ABSTRACT
Vermicompost tea (VCT) is the effluent or leachate with a honey-brown colour collected during vermicomposting, an ecologically significant process essential to today’s organic and regenerative agriculture. Dried earthworm, a.k.a. dilong (DL, meaning “earth dragon”) in traditional Chinese herbal medicine, has long been used as a key component to treat diverse skin diseases, including eczema. In the present study, we hypothesized that VCT might have a positive influence on eczema therapy. It was found that the oral application of 50% VCT in mouse model reduced the ear allergic scores and alleviated the histological changes caused by eczema. Furthermore, the levels of Th2-associated and pro-inflammatory cytokines (namely IL-4 and IL-13 in serum) and IgE (in serum and ear tissues) were significantly reduced by VCT. Therefore, oral administration of 50% VCT exerts immunomodulatory effects on the development of eczema, suggesting its potential as a nutraceutical candidate for eczema treatment.
GRAPHICAL ABSTRACT
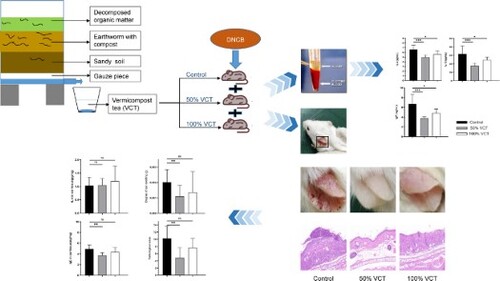
1. Introduction
Eczema, a cutaneous immune defect particularly prevalent in infancy, is a common, persisting, and currently incurable skin condition that causes flares of dry, itchy, and inflamed patches of varying severity (Camfferman et al., Citation2010; Langan et al., Citation2009; Lax et al., Citation2022). Atopic eczema in children is found to significantly impact sleep, behaviour, and multiple comorbid conditions (Silverberg & Simpson, Citation2013). With more than 90% of affected young children (less than 5 years old) requiring medical review (Hanifin et al., Citation2004), eczema is associated with high levels of healthcare utilization as well as psychological and educational interventions (Ersser et al., Citation2014). Although noninfectious, recurring eczema is estimated to impact 10-20% of children in industrialized countries (Beasley, Citation1998; Darsow et al., Citation2005; Kemp, Citation1999). A recent study encompassing clinical data of 193,912 adults across 17 countries identified that the overall prevalence of eczema is now 9.9% (Schmitt et al., Citation2011), a significant rise from the 2–5% reported by the World Allergy Organization (WAO) in 2003, indicating that eczema is a more severe global health burden than asthma (4.4%) (García-Marcos et al., Citation2022). Its treatment to date, unfortunately, has still been focused on applying emollients (moisturizers) combined with topical steroid cream such as corticosteroids to reduce inflammation and itching symptoms (Lax et al., Citation2022; Sutton et al., Citation2022; Wollenberg et al., Citation2022). However, fears around the use of topical steroids are very common among the parents of pediatric dermatology outpatients (Contento et al., Citation2021; Hon et al., Citation2006; Li et al., Citation2018). As such, there is a dire need to identify reliable and safe treatment options for atopic dermatitis.
In traditional Chinese medicine (TCM) it is believed that the pathogenesis of eczema is mostly caused by the accumulation of dampness and heat for a long time, and the dampness and heat infiltrate the skin (Aslam et al., Citation2018; Wan et al., Citation2016). Earthworm (Eisenia foetida), known as dilong (DL; direct translation: earth dragon) in TCM, is a mollusk with a juicy and soft body comprised of up to 70% protein content (Mihara et al., Citation1991). DL is considered a valuable medicinal ingredient and has long been prescribed as a key concoction ingredient for treatment of asthma and diuresis, and it is also suitable for strong fevers, convulsions, coughing due to lung heat, rheumatism-heat arthralgia, joint swelling and pain, and dysuria (Mihara et al., Citation1991). According to the Compendium of Materia Medica, it is the cold nature of DL that allows it to open the meridians, thereby relieving various heat diseases, facilitating urination, and curing joint diseases (Bilej et al., Citation2010; Cooper et al., Citation2004; Cooper & Hirabayashi, Citation2013). Moreover, DL concoctions have been historically used in TCM to treat eczema, mumps, suppurative otitis media, fixed erythema drug eruption, burns, and lower extremity ulcers (Balamurugan et al., Citation2007; Balamurugan et al., Citation2009). Traditionally, DL is made from harvested and dried earthworms. The process often involves inhumane procedures such as starvation, flooding or even electrification of soil to force earthworms out of their habitat. Given the vital role that earthworms play in the ecosystem, it is necessary to implement a method of little to no disruption to the earthworm’s natural function.
