Abstract
The excessive intake and bioaccumulation of sodium fluoride (NaF) through water, toothpaste or pesticides could trigger cardiac oxidative stress. The effects of Hibiscus sabdariffa (HS) fractions on biochemical markers of the heart in NaF-induced rats. Thirty male and female rats were grouped into five: Normal control (NC) received water only, Sodium fluoride (NaF, 300 mg L−1), the others were exposed to 300 mg L−1 NaF while the treated animals received100 mg kg−1d−1 of aqueous, n-hexane and butanol -H. sabdariffa (AQE-HS, HEE-HS, BTE-HS) fractions orally for 14 days respectively. This study revealed that NaF exposure increases TBARS, TC, TG, LDL-c, AI, AC, CRI-I, CRI-II and arginase activity with a decrease in catalase, GST, SOD, GSH, HDL-c, and NO level whilethe HS treatment stimulates antioxidant production which eventually reduce oxidative stress. The AQE-HS suppressed TBARS, lipid profile, and cardiac indices. Therefore, bioactive constituent of HS attenuates NAF-induced cardiac oxidative stress.
1. Background
Cardiovascular diseases (CVDs) are one of the leading causes of death globally with an estimated value of 23.6 million by year 2030 [Citation1]. It has been documented that several metabolic risk variables, such as hyperglycemia, dyslipidemia, and hypertension tend to play a significant role in the development and progression of CVDs [Citation1]. The prevalence of cardiovascular diseases in the health care system has become a critical economic burden in recent times which has resulted in a higher level of morbidity and mortality rate. However, excessive reactive oxygen species (ROS) production in the cell could also trigger molecular cell damage with numerous physiological and pathological disorders [Citation2]. Meanwhile, it has been revealed that irreplaceable functions are displayed by oxidative stress in the pathology and development of diverse cardiovascular diseases [Citation2]. Although, alterations in the DNA, lipids, and proteins by the oxidative species (OS) have led to inflammation and programmed cell death [Citation3], which perform a basic understanding of the development of diverse cardiovascular diseases [Citation4] which could be reduced by supplements [Citation5,Citation6]. Xu et al. reported that the conditions of cardiac pathology like dysregulation of cell death, cardiomyocyte hypertrophy, and extracellular matrix remodelling are connected to final heart failure [Citation2]. Furthermore, it has been established that intracellular ROS and its related signalling pathways participate actively in cardiac abnormalities [Citation1,Citation7]. Consequently, in addressing this, numerous therapeutic agents have been beneficial in managing or treating cardiac-associated disorders resulting from stress via the use of antioxidants-rich-supplements and synthetic drugs.
Interestingly, due to some side effects observed in the therapeutic drugs, there is an urgent need for a natural source of antioxidants that could suppress excessive ROS production. However, alternative remedies from natural substances like plant extracts have provided diverse opportunities for new drug discovery [Citation8].
Moreover, it has been documented by WHO that above 80% of the entire world population depends on alternative remedies for primary healthcare needs [Citation9–11]. Duraipandiyan et al. stated that various chronic and infectious diseases in time past have been treated with bioactive constituents contained in natural herbs [Citation12,Citation13]. Meanwhile, a range of bioactive components from plants has been classified as safe and effective alternatives with minimal side effects.
Hibiscus sabdariffa (roselle) belongs to the Malvaceae family and is often grown in many countries. Phytochemicals like anthocyanins, polysaccharides, and some organic acids are part of the polyphenols present in Hibiscus sabdariffa that support its traditional uses and thus numerous prospective in modern therapeutics [Citation14]. In the same vein, Lin et al. reported the inhibitory effect of roselle on the oxidation of LDL, thus it could serve as a bioactive agent in the treatment of atherosclerosis [Citation15]. Also, the antihyperlipidemic effect of roselle in patients suffering from metabolic syndrome has been documented [Citation16]. It was documented also that H. sabdariffa has high antioxidant properties that have been used as anticancer [Citation17], hypoglycemic [Citation18], antipyretic [Citation19], hypotensive properties [Citation20] and antiproliferative. It was also documented by Najafpour and co-authors that system blood pressure and glucose could be regulated via the consumption of H. sabdariffa tea [Citation1]. In 2018, Rason and the co-author also established the antiobesity and cholesterol-lowering potential of Hibiscus sabdariffa in obese-hypercholesterolemic rats [Citation21].
