Abstract
Background
Interruption of the enterohepatic circulation is regarded as an effective way to treat patients with amatoxin poisoning. Nonetheless, its effectiveness has not yet been systematically evaluated. Therefore, we performed a systematic review to investigate the role of enterohepatic circulation on patient outcome and clinical laboratory values. We specifically sought to evaluate the effect of activated charcoal, which absorbs drugs and toxins in the gastrointestinal tract.
Methods
A previously established database with data extracted from case reports and series from literature, supplemented with recent publications, was used. Patient characteristics, outcome, and laboratory values were evaluated.
Results
We included 133 publications describing a total of 1,119 unique cases. Survival was 75 per cent in the control group (n = 452), whereas in the group treated with single or multiple doses of activated charcoal (n = 667) survival was 83 per cent (P < 0.001, odds ratio 1.89 [95 per cent confidence interval 1.40–2.56]). Furthermore, no difference in peak values of alanine aminotransferase and aspartate aminotransferase activities were observed, whereas peak values of total serum bilirubin concentration and international normalized ratio were statistically significantly reduced in patients treated with activated charcoal.
Discussion
The ability of activated charcoal to enhance the elimination of amatoxin through interruption of the enterohepatic circulation offers a potentially safe and inexpensive therapy for patients in the post-absorptive phase.
Limitations
Limitations include the potential for publication bias, the lack of universal confirmation of amatoxin concentrations, and the inability to directly measure enterohepatic circulation of amatoxin.
Conclusion
Treatment with activated charcoal in patients with amatoxin poisoning was associated with a greater chance of a successful outcome. Additionally, activated charcoal was associated with a reduction in markers of liver function, but not markers of liver injury.
Introduction
Recently, our group performed a systematic review to determine the effectiveness of silibinin, acetylcysteine, and benzylpenicillin in patients with Amanita phalloides poisoning [Citation1]. In addition to antidote treatment, interrupting the enterohepatic circulation is a frequently used treatment. Previous studies have demonstrated that (naso)biliary drainage, interrupting the enterohepatic circulation, may have a positive impact on patient outcome in amatoxin intoxications [Citation2]. The effectiveness of activated charcoal, another well-recognized method of interrupting the enterohepatic circulation [Citation3], has not been systematically evaluated for amatoxin poisonings [Citation4,Citation5]. Therefore, we performed a systematic review on the effects of activated charcoal on patient outcome. In addition, we evaluated clinical laboratory values previously shown to be associated with outcomes in amatoxin poisonings [Citation1].
Materials and methods
Search strategy
For this article, published cases of Amanita spp. containing amatoxin poisonings spanning over more than 45 years were systemically reviewed, starting from 21 July 1975 for the first case until 18 April 2023. The cases presented in the article by Tan et al. [Citation1] were used as a starting point. Additional case reports and case series of poisoning with amatoxin-containing mushroom species, published after 31 July 2020 were retrieved from online databases, including MEDLINE (via PubMed), Embase, Google Scholar, and the University of Groningen database (via SmartCat). The keywords used for the search were: “Amanita intoxication”, “Amanita poisoning”, “amanitin”, and “amatoxin”. After 31 July until 18 April 2023, 17 new publications were found for the keywords used. For this study, mainly cases with amatoxin-containing Amanita spp. were included. Other mushroom species containing amatoxin and non-hepatotoxic Amanita spp. were excluded, except when the amatoxin concentration in urine or blood was measured suggesting involvement of an amatoxin-containing Amanita spp. as well. Furthermore, any additional references of the retrieved studies were checked. If these references were relevant, they were included. The articles obtained from the databases were grouped. Duplicate cases in these articles were removed. Articles that were in any other language than Chinese, Dutch, or English were excluded. Articles that had no full-text available or were in another language but did have an abstract in English or Dutch were included if the abstract provided information that met the inclusion criteria. Abstracts from case presentations at conferences were also added if they met the inclusion criteria. During the following screening, the primary researcher (JV) checked if the papers met the inclusion criteria. The secondary researchers (DT and BD) verified laboratory data and the unit conversion. See for the flowchart of the selection process.