Vermicompost tea (VCT), a collection of aqueous extract of the excretory products and mucus secretion of earthworms during vermicompost (Gudeta et al., Citation2022), is rich in minerals, organic acids, and living microorganisms and their metabolites (Hargreaves et al., Citation2008; Ingham, Citation2005). Commonly used as a nutrient-rich fertilizer and a soil conditioner to enrich soil biodiversity by promoting beneficial microbial organisms (Ansari & Ismail, Citation2012; Ingham, Citation2005), vermicompost is derived from the accelerated biological degradation of organic wastes through interactions between earthworms and microorganisms (Sugino Souffront et al., Citation2022). While the quality of VCT depends on the original organic matter used for vermicomposting and its environmental conditions (Nayak et al., Citation2019; Prasanth Bendalam, Citation2020; Zarei et al., Citation2018). VCT has been shown to be an ecologically safe and cost-effective alternative to synthetic plant growth promoters (Nayak et al., Citation2019). VCT can also be used as a potent input for both soil health and insect, pest and disease management in organic farming (Sugino Souffront et al., Citation2022; Yatoo et al., Citation2021). However, to date, studies on VCT are limited to improving the level of agricultural production and protecting crops from pests and diseases but have not considered further uses.
In the present study, we aimed to investigate the effects of VCT on alleviating eczema using a 1-chloro-2, 4-dinitrobenzene (DNCB)-sensitized mouse model. The severity of eczema was evaluated by ear allergenic scores, degree of ear swelling, and pathological scores, whereas Th2 immune responses (IgE, IL-4, and IL-13) were employed to elucidate its immunomodulatory characteristics. The significance is threefold: (1) this is the first study to report the use of VCT as an alternative or complimentary approach to steroid-based treatment of eczema; (2) knowledge about the immunomodulatory effect of VCT could trigger interest within the scientific community to further explore a wholistic approach that involves analysing the impact of environmental factors on eczema and illnesses alike; and (3) VCT can be prepared without harming earthworms and serves as an effective and sustainable alternative to DL in TCM.
2. Material and methods
2.1. Animals
Healthy male KM mice, weighing 25–30 g, 3 weeks old, were purchased from Guangdong Animal Center (Guangzhou, China) and kept in the animal room of the Institute for Advanced Study, Shenzhen University, Shenzhen, China. All mice were kept under constant temperature (23 ± 2°C), humidity (50 ± 5%), and 12-h light-and-dark cycle conditions. The air cleanliness of the laminar flow rack was 10,000 and the noise was kept under 60 decibels. The mice were fed with a standard diet and water ad libitum. The feed composition was crude protein of 18% and crude fat of 4%. The cages and drinking bottles used to house the mice were changed twice a week. The research was conducted in accordance with ethical animal practices. All animals were allowed more than one week to acclimate to the facility prior to any experimentation or testing.
2.2. Preparation of VCT
Each vermicompost bin was prepared using commercially available worm bins (40 × 40 × 50 cm) (length × width × height) (Guoyou Eco-Environmental Technology Co., Hangzhou, China). The bottom layer was covered with multiple layers of gauze to filter water that penetrated from the upper layer. The second layer was filled with a thin layer of sandy soil to absorb excess moisture from the compost pit above, and the third layer contained organic soil and old compost inoculated with earthworms (Eisenia foetida, Yuanhui Earthworm Co., Shenzhen, China). Fresh vegetable leaves obtained from the school cafeteria were added to the top layer as food for the earthworms. The lids were then placed on top of the worm bins to prevent exposure to direct sunlight and retain moisture. The internal environment of the vermicompost bins was maintained at 24°C–25°C, pH 7.5, and humidity level 7–9. During vermicomposting, the contents were turned over every two weeks to enhance ventilation. VCT was collected at the completion of a 30-day vermicomposting process and filtered by 40 μm Falcon cell strainers (352340, Thermo Fisher Scientific Inc.). All samples were stored at 4°C prior to use. After adaptive feeding for one week, mice were randomly divided into three groups of 13 (Control, 50% VCT and 100% VCT), and their drinking water was replaced with the corresponding proportion of VCT.
2.3. Construction of pathological model of eczema
To induce eczema, DNCB was used according to the method published by Fujii and coworkers with slight modifications (Fujii et al., Citation2009). The mice were weighed before sensitization and their abdomens were shaved (ca. 3 cm2) with an electric shaver (FS600, Beyotime, Shenzhen, China). On day 0, 150 μL of 7% 1-chloro-2, 4-dinitrobenzene (237329-10G, Sigma-Aldrich, USA) in acetone (10967A3-1, Guangtry Reagent Technology Co., Guangdong, China) was applied to the shaved area.
Five days after sensitization, 30 μL of 1% DNCB solution was applied to the inner side of the left ear of each mouse using a pipette to induce eczema while 30 μL of acetone was applied to the right ear as the control. Subsequent applications of DNCB occurred every three days for up to a total of 5 times (). Each mouse was weighed at prescribed time intervals and any changes to the ears of the mice were observed and recorded. All mice were analysed for degrees of allergic reaction on the ears using three metrics: epidermal erythema, edema, and scratch grades (Saunes et al., Citation2011; Sung & Kim, Citation2018; Yamamoto et al., Citation2007), as shown in .
Figure 1. Time schedule for the eczema induction and vermicompost tea (VCT) treatment. Mice were treated with different VCT (0, 50%, and 100%) for 7 weeks for adaptation. After that, mice were treated with topical 7% DNCB on day 0, and then challenged with 1.0% DNCB on days 5, 8, 11, 14, and 17. Mice were sacrificed on day 18 and both ear tissues and blood were collected.