Moreover, there is a dearth of information on the bioactivity of Hibiscus sabdariffa and its role in modulating cardiac induce toxicity. Therefore, this study intends to give an insight on the potency of various fractions of Hibiscus sabdariffa (HS) on how it could attenuate oxidative stress when investigated in both sexes of rats.
2. Methods
2.1. Plant collection
Hibiscus sabdariffa L. calyces were brought from Ikirun located at latitude 7° 55′ 34.5864″ N and longitude 4° 39′ 58.6368″ E Nigeria, while the identification was carried out in the herbarium of the University of Ilorin and a specimen was deposited with the voucher number UILH/002/1486/2022.
2.2. Chemicals and reagents
Thiobarbituric acid (TBA), sodium fluoride, hydrogen peroxide (H2O2), dithiobis (2-nitrobenzoic acid) (DTNB), trichloroacetic acid (TCA), sodium nitroprusside, o-dianisidine dihydrochloride, 1-chloro-2,4- dinitrobenzene (CDNB), and glutathione (GSH) were all Sigma Company products. Also, high-density lipoprotein (HDL), total cholesterol (TC), and triglycerides (TG) are the product of RANDOX lab Ltd.
2.3. Animals handling
Sixty (60) rats of 201 ± 13 g weight (30 males and 30 females) were procured from Osun State University animal house, Nigeria. The rats were maintained under standard humidity conditions, 12 h of light/dark cycle, and temperature respectively. The rats used during this experiment were fed with commercial pellet diet and water for two weeks of acclimatization. The experimental protocol used was approved by Osun State University Ethical Committee (UNIOSUNHREC/2021/005C).
2.4. Plant extract preparation
The plant extraction procedure was carried out following a procedure previously reported by Adebisi et al., [Citation22] with little modification. The Hibiscus sabdariffa (HS) were rinsed and oven dried at 40°C. The dried sample was milled to a fine powder using a NAKAI blender (Model No. NJ-463). 500 g of the HS powder was weighed and soaked in distilled water for 2 days. The filtrate was collected and concentrated at 40°C in a rotary evaporator. The crude extract was then subjected to fractionation with n-hexane, butanol, and aqueous in a separatory funnel to give n-hexane H. sabdariffa (HEE-HS), butanol H. sabdariffa (BTE-HS), and an aqueous H. sabdariffa (AQE-HS) fractions which were concentrated in a water bath at 45°C.
2.5. Experimental design
During this study the male and female rats thirty (30) each were grouped into five separate cages: Normal control (NC) receives water only, Sodium fluoride (NaF) group (exposed to 300 mg L−1 NaF according to Oyagbemi et al. [Citation23]), the other groups were exposed to 300 mg L−1 NaF and treated with 100 mg kg−1d−1 of AQE-HS, HEE-HS, and BTE-HS respectively via oral route using cannula for 14 days. The dose of the fractions administered was based on the methodology developed by Yuliastri et al. [Citation24].
2.6. Collection of blood and tissue homogenization
The rats were euthanized using diethyl-ether while the blood was collected into the bottle while the heart tissue was excised and homogenized using 0.25 M ice-cold sucrose and the resulting homogenate was centrifuged for 15 min at 10,000 rpm in a cold centrifuge to obtain the supernatant fraction used for the biochemical assays.