Inclusion and exclusion criteria
Patients were included if they experienced a poisoning due to consumption of Amanita spp. containing amatoxin, if the given treatment was described, and if the outcome was known. Subsequently, the treatment had to be linked to the outcome of that specific treatment. If treatment could not be linked, the case (series) was excluded. Additionally, if an article contained a case series of amatoxin poisoning including mushroom species with amatoxin other than Amanita spp., but amatoxin poisoning was classified as proven in the article, then the cases were included. Individual patients were excluded if the mushroom they ate did not contain amatoxin, if they were not hospitalized, if the treatment or the outcome of the treatment is unknown, if they had no gastrointestinal symptoms, if they had a previously diagnosed liver disease, or if they had a normal liver function determined by laboratory testing and symptoms.
Extraction of the data
Data were extracted for all included publications. Extracted data included the treatment given, including the use of activated charcoal, outcome, demographic parameters, and clinical laboratory values. Data were only extracted if described and traceable to the specific case. The laboratory data retrieved consisted of aspartate aminotransferase (AST) activity, alanine aminotransferase (ALT) activity, total serum bilirubin concentration, and international normalized ratio (INR). Additional data consist of the time from ingestion to gastrointestinal symptoms, the time until clinical care was provided, the time spent in the hospital, and if hepatoxicity was present. The latter was determined either by elevated activity of liver enzymes, AST or ALT, by the description of the symptoms, or clinical confirmation.
Classification of the data
The outcome of the treatments was either classified as a “success” or “failure”. The treatment was classified as a “success” when the patient survived without a liver transplant. Outcomes were classified as a “failure” when the patient survived with a liver transplant, when the patient died, or when the patient died with a liver transplant. Furthermore, patients were grouped in treatment with activated charcoal and treatment without activated charcoal in order to analyze the effects of the treatment with activated charcoal on the outcome. Lastly, treatments with the most frequently used antidotes were scored. This includes acetylcysteine, benzylpenicillin, silibinin or combinations.
Statistical analysis
For the statistical analysis, SPSS statistics software (version 28.0.1.0) was used. Descriptive analysis was used in order to describe the patient characteristics. For each characteristic, the total population, the median, and the range were calculated. To determine if a dataset is normally distributed, the Kolmogorov-Smirnov test was performed. If the dataset was normally distributed, an independent samples T-test was carried out to test for significant differences in continuous datasets between treatments (use of activated charcoal or no use of activated charcoal). However, if the dataset was not normally distributed then the Mann-Whitney U-test was used to test for significant differences. Additionally, a chi-squared test was performed to test for the correlation between the categorical datasets and patient outcome. The independent samples T-test was used for the determination of possible covariables when the continuous dataset was normally distributed. The Mann-Whitney U-test was performed to test for possible covariables when the dataset is not normally distributed. At last, logistic regression was performed to be able to compare the successful and failed treatment outcome rates with the usage of activated charcoal. On the basis of the adjusted odds ratio at 95% confidence interval (CI) calculated with the logistic regression, the treatments and outcomes were compared. Differences between treatment groups for all tests were regarded significant if P < 0.05.
Results
Descriptive analysis
We included 133 publications, six new studied were identified and 129 studies were included from the publication of Tan et al. [Citation1]. Two studies were excluded as they did not meet the inclusion criteria (). In the end, 4 additional publications were included [Citation6–9]. In total, 1,119 patients were identified. An overview of the patient characteristics and clinical laboratory values is presented in . The clinical laboratory values and characteristics of the patients per treatment group is presented in . Most parameters were comparable between the groups. Hepatotoxicity and antidote use were different between groups.
Table 1. Clinical laboratory values and patient properties of the whole population (n = 1,119).
Table 2. Clinical laboratory values and characteristics of the patients treated with or without activated charcoal from the whole population (n = 1,119).
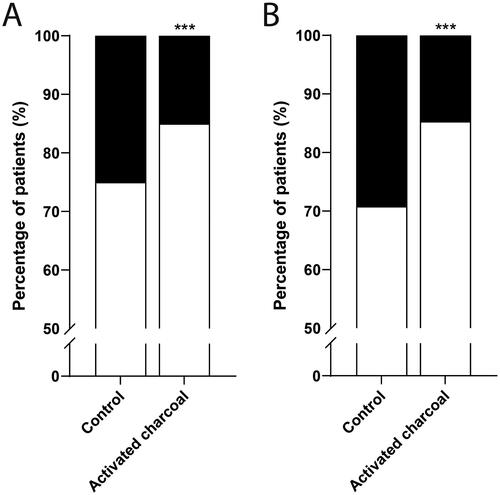
The majority of patients received activated charcoal as multiple doses (59%). Only two patients (0.4%) received a single dose of activated charcoal. For the remainder of cases (41%) the dose was not specified. Overall treatment success (survival) was 81% (925 of 1,119 patients). In the control group (n = 452), treatment success was 75% (). In the group with treated with activated charcoal (n = 667), treatment success was 83%. Treatment with activated charcoal was associated with increased treatment success (P < 0.001, OR 1.89 [95% CI 1.40–2.56]).