Table 1. The scoring criteria for mouse ear allergy (Sung & Kim, Citation2018; Yamamoto et al., Citation2007).
2.4. Degree of ear swelling and skin histopathological evaluation
To evaluate the swelling of ears in which eczema was induced by DNCB, skin samples were taken after 18 days of sensitization from the left and right ear of each mouse after euthanization by an overdose of isoflurane (Anfenghua Biotechnology Co., Shenzhen, China). Circular ear pieces were removed with a 4-mm diameter skin punch (25959, Zhencheng Technology Co., Shenzhen, China), and weighed by an analytical balance (Yoke FA2004B). The degree of ear swelling of each mouse was calculated by subtracting the weight of the right ear from the weight of the left ear.
The circular ear pieces (4-mm diameter) were then preserved in 10% formalin (Nanchang Rain and Dew, Jiangxi, China) and embedded in paraffin. Subsequently, the ear tissues were stained with hematoxylin (G1004, Servicebio, Wuhan, China) and eosin (G1108, Soleibao, Beijing, China) (HE) as previously described (Huang et al., Citation2007; Tabatabaei & Ahmed, Citation2022). Each sample was observed at different magnifications (100X and 400X) under an optical microscope (Eclipse-E100, Nikon, Japan) to identify the pathological changes of the skin at the induction site. After observation, pathological scores were given depending on the severity of pathological changes (the number of infiltrating inflammatory cells in the epidermis and dermis, epidermal thickness, degree of keratinization, necrosis, hyperplasia, and fibrosis), according to the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) for Lesions in Rats and Mice, as shown in (Mann et al., Citation2012).
Table 2. The 5-point (0–4) lesion scoring system employed in the present study based on the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) for Lesions in Rats and Mice (Mann et al., Citation2012).
2.5. Cytokines and antibody assessment
Blood samples were collected from the orbital vascular plexus of the mice and placed at room temperature for more than one hour. All samples were then centrifuged at 3000 rpm for 10 min at 4°C, and the supernatant (serum) was collected and stored at −80°C.
The ear tissues were minced and added with a corresponding volume of PBS (P1020-10*500 ml, Solarbio, China) (at a weight/volume ratio of 1:9), and repeatedly ground by a glass rod (YM001, KangBEI Scientific Instrument Co., Shenzhen, China) on the ice. Finally, the homogenate was centrifuged at 5000 g for 10 min, and the supernatant was used for testing. The levels of IL-4, IL-13, and IgE both in serum and ear tissues were tested using ELISA kits (Huaying Institute of Biotechnology, Beijing, China).
2.6. Survival analysis, body weight, and organ coefficient
Survival analysis was constructed based on the waiting time until the occurrence of an event (dead: 1, alive or censored: 0) (Ayalew et al., Citation2019; Dudley et al., Citation2016). Mice were monitored throughout the experiment and GraphPad Prism 8 was used for creating a Kaplan-Meier survival curve. Mice were weighed with an analytical balance (Yoke FA2004B) at a fixed time every week and the growth curve of body weight was drawn using GraphPad Prism 8. The organs, including heart, lung, liver, spleen, and kidneys, were removed from sacrificed mice. All organs were washed in 0.9% saline solution (MPB180353, Zhencheng Technology Co., Shenzhen, China), dried with normal filter paper, and then weighed with an analytical balance (Yoke FA2004B). Respective organ coefficient (e.g. liver coefficient, kidney coefficient, etc.) which equals organ weight divided by body weight, was recorded (Zhang et al., Citation2014).
2.7. Statistical analysis
Experimental data are presented as mean ± standard deviation (SD) and analysed in GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA). Groups were compared using unpaired Student’s t-tests and/or one-way analysis of variance (ANOVA); P < 0.05 was considered statistically significant.
3. Results
3.1. Construction results of pathological model of eczema
Representative images of DNCB-induced ear skin without VCT treatments were shown in (A). DNCB, a type of hapten that penetrates the skin barrier and induces a delayed-type hypersensitivity reaction, is used to cause skin barrier dysfunctions and to further induce the development and severity of eczema (Strick, Citation2013; Voisin & Chiu, Citation2019). These eczematous lesions include dry skin, erythema, exfoliation, and edema were observed in DNCB-induced left ears, while right ears treated with acetone appeared normal. (B) showed the effects of DNCB induction on histopathological changes in the control group. Unlike the right ear treated with acetone that showed no sign of inflammation, significant changes such as connective tissue hyperplasia in the dermis (black arrow); severe inflammatory reaction with abundant inflammatory cell infiltration in the dermis, subcutaneous, and muscular layers (blue arrow); and new capillaries (green arrows) were observed in the DNCB-induced left ear ((B)).
Figure 2. Construction of pathological eczema model in the left ears of mice. (A) Representative images of ear skins obtained from mice in control group. (B) Effects of DNCB induction on histopathological changes in ear. Representative microphotographs of ear sections stained with hematoxylin and eosin (HE), (a) right ear treated with acetone and (b) left ear induced by DNCB (HE staining, × 400). The photos showed that the connective tissue in the dermis had hyperplasia (black arrow); the inflammatory cells infiltrated in the dermis (blue arrow), and the new capillaries appeared (green arrows).