2.7. Biochemical analysis
Chance and Maehly method were used to determine catalase activity [Citation25]. The activity of glutathione -S-transferase was assessed following the protocol developed by Habig et al. [Citation26]. Kakkar et al. protocol was used to determine superoxide dismutase activity [Citation27]. Nitric oxide was measured using Miranda et al method [Citation28]. Arginase activity was assessed with the Kaysen and Strecker protocol [Citation29]. Reduced glutathione (GSH) level was assessed with Jollow et al. method [Citation30]. Lipid peroxidation (TBARS) was estimated using the standard protocol. Serum lipid profiles were analysed using standard diagnostic kits and following the manufacturer's instructions while low-density lipoprotein cholesterol (LDL-c) was estimated by the Friedewald equation [Citation31]. Atherogenic index (AI) = log TG/HDLc [Citation32], Atherogenic coefficient (AC) = (TC– HDLc)/HDLc [Citation33], CRI-I = TC/HDLc and CRI-II = LDLc/HDLc [Citation33] were all evaluated from the results of the lipid profile analysis. The active constituents characteristics of the fractions functional group were assessed following Ouhaddouch et al., a protocol using SHIMADZU Fourier transform-infrared (FTIR) spectroscopy 8400S, and the spectra were determined in the region of 4000–500 cm−1 [Citation34].
2.8. Data analysis
The values were mean ± SEM (n = 6). The mean difference was assessed using one-way ANOVA on the Graph pad prism 5.0 and then followed by Duncan multiple range tests at level p ˂ 0.05.
3. Results
3.1. Thiobarbituric acids (TBARs)
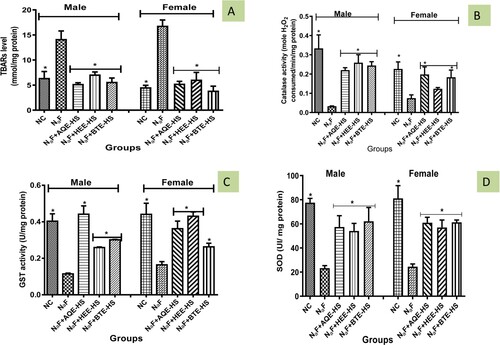
During this study it was observed that NaF administered to rats elevated the level of TBARS significantly (p < 0.05) in NaF-induced group while treatment with Hibiscus sabdariffa (HEE, BTE, and AQE) fraction reversed TBARS increase in NaF-induced rats in both sexes when compared with the NC (Figure (A)). However, a significant decline in the value of TBARS was seen in the female rat treated with BTE when compared with NC and NaF-induced male and female rats (Figure (A)).
3.2. Catalase
Catalase activity of NaF-induced rats was significantly decreased (p<0.05) when compared to the NC and treatment groups while treatment with HS (AQE, HEE, BTE) elevated the activity of the antioxidant enzyme catalase when compared with NaF-induced rats (Figure (B)).
3.3. Glutathione-S-transferase (GST)
The activity of GST of NaF-induced was significantly reduced (p < 0.05) in comparison with NC and treatment groups except for the AQE, treatment with Hibiscus sabdariffa (AQE, HEE, BTE) increases the activity of GST when compared with NaF (Figure (C)).
3.4. Superoxide dismutase (SOD)
The activity of SOD declined in male and female rats induced with NaF in comparison with the NC while treatment with Hibiscus sabdariffa (AQE, HEE, and BTE) increases SOD activity in comparison with the NaF-induced rats (Figure (D)).
3.5. Nitric oxide (NO) level
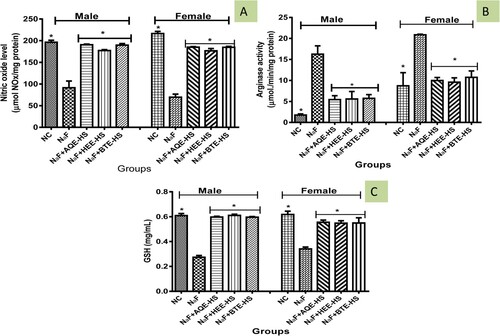
Nitric oxide level was observed to be significantly reduced (p < 0.05) in NaF-induced rats when compared with the NC and the treatment groups. However, treatment with HS (AQE, HEE, BTE) significantly increases the level of nitric oxide when compared with NaF-induced rats (Figure (A)) but shows no significant difference from the NC.