Figure 2. Effect of activated charcoal on survival outcomes of patients with amatoxin poisonings. (A) whole database. Patients treated without activated charcoal (control, n = 452) and with activated charcoal (n = 667). (B) subgroup analysis of reasonably certain cases. Patients treated without activated charcoal (control, n = 329) and with activated charcoal (n = 667). Percentage of patients with treatment success (white) and treatment failure (black). ***P < 0.001 compared to control.
Comparison of clinical laboratory values
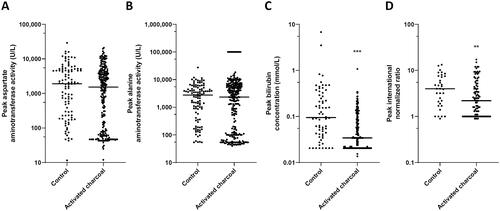
Acute liver failure is a characteristic feature of amatoxin poisoning. In our previous study, peak values of AST activity, ALT activity, bilirubin concentration, and INR were found to be associated outcomes [Citation1]. Therefore, we compared ALT activity, AST activity, bilirubin concentration, and INR peak values between the two treatment groups (). The use of activated charcoal was not associated with significant differences in peak AST or peak ALT activities. However, the liver function markers of the group treated with activated charcoal were associated with a decrease in peak bilirubin concentration (P < 0.001) and peak INR (P < 0.05) when compared to the group not treated with activated charcoal.
Figure 3. Clinical laboratory peak values of important liver markers in 1,119 patients divided in treated without activated charcoal (control) and with activated charcoal. (A) Aspartate aminotransferase activity, (B) Alanine aminotransferase activity, (C) Total serum bilirubin concentration, and (D) International normalized ratio. *P < 0.05, ** P < 0.01, and ***P < 0.001 compared to the control.
Potential confounders
Potential confounders were tested for their influence on the treatment outcome (). Time from ingestion to gastrointestinal symptoms, hepatoxicity and antidote treatment were identified as confounders (P < 0.01). Confounding was not present for age, gender, time to clinical care, length of hospital stay or publication date. The adjusted odds ratio (AOR) 2.12 [95% CI 1.26–3.58] indicates that treatment with activated charcoal was still associated with an increased treatment success after adjusting for these confounders (P < 0.01).
Table 3. Association of potential confounders on treatment outcome.
Subgroup analysis
Treatment with activated charcoal is a routine intervention in the treatment of multiple intoxications. For this reason, it could be that activated charcoal was administered but was not mentioned in the case description. Therefore, a subgroup analysis of cases of which it is reasonably certain that the patients did or did not receive activated charcoal was performed. This analysis included 996 patients. An overview of the characteristics of these patients are shown in Supplementary Table S1. The clinical laboratory values and characteristics of the patients per treatment group are displayed in Supplementary Table S2. The results of this analysis indicate a success rate of 85%. The results from the analysis also demonstrate that treatment with activated charcoal was still associated with an increased treatment success (P < 0.01) with a slightly higher odds ratio (OR 2.39 [95% CI 1.74–3.30]) and an increased adjusted odds ratio (AOR 2.30 [95% CI 1.36–3.89]) when uncertain cases are removed. Furthermore, the use of activated charcoal was still associated with a decrease in peak bilirubin concentration (P < 0.001) and peak INR (P < 0.01). However, an association of activated charcoal treatment with a decrease in peak ALT activity (P < 0.05) was also identified.
Discussion
In this study, we demonstrate that the use of activated charcoal was associated with a positive effect on outcome of patients with amatoxin poisoning. This effect was accompanied with a reduction of peak values of bilirubin concentration and INR, but not AST or ALT activity.