3.2. Oral administration of VCT does not cause harm to mice
Survival analysis is a good indication to analyse whether oral administration of VCT has any side effects on the survival of mice (Dudley et al., Citation2016). (A) shows the survival analysis on mice fed with different proportions of VCT. Since there were no human-caused deaths in any mice from these three groups throughout the experimental period, the survival rate was 100% (P < 0.05). It was also observed that there were no differences in survival rate among the control mice, 50% VCT mice, and 100% VCT mice (P < 0.05).
Figure 3. Oral administration of vermicompost tea (VCT) did not cause harm to mice. (A) The percent survival of mice fed with different proportions of VCT (mean ± SD, n = 13; P > 0.05). (B) Changes in body weight of mice fed with different ratios of VCT (mean ± SD, n = 5; P > 0.05). (C) The organ coefficients of mice fed with different ratios of VCT (each organ coefficient = organ weight (g)/body weight (g)*100). Data were presented as mean ± SD, n = 5; *P < 0.05; **P < 0.01.

Changes in body weight of mice fed with different proportions of VCT are shown in (B). The body weight of mice in the control group gradually increased and remained relatively steady over the modelling time (before and after DNCB induction) (P < 0.05). Also, seen in (B), mice fed with different proportions of VCT had a consistent growth trend compared to the control (P < 0.05).
Equally noteworthy is that the organ coefficient, an index in pathological anatomy and a necessary item for measuring drug chronic toxicity, were critical in assessing the safety and health of mice fed with different ratios of VCT ((C)). The results showed that there was no significant difference in the organ coefficients between control mice and mice fed with VCT (P > 0.05).
3.3. VCT alleviates symptoms of DNCB-induced eczema mice
Obvious eczema symptoms of skin erythema, increased ear thickness, scarring, exfoliation, and dry crusts were apparent on the left ears of control mice ((A)). However, the mice fed with 50% VCT had no obvious red and swollen or any scarring and exfoliation in DNCB-induced left ears. Mice fed with 100% VCT had less ear redness and swelling, but the condition was better than the control group.
Figure 4. Vermicompost tea (VCT) ameliorated the symptoms of eczema in mice. (A) Representative images of dermatitis on the left ears of mice fed with different proportions of VCT. (B) The ear allergenic scores of mice. Allergenic scores were evaluated on day 10, day 12, day 16, and day 18 (mean ± SD, n = 8; * P < 0.05; ** P < 0.01; *** P < 0.001). (C) The degree of ear swelling, which equals to the weight of the left ear piece minus the right ear piece (mean ± SD, n = 13; *P < 0.05; **P < 0.01; ***P < 0.001). (D) Effects of VCT on histopathological changes in ear. Representative histopathological images of ear tissue were obtained under light microscope (HE staining, × 400). (E) The pathological scores were given based on the severity of pathological changes (mean ± SD, n = 8; *P < 0.05; **P < 0.01; ***P < 0.001).

The ear allergenic scores ((B)) indicated that, with repetitive application of DNCB to the left ears of KM mice, the ear allergenic scores of the mice in control group was significantly increased and reached its peak on day 18 (P < 0.01). Compared with DNCB-induced left ears in control mice, the clinical allergenic scores of mice fed with 50% VCT were significantly lowered (P < 0.01) at almost every measurement point, whereas the dry skin peeling, swelling, and ulceration were improved. It is worth noting that 100% VCT did not show significant reduction on the allergenic scores. Thus, oral administration of 50% VCT was more effective in lowering the DNCB-induced ear allergenic scores and degree of ear swelling in the mouse model ((C), P < 0.01) than the 100% VCT (P > 0.05).
Histopathologically, as shown in (D), repeated DNCB exposure caused remarkable inflammatory changes, such as thickening of the epidermis, infiltrating of eosinophil in the ear tissues of the DNCB-induced left ear when compared with the acetone-treated right ear in control mice. Oral administration of 50% VCT markedly reduced the epidermal thickness and eosinophil infiltration in DNCB-induced left ear while treatment with 100% VCT did not differ significantly compared with control group (P > 0.05). The pathological scores in mice fed with 50% VCT were significantly lower in comparison with the control group (P < 0.01) ((E)).
3.4. Th2 immune response was reduced in mice fed with VCT
Th2-associated and pro-inflammatory cytokines (IL-4 and IL-13) enhanced eosinophil activity and activated B cells and induced B cells to produce IgE, and further exacerbated the pathological symptoms of eczema (Brandt & Sivaprasad, Citation2011; Nakajima et al., Citation2019). To explore whether VCT affects the severity of eczema by influencing Th2 immune response, the levels of IL-4, IL-13 and IgE both in serum and ear tissues were tested (). The control group showed the highest levels of IL-4, IL-13, and IgE in serum. The mice fed with 50% VCT showed significantly decreased levels of IL-4 and IL-13 ((A)–(B), P < 0.001). The mice fed with 100% VCT mitigated the levels of IL-13 and IL-4 in the serum markedly as well ((A)–(B), P < 0.05). However, there were no significant differences of levels of IL-4 and IL-13 in ear tissues between control and the mice fed with VCT ((D)–(E), P > 0.05). Therefore, the Th2-associated and pro-inflammatory cytokines (IL-4 and IL-13) were suppressed when DNCB-sensitized mice were administered with VCT (both 50% and 100%).