3.6. Arginase activity
In Figure (B) the activity of arginase was significantly increased (p > 0.05) in NaF-induced rats in comparison with the NC while HS (AQE, HEE, BTE) treatment reversed the increase when compared with NaF-induced rats but the activity of arginase in the female treated group shows no significant difference from the NC.
3.7. Glutathione (GSH)
Administration of NaF to rats significantly decreased (p < 0.05) the GSH level in the NaF-induced rat as compared with the NC while treatment with HS (AQE, HEE, and BTE) increased the GSH level in rats when compared with the NaF (Figure (C)).
3.8. Lipid profiles
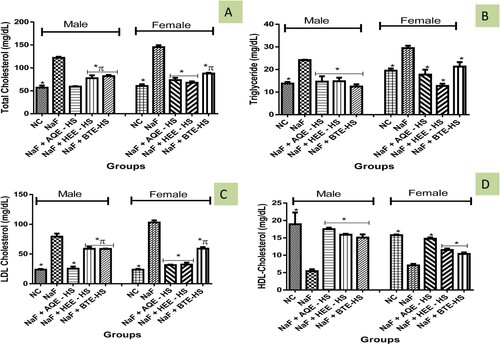
The concentration of TG, TC, and LDL-c in the group of rats induced with NaF was significantly elevated (p > 0.05) in rats when compared with the NC while the reverse was observed in HDL-c (Figure (A–D)). However, Hibiscus sabdariffa (AQE, HEE, BTE) treatment reverse the increase observed in TC, TG, and LDL-c concentration when compared with NaF-induced rats while boosting HDL-c concentration (Figure (A–D)).
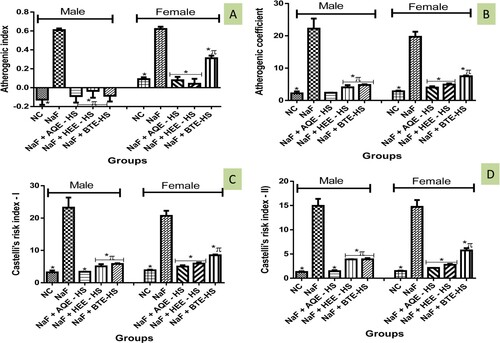
3.9. Atherogenic indices
The AI, AC, CRI-I, and CRI-II were observed to be significantly elevated in NaF-induced rats when compared with the NC. Consequently, treatment with fractions of Hibiscus sabdariffa (AQE, HEE, BTE) caused a reduction in the AI, AC, CRI-I, and CRI-II (Figure (A–D)).
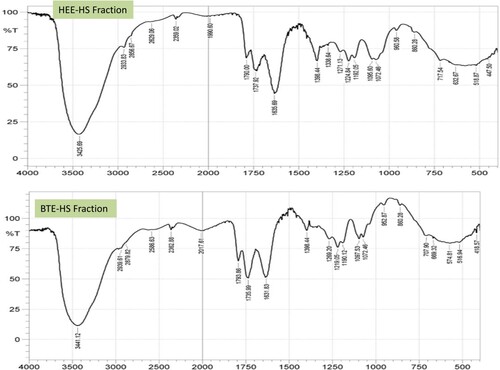
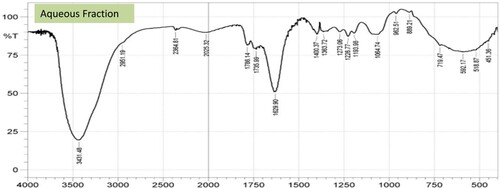
3.10. FTIR analysis
The FTIR characteristic functional group analysis carried out revealed a broad band around 3441.12, 3425.69, and 3431.48 cm−1 for BTE-HS, HEE-HS, and AQE-HS respectively, which are attributable to carboxylic acid v(O–H) stretching vibrations. IR spectra of the fractions showed stretching vibrational frequencies of 2856.67–2951.19 cm−1 and 2586.63–2629.06 cm−1 in the spectrum of HEE-HS, AQE-HS, and BTE-HS fractions assignable to the symmetric and asymmetric aliphatic ν(CH2/CH3) and thiol or a sulfhydryl group. The vibrational frequencies from 1736cm−1 to 1744cm−1 have been designated to the attachment of unsaturated oxygen of the carbonyl group of the different fractions. The vibrations ν(C–N) observed at 1219.05 cm−1 1224.84, and 1226.77 cm−1 for BTE-HS, HEE-HS, and AQE-HS respectively, are attributed to amine stretching vibrations.
