14-3-3 proteins protects against cell death and increases survival when cells are faced with apoptotic stimuli [Citation8–10]. Indeed, previous research has identified a 14-3-3 protein as a putative oncogene whose activation was common and driven by its genomic amplification in lung adenocarcinoma, and it further indicated that increased 14-3-3 expression was positively associated with advanced pathologic stage and grade of lung adenocarcinoma and related to poor outcomes of patients [Citation8]. These notions offer an attractive opportunity for therapeutic approaches that inhibit 14-3-3 proteins. The inhibitory effects of curcumin on 14-3-3 proteins may be supported by previous reports that curcumin decreased the expression of 14-3-3 proteins in human cancer cell lines, [Citation39,Citation40] whereas recent study has indicated that the expression of 14-3-3 in primary cardiomyocytes was up-regulated after the treatment with curcumin [Citation41]. Some of this confusion may be due to different experimental systems, or reflects the variability of apoptotic pathways among different cell lines. The natural compounds that decrease the expression of 14-3-3 proteins, such as curcumin, may represent a promising new class of cancer chemotherapeutic agents with broad application.
The present study showed that seven 14-3-3 isoforms are differentially expressed after exposure of A549 cells to curcumin. Among the seven isoforms examined, curcumin selectively decreased the expression of 14-3-3 β, γ, and η, indicating that different isoforms are regulated differently and may serve distinct functions. It has been demonstrated that the key regulators of apoptosis include the antiapoptotic Bcl-2 proteins, which control the activation of proapoptotic molecules and, thereby, affect mitochondria’s outer membrane permeability [Citation42]. The balance between antiapoptotic and proapoptotic Bcl-2 family members determines whether a cell lives or dies. In fact, elevated expression of death-inhibitory members (Bcl-2, Bcl-xL) and/or reduced expression of death-inducing members (Bax) have been reported in various cancer types and malignancies [Citation11]. Among these, proapoptotic Bax plays an important role in both inhibiting cancer progression and promoting the apoptosis of some types of cancer. Interestingly, earlier reports have shown that curcumin increases Bax expression and decreases Bcl-2 expression in cancer cells [Citation43,Citation44], although the specific mechanisms modulating the ratio of Bax and Bcl-2 remain unclear. In this study, we observed similar suppression of Bcl-2 expression and upregulation and activation of Bax in A549 cells treated with curcumin (data not shown). On the other hand, less is known about the role of another death-inducing Bcl-2 family member in curcumin-induced apoptosis. It is noteworthy that curcumin inhibits PI3K/AKT signaling by downregulating the expression of AKT, which might be linked to the activation of a downstream apoptosis-related protein, Bad, in cancer cells. Although the fundamental mechanism of curcumin-mediated downregulation of AKT remains unclear, a recent report has shown that SNAIL, which acts as a transcription factor, binds to the AKT promotor and directly regulates the expression of AKT [Citation45]. Further studies on the actions of curcumin toward AKT are currently in progress and likely to clarify it. In the absence of apoptotic stimuli, survival factors, acting through kinases such as AKT and PKA, induce endogenous Bad phosphorylation at two evolutionarily conserved sites, which blocks the translocation of Bad to the mitochondria and inhibits Bad-dependent cell death [Citation32].
The present study revealed that curcumin induces mitochondria-dependent apoptosis through the dephosphorylation of Bad resulting from the inhibition of PI3K/AKT signaling. Furthermore, given that 14-3-3 proteins actively protect Bad against dephosphorylation and associate with phosphorylated Bad to sequestrate it in the cytosol, the suppression of 14-3-3 proteins by curcumin accelerates the apoptosis of A549 cells. Our experiments also provided the first evidence that 14-3-3 proteins are critical for determining the cellular decision to either initiate or suppress apoptosis mediated by curcumin. These findings may contribute toward the development of new and less toxic chemotherapies that use natural compounds against cancer, and they also highlight the putative merits of developing anticancer treatments targeting 14-3-3 proteins.
Author contributions
MY and HE designed the research and experiment, and supervised the experimental analysis. II and KM carried out the experiments. II performed cell viability assay and manufactured the samples and characterized them by flow cytometry. KM performed almost all of other experiments. HE encouraged II and KM to investigate and supervised the findings of this work. MT contributed to the design and implementation of the research. MY wrote the paper with input from all authors. All authors discussed the results. All authors read and approved the manuscript.
Final_draft_BBB_suplimental_figures__H._Endo_et_al.pdf
Download PDF (146.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
- Nakano K, Vousden KH. Puma, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–689.
- Igney FH, Krammer PH. Death and anti-death: tumor resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–288.
- Fu H, Xia K, Pallas DC, et al. Interaction of the protein kinase Raf-1 with 14-3-3 proteins. Science. 1994;266:126–129.
- Reuther GW, Fu H, Crip LD, et al. Association of the protein kinase-c-Bcr and Bcr-Abl with proteins of the 14-3-3 family. Science. 1994;266:129–133.
- Yaffe MB. How to 14-3-3 protein work?–Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002;513: 53–57.
- Yano M, Nakamuta S, Wu X, et al. A novel function of 14-3-3 protein: 14-3-3 is a heat shock-related molecular chaperone that dissolves thermal-aggregated proteins. Mol Biol Cell. 2006;17:4769–4779.
- Fan T, Li R, Todd NW, et al. Up-regulation of 14-3-3 in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007;67(16):7901–7906.
- Neal CL, Yao J, Yang W, et al. 14-3-3 overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69(8):3425–3432.