Figure 5. Vermicompost tea (VCT) decreased IgE and Th2-associated cytokine levels in DNCB-induced mice. Effects of VCT on serum IL-4 (A) and IL-13 (B) levels, serum antibody-IgE level (C); Effects of VCT on levels of Th2-associated cytokines and antibody in ear tissues, IL-4 (D), IL-13 (E) and IgE (F). Data were presented as mean ± SD, n = 8; *P < 0.05; **P < 0.01; ***P < 0.001.

On the other hand, since IgE binds with allergens and mast cells to induce mast cell degranulation and the release of inflammatory mediators, it ultimately promotes the development of eczema (Frew, Citation2010). Therefore, the level of IgE is an important indicator to monitor the effectiveness of eczema therapy. Serum from mice in the control group had the highest level of DNCB-specific IgE, but the levels were significantly reduced in serum from mice fed with 50% VCT ((C), P < 0.001) and 100% VCT ((C), P < 0.05). Subsequently, the levels of IgE in ear tissues were also examined by ELISA ((F)). DNCB-specific IgE in ear tissue was significantly lowered in mice fed with 50% VCT compared with control mice. However, there was no significant difference in IgE levels in ear tissues between control and the mice fed with 100% VCT. These results showed that 50% VCT reduced the expression of DNCB-specific IgE both in serum and ear tissues.
4. Discussion
VCT has traditionally been used as an ecofriendly and organic alternative to inorganic pesticides and fungicides, helping to prevent plant diseases and pests (Gudeta et al., Citation2022; Nayak et al., Citation2019) as well as having positive effects on the growth and productivity of various crops (Yatoo et al., Citation2021). However, the benefits of VCT on the immune response in animals had yet to be explored. The present study is the first to elucidate the impact of VCT on alleviating symptoms of DNCB-induced eczema. This can be seen in the following areas: reduction in ear redness and swelling ((A), (C)), lowered ear allergenic scores ((B)), and a decrease in pathological scores ((E)). It is worth noting that 50% VCT showed better results as an eczema treatment than 100% VCT.
According to previous studies (Brandt & Sivaprasad, Citation2011; Nakajima et al., Citation2019), Th2-associated and pro-inflammatory cytokines (IL-4 and IL-13) enhanced eosinophil activity, activated B cells, and induced B cells to produce IgE, further exacerbating the pathological symptoms of eczema. To explore whether VCT actually affects the severity of eczema by influencing Th2 immune response, the levels of IL-4, IL-13 and IgE both in serum and ear tissues were tested. Our results showed that 50% VCT reduced the expression of DNCB-specific IgE both in serum and ear tissues ((C) and 5(F)) and suppressed the expression of Th2-associated and pro-inflammatory cytokines (IL-4 and IL-13) in serum ((A)–(B)), consequently attenuating the development of eczema. Therefore, the ability of VCT to alleviate eczema symptoms could be attributed to the inhibition of DNCB-induced Th2 immune response.
As the first study to report the use of VCT as an alternative or complimentary approach to steroid-based treatment of eczema, it was necessary to validate the safety of VCT before proceeding further. According to Jiang et al. (Citation2023), the microflora in VCT offers beneficial effects in hydroponic cultivation of maple peas, and the physicochemical analysis also indicates rich nutrients without heavy metals. Oral administration of VCT showed no effect on the survival rate of mice, as indicated by the 100% survival rate across the control, 50% VCT, and 100% VCT groups ((A)). Furthermore, VCT had no significant impact on the growth and organ coefficients of mice ((B)–(C)). All of these results indicated that oral administration of VCT does not cause harm to mice. Thus, VCT could be considered as a safe alternative or a supplemental nutraceutical for eczema treatment. However, several questions remain to be investigated. Why does 50% VCT show more potency in treating eczema than 100% VCT? Could it be an overexpression of immune response when the VCT concentration is too high? A full spectrum assessment on all possible immune factors involved, as well as dose-dependence, are needed to provide a clear picture about the optimal VCT concentration to use for eczema treatment. Furthermore, a detailed chemical analysis on the composition of VCT would also elucidate why it is effective in treating such a persistent, incurable skin condition. It is the authors’ intention to raise the awareness of experts specialized in immunology and to encourage these experts to conduct in-depth analysis on the relationship between VCT and eczema.
On the other hand, this research also serves as a milestone in providing scientific evidence on the efficacy of a critical ingredient (dried earthworms, aka Dilong) in TCM to treat skin diseases. The ability to alleviate eczema without sacrificing earthworms is important as the earthworms can now continue to play their role in the ecosystem while providing the necessary components of VCT. Alternative medicine is a unique area worthy of further exploration. The authors of this study urge all scientists to assist in expanding the knowledge around natural alternatives.