Also, the presence of the ν(C–O–H) band was detected at the 1097.53, 1072.46, and 1064.75 cm−1 stretching vibrations of the BTE-HS, HEE-HS, and AQE-HS respectively (Figures and ).
4. Discussion
Daily exposure to sodium fluoride is on the increase in humans and this could be via the toothpaste usage, water purification, and pesticide production [Citation23]. The metabolism and route of excretion of sodium fluoride increase the risk of renal toxicity [Citation35]. Basha and Sujitha documented the potential of NaF in inducing cardiac toxicity, myocardial damage, and hypertension [Citation36]. The current study investigates the beneficial role of HS fractions on NaF-induced cardiac toxicity in rats.
The mechanism of sodium fluoride-induced toxicity could be linked to with oxidative stress which triggers the nuclear factor kappa B pathway [Citation23]. However, oxidative stress reflects an unevenness in the degree of oxidative species generated in the body via various mechanisms and the ability of the body to detoxify them [Citation22,Citation37]. The numerous enzymatic and non-enzymatic antioxidants in the body's defence system counteract the exposure to reactive species derived from oxygen and nitrogen [Citation38]. In this study, our results revealed that exposure to sodium fluoride led to a significant decline in the activities of catalase, glutathione, SOD, and GST which is reflective of oxidative stress. These results are in tandem with Lu et al. reports that documented the induction of oxidative stress and apoptosis in the liver of mice upon sodium fluoride administration [Citation39]. Catalase is an enzymatic antioxidant that catalyses hydrogen peroxide degradation to water and superoxide radicals. The ensuing superoxide radical is further degraded through the dismutation process by superoxide dismutase to form water and oxygen. Glutathione is a molecule with three peptides such as cysteine, glutamate, and glycine which directly act as an antioxidant or conjugate with xenobiotics. Glutathione-s-transferase is a phase two enzyme for detoxification that triggers the conjugation of glutathione to xenobiotics to make them bulkier and easy to excrete. Treatment with different fractions of H. sabdariffa leads to significant upregulation of these antioxidants in the sex of rats. These results indicate HS's potential in combating oxidative stress induced by sodium fluoride. These results are largely correlated with Ekor et al. who document the protective roles of H. sabdariffa against induced oxidative stress via chronic cholesterol administration [Citation1,Citation40].
Furthermore, lipid peroxidation occurred when the carbon–carbon double bonds contained in lipids usually polyunsaturated fatty acids (PUFAs) are destroyed by free radicals. The ensuing peroxidation products include 4-hydroxynonenal (HNE) and TBARS. Results from this study document an increased level of TBARS upon exposure of experimental animals to sodium fluoride. However, treatment with different fractions of H. sabdariffa significantly reduces the number of oxidative stress products in both sexes of rats.