Our results suggest that the enterohepatic circulation may contribute to toxicity in amatoxin poisonings. It is important to notice that we only indirectly studied the enterohepatic circulation in patients. Previously, it was assumed that interrupting the enterohepatic circulation is only beneficial in the early stages of amatoxin poisonings [Citation10]. However, in that study, pigs were sedated by high doses of fentanyl, well known for its gastrointestinal side effects that may have impaired the enterohepatic circulation of amatoxin, which may have led to false conclusions. These considerations were recently communicated [Citation11]. This is especially important in light of multiple studies which concluded that amatoxins are reprocessed through the enterohepatic circulation and that interruption of this cycle may lead to improved outcomes [Citation2,Citation12–14]. Future studies using radiolabelled amatoxins in animal models could address the contribution of the enterohepatic circulation to amatoxin poisonings in more detail. Other treatments that interrupt the enterohepatic circulation, like (naso)biliary drainage, may also have beneficial effects on patient outcome. Madhok et al. showed that the use of continuous biliary drainage reduced the concentration of α- and β-amanitin in the bile [Citation2]. However, this intervention and similar treatments were not taken into account in our study due to the limited number of cases. Biliary drainage has been studied in a dog model of Amanita exitialis poisonings. In this model biliary drainage reduced the severity of the poisoning and a reduced increase in biochemical markers of liver injury which was associated with a reduced maximal concentrations and exposure to amatoxins. These findings suggest a prominent role of the enterohepatic circulation in patients with amatoxin poisoning [Citation14]. In addition to absorption of toxins excreted via the bile, activated charcoal has been shown to also absorb toxins secreted by the intestinal mucosa suggesting that this intervention may be even more effective [Citation14].
Activated charcoal was administered in the majority of cases, typically as multiple doses. The dose and frequency of administration, however, were quite variable. In some publications activated charcoal was administered every hour, whereas in others activated charcoal was administered every 4–6 hours [Citation15,Citation16]. Also, the dose varied. In some cases, dosing was weight-based [Citation16], whereas in other fixed doses were used [Citation17]. Future studies are required to further investigate the appropriate dose and frequency.
Moreover, the effect of activated charcoal on the liver markers is interesting. Peak bilirubin concentration and INR values are significantly reduced in patients treated with activated charcoal, whereas no significant reduction in peak AST or ALT activities were observed. Therefore, use of activated charcoal did not appear to reduce cell damage of hepatocytes. An explanation for this could be that patients are hospitalized primarily after 24 hours. During this time most of the damage to the hepatocytes seems to have occurred. Nonetheless, the significant reduction in INR, suggests a recovery of the capability of the liver for biosynthesis. Accordingly, the decrease in bilirubin concentration compared to the control group suggests that the liver remains more functional due to activated charcoal treatment [Citation18], suggesting that an extended exposure of the liver to the toxin may limit the recovery of the organ. A reduced increase in liver function parameters may be clinically more relevant as these patients may be at reduced risk of restrictions in coagulation or encephalopathy. Furthermore, we previously found that peak activities of AST and ALT, bilirubin concentration, and INR values were more elevated in patients with fatal outcomes, indicating these to be possible prognostic markers for patient survival [Citation1]. Therefore, a decrease in these values imply a positive effect of activated charcoal on survival after amatoxin poisonings. One limitation is the use of published case series and reports, resulting in publication bias because publications are more inclined to highlight noteworthy cases. As a result, extreme outcomes are more likely to be reported, leading to a possible over- or underestimation of the treatment effect. In most published cases, a positive outcome has likely been reported. Therefore, the overestimation of results is possible. Quality of care provided to the patients is also a bias which we could not account for. Patients with intensive supportive treatment are more likely to survive. The setup of the study, using individual cases and series, may, however, have compensated for this. Another limitation is the accuracy of the data. This is primarily the result of missing data. However, this also applies to reported data due to incomplete data collection and variation in time between the poisoning and time to clinical care. It would be preferred to look at clinical laboratory values before and after treatment of activated charcoal. However, this was not possible because this data is not always reported.
Conclusion
Our study suggests that treatment with activated charcoal was associated with increased successful treatment outcomes, including a two times higher chance to have a successful outcome compared with the control group. The use of activated charcoal was also associated with a decrease in peak bilirubin concentration and INR, providing a potential explanation for the observed effects. Even though there are multiple ways to interrupt the enterohepatic circulation, use of activated charcoal is preferable as it is easy to use and less invasive for patients.
Authors’ contributions
JV: Conceptualization, data collection, analysis of the data and writing of the paper; JLT: data collection, review, and approval of the manuscript; JS: data collection, review, and approval of the manuscript; AvdB: review and approval of the manuscript; PFvR: review and approval of the manuscript; DJT: Conceptualization, supervision, writing, review, and approval of the paper, BGJD: conceptualization, supervision, writing, review, and approval of the paper
Supplemental Material
Download MS Word (27.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data sharing statement
Upon reasonable request, and subject to review, the authors will provide the data that support the findings of this study.