In summary, oral administration of 50% VCT was able to decrease ear swelling and skin inflammatory symptoms in eczema-induced mice. Drinking water containing 50% VCT showed the most pronounced effects on Th2-associated and pro-inflammatory cytokines (IL-4 and IL-13) and reducing eosinophil infiltration and the expression of IgE. These anti-inflammatory and anti-eczema effects of VCT suggest its potential as an alternative or a supplemental nutraceutical for eczema treatment.
Contributions
H.W., Y.L. and S.J. conceived the study and designed models. H.W. implemented models, performed data analysis, and wrote the manuscript. Y.L. and S.J. supervised the project and modified the manuscript. The another author set up earthworm bins and took care of the earthworms. All Authors Read and Approved the Final Paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ansari, A., & Ismail, S. A.. (2012). Earthworms and Vermiculture Biotechnology. In Sunil Kumar (Ed.), Management of Organic Waste (pp. 87–96). InTech. ISBN: 978-953-307-925-7.
- Aslam, H. M., Shahzad, M., Shabbir, A., & Irshad, S. (2018). Immunomodulatory effect of thymoquinone on atopic dermatitis. Molecular Immunology, 101, 276–283. https://doi.org/10.1016/j.molimm.2018.07.013
- Ayalew, A. S., Erango, M. A., & Gergiso, K. T. (2019). Survival analysis of factor affects survival time of hypertension patients. Open Journal of Modelling and Simulation, 07, 177–189. https://doi.org/10.4236/ojmsi.2019.74010
- Balamurugan, M., Parthasarathi, K., Cooper, E. L., & Ranganathan, L. S. (2007). Earthworm paste (lampito mauritii, kinberg) alters inflammatory, oxidative, haematological and serum biochemical indices of inflamed rat. European Review for Medical and Pharmacological Sciences, 11(2), 77–90.
- Balamurugan, M., Parthasarathi, K., Cooper, E. L., & Ranganathan, L. S. (2009). Anti-inflammatory and anti-pyretic activities of earthworm extract—Lampito mauritii (Kinberg). Journal of Ethnopharmacology, 121(2), 330–332. https://doi.org/10.1016/j.jep.2008.10.021
- Beasley, R. (1998). Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The Lancet, 351(9111), 1225–1232. https://doi.org/10.1016/S0140-6736(97)07302-9
- Bilej, M., Procházková, P., Silerová, M., & Josková, R. (2010). Advances in experimental medicine and biology. Advances in Experimental Medicine and Biology, 708, 66–79. https://doi.org/10.1007/978-1-4419-8059-5_4
- Brandt, E. B., & Sivaprasad, U. (2011). Th2 cytokines and atopic dermatitis. Journal of Clinical & Cellular Immunology, 02(3), 110. https://doi.org/10.4172/2155-9899.1000110
- Camfferman, D., Kennedy, J. D., Gold, M., Martin, A. J., & Lushington, K. (2010). Eczema and sleep and its relationship to daytime functioning in children. Sleep Medicine Reviews, 14(6), 359–369. https://doi.org/10.1016/j.smrv.2010.01.004
- Contento, M., Cline, A., & Russo, M. (2021). Steroid phobia: A review of prevalence, risk factors, and interventions. American Journal of Clinical Dermatology, 22(6), 837–851. https://doi.org/10.1007/s40257-021-00623-6
- Cooper, E. L., & Hirabayashi, K. (2013). Origin of innate immune responses: Revelation of food and medicinal applications. Journal of Traditional and Complementary Medicine, 3(4), 204–212. https://doi.org/10.4103/2225-4110.119708
- Cooper, E. L., Ru, B., & Weng, N. (2004). Advances in experimental medicine and biology. Advances in Experimental Medicine and Biology, 546, 359–389. https://doi.org/10.1007/978-1-4757-4820-8_25
- Darsow, U., Laifaoui, J., Kerschenlohr, K., Wollenberg, A., Przybilla, B., Wüthrich, B., Borelli, S., F, F., Seidenari, S., Drzimalla, K., Simon, D., Disch, R., Devillers, A., Oranje, A., Raeve, L., Hachem, J. P., Dangoisse, C., Blondeel, A., Song, M., & Ring, J. (2004). The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: A European multicenter study. Allergy, 59(12), 1318–1325. https://doi.org/10.1111/j.1398-9995.2004.00556.x
- Dudley, W. N., Wickham, R., & Coombs, N. (2016). An introduction to survival statistics: Kaplan-meier analysis. Journal of the Advanced Practitioner in Oncology, 7(1), 91–100. https://doi.org/10.6004/jadpro.2016.7.1.8
- Ersser, S. J., Cowdell, F., Latter, S., Gardiner, E., Flohr, C., Thompson, A. R., Jackson, K., Farasat, H., Ware, F., & Drury, A. (2014). Psychological and educational interventions for atopic eczema in children. Cochrane Database of Systematic Reviews, 2014(1), 1–51. https://doi.org/10.1002/14651858.CD004054.pub3
- Frew, A. J. (2010). Allergen immunotherapy. Journal of Allergy and Clinical Immunology, 125, (2 Suppl 2), S306–S313. https://doi.org/10.1016/j.jaci.2009.10.064