Nitric oxide is a multifunctional effector molecule that modulates vascular tone and inhibits platelet and leukocyte adherence to endothelial cells, as well as maintaining vascular homeostasis. Results from this experiment reveal a significant reduction in nitric oxide upon administration of sodium fluoride. The decline in nitric oxide is in collaboration with previous studies by Miranda et al. that document the reduction in nitric oxide upon exposure to sodium fluoride in mice [Citation41]. Another closely related enzyme is arginase an enzyme that triggers the cation-dependent catabolism of arginine to produce urea and l-ornithine. Arginase competes for l-arginine, a substrate for nitric oxide and ornithine synthesis [Citation42]. Several pathological cardiovascular disorders have been linked to elevated arginase activity and expression, including hypertension, myocardial ischemia, atherosclerosis, pulmonary arterial hypertension, and congestive heart failure [Citation43]. Administration of sodium fluoride upregulates the activity of arginase as shown in this study and supported by the findings of Adetunji et al. [Citation44]. This upregulation is counteracted in rats that received HS treatments. The excessive production of thiobarbituric acid led to concomitant increased conversion of LDL-cholesterol to oxidized LDL cholesterol, a key player in the pathogenesis of plaque formation, and the enhancement of vascular smooth muscle (VSMC) migration, adhesion, and proliferation, which both ultimately result in arteriosclerosis [Citation45]. More so, excess LDL-cholesterol bioavailability increases lipid concentrations in the tissue system, which results in notable lethal conditions that also result in oxidative damage and arteriosclerosis [Citation46]. Hyperlipidemia was previously established to play a central role in the development of cardiovascular diseases and atherosclerosis [Citation47]. These elevated lipid profiles have been linked to a shortage in nitric oxide concentrations in NaF-induced rats as observed. Also, the results obtained reveal that administration of sodium fluoride leads to an up-regulation of low-density lipoprotein, triglyceride, and total cholesterol with a concomitant decline in high-density lipoprotein which is indicative of atherosclerosis. This atherosclerosis is correlated to plaque and different coronary heart diseases. Emejulu et al. reported the induction of dyslipidemia in rats by the induction of sodium fluoride [Citation48]. Omóbòwálé et al. also reported hypertension induction in rats via consecutive sodium fluoride administration for seven days [Citation49].
Our findings revealed the ability of H. sabdariffa to prevent the alteration in lipid profiles and improve HDL-cholesterol concentrations in the NaF-induced groups suggesting it anti-hyperlipidemic potential. Interestingly, the beneficial role of HDL-cholesterol has been shown to include the mopping of excess cholesterol from the tissue system into the liver and the reduction of the atherogenic index [Citation46]. Therefore, inhibition of enzymes involved in HMG-CoA reductase deactivation and cholesterol synthesis, as well as a decrease in lipogenic enzymes activities, has been implicated as a possible mechanism of action employed by H. sabdariffa to ameliorate the altered lipid profiles [Citation50]. The atherogenic index represents the levels of TG and HDL-C cholesterol and is computed as a log (TG/HDL). AIP has been utilized to quantify complete lipid levels as a reliable biomarker of dyslipidemia and atherosclerosis [Citation51]. It represents a key indicator for coronary artery disease and metabolic syndrome and has been positively correlated with the risk of developing cardiovascular diseases. Our studies document a significant elevation in the atherogenic index in NaF-induced rats, however, treatment with H. sabdariffa modulated this increase indicating the potential antilipidemic potentials of HS. These findings lend credence to that of Bule et al. that called for more investigation into the HS antilipidemic potentials [Citation52].
The Castelli risk index-I (CRI-I) is used to assess cardiac risk through the determination of coronary plaque formation with comparable diagnostic value to total cholesterol assessment [Citation53]. Sodium fluoride bioaccumulation corresponds to an increase in both Castelli index I and II. This increase indicates the potential of NaF in cardiac-related disease induction as documented in previous studies [Citation54].
The presence of carboxylic acid v(OH) in any organic substance has been documented to contribute to its high antioxidant activity [Citation55,Citation56]. Jantan et al. also established that the OH present in xanthorrhizol contributed to its high physiological mechanism [Citation57]. Therefore, the observed carboxylic functional groups in AQE-HS might contribute to the bioavailability of the fraction as well as mimicking the captopril an L-proline derivative in the attenuation of the induced stress. Furthermore, the carbonyl could also enhance the potency of the fraction, this is in support of the findings of Mansoori et al. [Citation58].