Additional information
Funding
References
- Tan JL, Stam J, van den Berg AP, et al. Amanitin intoxication: effects of therapies on clinical outcomes - a review of 40 years of reported cases. Clin Toxicol. 2022;60(11):1251–1265. doi: 10.1080/15563650.2022.2098139.
- Madhok M, Scalzo AJ, Blume CM, et al. Amanita bisporigera ingestion: mistaken identity, dose-related toxicity, and improvement despite severe hepatotoxicity. Pediatr Emerg Care. 2006;22(3):177–180. doi: 10.1097/01.pec.0000202459.49731.33.
- Zellner T, Prasa D, Färber E, et al. The use of activated charcoal to treat intoxications. Dtsch Arztebl Int. 2019;116:311–317.
- Neuschwander-Tetri BA, Scalzo AJ. Comment on: amanitin intoxication. Clin Toxicol. 2023;61(2):141–141. doi: 10.1080/15563650.2022.2132167.
- Dekkers BGJ, Touw DJ. Authors” reply to amanita intoxication. Clin Toxicol. 2023;61(5):411–412. doi: 10.1080/15563650.2023.2206944.
- Angioi A, Floris M, Lepori N, et al. Extensive proximal tubular necrosis without recovery following the ingestion of amanita phalloides: a case report. J Nephrol. 2021;34(6):2137–2140. doi: 10.1007/s40620-021-01018-w.
- Dluholucký S, Snitková M, Knapková M, et al. Results of diagnostics and treatment of amanita phalloides poisoning in Slovakia (2004-2020). Toxicon. 2022;219:106927. doi: 10.1016/j.toxicon.2022.09.013.
- Sabee AI, Kurkus J, Lindholm T. Intensive hemodialysis and hemoperfusion treatment of Amanita mushroom poisoning. Mycopathologia. 1995;131(2):107–114. doi: 10.1007/BF01102888.
- Kantola T, Kantola T, Koivusalo AM, et al. Early molecular adsorbents recirculating system treatment of amanita mushroom poisoning. Ther Apher Dial. 2009;13(5):399–403. doi: 10.1111/j.1744-9987.2009.00758.x.
- Thiel C, Thiel K, Klingert W, et al. The enterohepatic circulation of amanitin: kinetics and therapeutical implications. Toxicol Lett. 2011;203(2):142–146. doi: 10.1016/j.toxlet.2011.03.016.
- Mitchell ST. Letter to the editor: regarding the toxicology letters publication: “The enterohepatic circulation of amanitin: kinetics and therapeutical implications (thiel et al 2011). Toxicol Lett. 2022;367:1–2. doi: 10.1016/j.toxlet.2022.06.011.
- Frank I, Cummins L. Amanita poisoning treated with endoscopic biliary diversion. J Emerg Nurs. 1987;13(3):132–136.
- Fauser U, Faulstich H. Therapy of Amanita phalloides poisoning. Improvement of the prognosis due to interruption of the enterohepatic circulation (common bile duct drainage). Dtsch Med Wochenschr. 1973;98(47):2259. German
- Sun J, Niu YM, Zhang YT, et al. Toxicity and toxicokinetics of Amanita exitialis in beagle dogs. Toxicon. 2018;143:59–67. doi: 10.1016/j.toxicon.2018.01.008.
- Giannini L, Vannacci A, Missanelli A, et al. Amatoxin poisoning: a 15-year retrospective analysis and follow-up evaluation of 105 patients. Clin Toxicol. 2007;45(5):539–542. doi: 10.1080/15563650701365834.
- Vesconi S, Langer M, Iapichino G, et al. Therapy of cytotoxic mushroom intoxication. Crit Care Med. 1985;13(5):402–406. doi: 10.1097/00003246-198505000-00007.
- Ennecker-Jans S, van Daele P, Blonk M, et al. [Amatoxin poisoning due to soup from personally picked deathcap mushrooms (amanita phalloides)]. Ned Tijdschr Geneeskd. 2007;151(13):764–768. Dutch
- Preedy V. Biomarkers in liver disease. Patel V, Preedy V, editors. Dordrecht (The Netherlands): Springer Netherlands; 2017.