- Fujii, Y., Takeuchi, H., Sakuma, S., Sengoku, T., & Takakura, S. (2009). Characterization of a 2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats. Skin Pharmacology and Physiology, 22(5), 240–247. https://doi.org/10.1159/000235551
- García-Marcos, L., Asher, M. I., Pearce, N., Ellwood, E., Bissell, K., Chiang, C. Y., El Sony, A., Ellwood, P., Marks, G. B., Mortimer, K., Martínez-Torres, A. E., Morales, E., Perez-Fernandez, V., Robertson, S., Rutter, C. E., Silverwood, R. J., & Strachan, D. P. (2022). The burden of asthma, hay fever and eczema in children in 25 countries: GAN phase I study. European Respiratory Journal, 60(3), 2102866. https://doi.org/10.1183/13993003.02866-2021
- Gudeta, K., Bhagat, A., Julka, J. M., Sinha, R., Verma, R., Kumar, A., Kumari, S., Ameen, F., Bhat, S. A., Amarowicz, R., & Sharma, M. (2022). Vermicompost and Its derivatives against phytopathogenic fungi in the soil: A review. Horticulturae, 8(4), 311. https://doi.org/10.3390/horticulturae8040311
- Hanifin, J. M., Cooper, K. D., Ho, V. C., Kang, S., Krafchik, B. R., Margolis, D. J., Schachner, L. A., Sidbury, R., Whitmore, S. E., Sieck, C. K., & Van Voorhees, A. S. (2004). Guidelines of care for atopic dermatitis. Journal of the American Academy of Dermatology, 50(3), 391–404. https://doi.org/10.1016/j.jaad.2003.08.003
- Hargreaves, J. C., Adl, M., & Warman, P. R. (2008). A review of the use of composted municipal solid waste in agriculture. Agriculture, Ecosystems & Environment, 123(1-3), https://doi.org/10.1016/j.agee.2007.07.004
- Hon, K. L., Kam, W. Y., Leung, T. F., Lam, M. C., Wong, K. Y., Lee, K. C., Luk, N. M., Fok, T. F., & Ng, P. C. (2006). Steroid fears in children with eczema. Acta Paediatrica, 95(11), 1451–1455. https://doi.org/10.1080/08035250600612298
- Huang, W.-C., Kuo, M.-L., Li, M.-L., Yang, R.-C., Liou, C.-J., & Shen, J.-J. (2007). The extract of <i>gynostemma pentaphyllum</i>enhanced the production of antibodies and cytokines in mice. YAKUGAKU ZASSHI, 127(5), 889–896. https://doi.org/10.1248/yakushi.127.889
- Ingham, E. R. (2005). The compost Tea brewing manual chapter 3 (5th ed.)., p. 11–12. Soil Foodweb Incorporated Press.
- Jiang, X., Lu, C., Hu, R., Shi, W., Zhou, L., Wen, P., Jiang, Y., & Lo, Y. M. (2023). Nutritional and microbiological effects of vermicompost tea in hydroponic cultivation of maple peas (Pisum sativum var. Arvense L.). Food Science & Nutrition, 127(5), 889–896. https://doi.org/10.1002/fsn3.3299
- Kemp, A. S. (1999). Atopic eczema: Its social and financial costs. Journal of Paediatrics and Child Health, 35(3), 229–231. https://doi.org/10.1046/j.1440-1754.1999.00343.x
- Langan, S. M., Silcocks, P., & Williams, H. C. (2009). What causes flares of eczema in children? British Journal of Dermatology, 161(3), 640–646. https://doi.org/10.1111/j.1365-2133.2009.09320.x
- Lax, S. J., Harvey, J., Axon, E., Howells, L., Santer, M., Ridd, M. J., Lawton, S., Langan, S., Roberts, A., Ahmed, A., Muller, I., Ming, L. C., Panda, S., Chernyshov, P., Carter, B., Williams, H. C., Thomas, K. S., & Chalmers, J. R. (2022). Strategies for using topical corticosteroids in children and adults with eczema. Cochrane Database of Systematic Reviews, 3(3), 1–524. https://doi.org/10.1002/14651858.CD013356.pub2
- Li, Y., Han, T., Li, W., Li, Y., Guo, X., & Zheng, L. (2018). Awareness of and phobias about topical corticosteroids in parents of infants with eczema in Hangzhou, China. Pediatric Dermatology, 35(4), 463–467. https://doi.org/10.1111/pde.13527
- Mann, P. C., Vahle, J., Keenan, C. M., Baker, J. F., Bradley, A. E., Goodman, D. G., Harada, T., Herbert, R., Kaufmann, W., Kellner, R., Nolte, T., Rittinghausen, S., & Tanaka, T. (2012). International harmonization of toxicologic pathology nomenclature. Toxicologic Pathology, 40(4 Suppl), 7S–13S. https://doi.org/10.1177/0192623312438738
- Mihara, H., Sumi, H., Yoneta, T., Mizumoto, H., Ikeda, R., Seiki, M., & Maruyama, M. (1991). A novel fibrinolytic enzyme extracted from the earthworm, lumbricus rubellus. The Japanese Journal of Physiology, 41(3), 461–472. https://doi.org/10.2170/jjphysiol.41.461
- Nakajima, S., Nomura, T., Common, J., & Kabashima, K. (2019). Insights into atopic dermatitis gained from genetically defined mouse models. Journal of Allergy and Clinical Immunology, 143(1), 13–25. https://doi.org/10.1016/j.jaci.2018.11.014
- Nayak, H., Rai, S., Mahto, R., Rani, P., Yadav, S., Prasad, S., & Singh, R. (2019). Vermiwash: A potential tool for sustainable agriculture. Food and Scientific Reports, 2(5), 308–312.