- Liu TA, Jan YJ, Ko BS, et al. Increased expression of 14-3-3β promotes tumor progression and predicts extrahepatic metastasis and worse survival in hepatocellular carcinoma. Am J Pathol. 2011;179(6):2698–2708.
- Ismail NI, Othman I, Abas F, et al. Mechanisms of apoptosis induced by curcumin in colorectal cancer. Int J Sci. 2019;20:2454.
- Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? Aaps J. 2009;11:495–510.
- Yallapu MM, Nagesh PKB, Jaggi M, et al. Therapeutic application of curcumin nanoformulations. Aaps J. 2015;17:1341–1356.
- Bhaumik S, Anjum R, Rangaraj N, et al. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–314.
- Woo JH, Kim YH, Choi YJ, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-xL and IAP, the release of cytochrome c and inhibition of AKT. Carcinogenesis. 2003;24:1199–1208.
- Moussavi M, Assi K, Gomez-Munoz A, et al. Curcumin mediates ceramide generation via the de novo pathway in colon cancer cells. Carcinogenesis. 2006;27:1636–1644.
- Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cell via AP-1 and NF- B transcription factors. J Neurochem. 2007;102:522–538.
- Shakibaei M, Mobasheri A, Leuders C, et al. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF- B and src protein kinase signaling pathways. Plos ONE. 2013;8:e57218.
- Shankar S, Srivastava RK. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcumma longa. Carcinogenesis. 2007;28:1277–1286.
- Li YL, Liu L, Xiao Y, et al. 14-3-3 an independent prognostic biomarker for gastric cancer and is associated with apoptosis and proliferation in gastric cancer. Oncol Lett. 2015;9:290–294.
- Hashemi M, Zail A, Hashemi J, et al. Down-regulation of 14-3-3 zeta sensitizes human glioblastoma cells to apoptosis induction. Apoptosis. 2018;23:616–625.
- Waterman MJ, Stavridi ES, Waterman JL, et al. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet. 1998;19:175–178.
- Zhang L, Chen J, Fu H. Suppression of apoptosis signaling-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc Natl Acad Sci USA. 1999a;96: 8511–8515.
- Nomura M, Shimizu S, Sugiyama T, et al. 14-3-3 interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem. 2003;278:2058–2065.
- Yano M, Nakamuta S, Shiota M, et al. Gatekeeper role of 14-3-3 protein in HIV-1 gp120-mediated apoptosis of human endothelial cells by inactivation of Bad. AIDS. 2007;21:911–920.
- Li Z, Zhao J, Du Y, et al. Down-regulation of 14-3-3 suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci USA. 2008;105:162–167.
- Muslin AJ, Tanner JW, Allen PM, et al. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897.
- Yaffe MB, Rittinger K, Volinia S, et al. The structural basis for 14-3-3: phosphopeptidebinding specificity. Cell. 1997;91:961–971.
- Bonnefoy-Berard N, Liu YC, von Willebrand M, et al. Inhibition of phosphatidylinositol 3-kinase activity by association with 14-3-3 proteins in T cells. Proc Natl Acad Sci USA. 1995;92:10142–10146.
- Tzivion G, Shen YH, Zhu J. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene. 2001;20:6331–6338.
- Ohi N, Nishikawa Y, Tokairin T, et al. Maintenance of Bad phoshorylation prevents apoptosis of rat hepatic sinusoidal endothelial cells in Vitro and in Vivo. Am J Pathol. 2006;168:1097–1106.
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927.
- Nakamuta S, Endo H, Higashi Y, et al. Human immunodeficiency virus type 1 gp-120mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and −2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195.
- Shao Z, Cai Y, Xu L, et al. Loss of the 14-3-3 is essential for LASP1-mediated colorectal cancer progression via activating PI3/AKT signaling pathway. Sci Rep. 2016;9(6):25631.
- Li J, Xu H, Wang Q, et al. 14-3-3 promotes glioma cells invasion by regulating snail through the PI3K/AKT signaling. Cancer Med. 2019;8:783–794.
- Vehlow A, Klapproth E, Jin S, et al. Interaction of discoidin domain receptor 1 with a 14-3-3-Beclin-1-Akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. 2019;6:3672–3683.
- Nagahama K, Utsumi T, Kumano T, et al. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci Rep. 2016;6:30962.
- Heger M, van Golen RF, Broekgaarden M, et al. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol Rev. 2014;66:222–307.
- Ting CY, Wang HE, Yu CC, et al. Curcumin trigers DNA damage and inhibits expression of DNA repair proteins in human lung cancer cells. Anticancer Res. 2015;35:3867–3873.
- Lu K, Rui G, Liu F, et al. 14-3-3 is a nuclear matrix protein, and its altered expression and localization are associated with curcumin-induced apoptosis of MG-63 cells. Oncol Lett. 2018;15:338–346.
- He H, Luo Y, Qiano Y, et al. Curcumin attenuates doxorubicin-induced cardiotoxicity via suppressing oxidative stress and preventing mitochondrial dysfunction mediated by 14-3-3. Food Funct. 2018;9(8):40404–44418.
- Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome C from mitochondria blocked. Science. 1997;275:1129–1132.
- Moragoda L, Jaszewski R, Majumdar APN. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001;21:873–878.
- Su CC, Lin JG, Li TM, et al. Curcumin-induced apoptosis of human colon cancer COLO-205 cells through the production of ROS, Ca2+ and the activation of caspase 3. Anticancer Res. 2006;26:4379–4389.
- Skrzypek K, Kot M, Konieczny P, et al. SNAIL promotes metastatic behavior of rhabdomyosarcoma by increasing EZRIN and AKT expression and regulating MicroRNA networks. Cancers (Basel). 2020;12:1870.