- Prasanth Bendalam, V. L. K. (2020). Vermiwash. Just Agriculture, 1, 42–43.
- Saunes, M., Øien, T., Storrø, O., & Johnsen, R. (2011). Family eczema-history in 2-year olds with eczema; a prospective, population-based study. The PACT-study, Norway. BMC Dermatology, 11(1), 11. https://doi.org/10.1186/1471-5945-11-11
- Schmitt, J., Apfelbacher, C. J., & Flohr, C. (2011). Eczema. BMJ Clinical Evidence, 1–41.
- Silverberg, J. I., & Simpson, E. L. (2013). Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatric Allergy and Immunology, 24(5), 476–486. https://doi.org/10.1111/pai.12095
- Strick, R. A. (2013). Dncb Use in treating extensive alopecia areata. Journal of Investigative Dermatology Symposium Proceedings, 16(1), S45. https://doi.org/10.1038/jidsymp.2013.15
- Sugino Souffront, D. K., Salazar-Amoretti, D., & Jayachandran, K. (2022). Influence of vermicompost tea on secondary metabolite production in tomato crop. Scientia Horticulturae, 301, 111135. https://doi.org/10.1016/j.scienta.2022.111135
- Sung, Y. Y., & Kim, H. K. (2018). Crocin ameliorates atopic dermatitis symptoms by down regulation of Th2 response via blocking of NF-κB/STAT6 signaling pathways in mice. Nutrients, 10(11), 1625. https://doi.org/10.3390/nu10111625
- Sutton, E., Shaw, A. R., Ridd, M. J., Santer, M., Roberts, A., Baxter, H., Williams, H. C., & Banks, J. (2022). How parents and children evaluate emollients for childhood eczema: A qualitative study. British Journal of General Practice, 72(719), e390–e397. https://doi.org/10.3399/BJGP.2021.0630
- Tabatabaei, M. S., & Ahmed, M. (2022). Methods in molecular biology. Methods in Molecular Biology, 2508, 115–134. https://doi.org/10.1007/978-1-0716-2376-3_10
- Voisin, T., & Chiu, I. M. (2019). Mast cells Get on your nerves in itch. Immunity, 50(5), 1117–1119. https://doi.org/10.1016/j.immuni.2019.04.007
- Wan, H. L., Chen, H. Z., & Shi, X. Q. (2016). Study on effect of traditional Chinese medicine jianpi chushi decoction and ointment on chronic eczema. Asian Pacific Journal of Tropical Medicine, 9(9), 920–923. https://doi.org/10.1016/j.apjtm.2016.07.019
- Wollenberg, A., Kinberger, M., Arents, B., Aszodi, N., Avila Valle, G., Barbarot, S., Bieber, T., Brough, H. A., Calzavara Pinton, P., Christen-Zäch, S., Deleuran, M., Dittmann, M., Dressler, C., Fink-Wagner, A. H., Fosse, N., Gáspár, K., Gerbens, L., Gieler, U., Girolomoni, G., … Flohr, C. (2022). European guideline (EuroGuiDerm) on atopic eczema: Part I – European guideline (EuroGuiDerm) on atopic eczema: Part I – systemic therapy. Journal of the European Academy of Dermatology and Venereology, 36(9), 1409–1431. https://doi.org/10.1111/jdv.18345
- Yamamoto, M., Haruna, T., Yasui, K., Takahashi, H., Iduhara, M., Takaki, S., Deguchi, M., & Arimura, A. (2007). A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergology International, 56(2), 139–148. https://doi.org/10.2332/allergolint.O-06-458
- Yatoo, A. M., Ali, M. N., Baba, Z. A., & Hassan, B. (2021). Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agronomy for Sustainable Development, 41, 1–26. https://doi.org/10.1007/s13593-020-00657-w
- Zarei, M., Jahandideh Mahjen Abadi, V. A., & Moridi, A. (2018). Comparison of vermiwash and vermicompost tea properties produced from different organic beds under greenhouse conditions. International Journal of Recycling of Organic Waste in Agriculture, 7(1), 25–32. https://doi.org/10.1007/s40093-017-0186-2
- Zhang, R., Zhang, L., Jiang, D., Zheng, K., Cui, Y., Li, M., Wu, B., & Cheng, S. (2014). Mouse organ coefficient and abnormal sperm rate analysis with exposure to tap water and source water in Nanjing reach of Yangtze river. Ecotoxicology, 23(4), 641–646. https://doi.org/10.1007/s10646-014-1228-4