5. Conclusion
In summary, our studies revealed the cardioprotective potential of H. sabdariffa in NaF induces cardiotoxicity in rats. Induction of toxicity with NaF promotes the accumulation of TBARS, TC, TG, LDL-c, AI, AC, CRI-I, and CRI-II. However, the fraction AQE-HS suppressed cardiac biomarkers measurement and boosted the availability of HDL-c, NO, and the antioxidant GSH, catalase, SOD, and GST in the tissues when compare to the other two fractions. Moreover, the functional group characteristics identify revealed the captopril-mimicking nature of the fraction. Therefore, AQE-HS fractions significantly modulate the biomarkers of oxidative stress and lipid profile. These data obtained during this study will go a long way in sustainable production of a novel drugs derived from H. sabdariffa for effective management of cardiotoxicity and other related diseases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Najafpour Boushehri S, Karimbeiki R, Ghasempour S, et al. The efficacy of sour tea (Hibiscus sabdariffa L.) on selected cardiovascular disease risk factors: a systematic review and meta-analysis of randomized clinical trials. Phyther Res. 2020;34:329–339.
- Xu T, Ding W, Ji X, et al. Oxidative stress in cell death and cardiovascular diseases. Oxid Med Cell Longev. 2019;2019:1–11.
- D’Oria R, Schipani R, Leonardini A, et al. The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid Med Cell Longev. 2020;2020:1–29.
- Wang W, Kang PM. Oxidative stress and antioxidant treatments in cardiovascular diseases. Antioxidants. 2020;9(12):1292.
- Pahlavani N, Malekahmadi M, Sedaghat A, et al. Effects of melatonin and propolis supplementation on inflammation, oxidative stress, and clinical outcomes in patients with primary pneumosepsis: a randomized controlled clinical trial. Complement Med Res. 2022;29(4):275–285.
- Malekahmadi M, Shadnoush M, Islam SMS, et al. The effect of French maritime pine bark extract supplementation on inflammation, nutritional and clinical status in critically ill patients with traumatic brain injury: a randomized controlled trial. Phytother Res Ptr. 2021;35(9):5178–5188.
- Peoples JN, Saraf A, Ghazal N, et al. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 2019;51(12):1–13.
- Cosa P, Vlietinck AJ, Berghe DV, et al. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol. 2006;106:290–302.
- Mahomoodally MF. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid-Based Complement Altern Med. 2013;2013:617459. 14 pages.
- Pahlavani N, Rostami D, Ebrahimi F, et al. Nuts effects in chronic disease and relationship between walnuts and satiety: review on the available evidence. Obesity Med. 2019;17:100173.
- Mansouri M, Pahlavani N, Sharifi F, et al. Dairy consumption in relation to hypertension among a large population of university students: the MEPHASOUS study. Diabetes, Metab Syndr Obes Targets Ther. 2020;13:1633–1642.
- Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:35–41.
- Pahlavani N, Roudi F, Zakerian M, et al. Possible molecular mechanisms of glucose-lowering activities of Momordica charantia (karela) in diabetes. J Cell Biochem. 2019;120(7):10921–10929. doi:10.1002/jcb.28483.
- Riaz G, Chopra R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018;102:575–586.
- Lin H, Charles AL, Hsieh C, et al. Antioxidant effects of 14 Chinese traditional medicinal herbs against human low-density lipoprotein oxidation. J Tradit Complemen Med. 2014;5(1):51–55.
- Gurrola-Díaz CM, García-López PM, Sánchez-Enríquez S, et al. Effects of Hibiscus sabdariffa extract powder and preventive treatment (diet) on the lipid profiles of patients with metabolic syndrome (MeSy). Phytomedicine. 2010;17(7):500–505.
- Huang C, Hung C, Chen C, et al. Hibiscus sabdariffa polyphenol-enriched extract inhibits colon carcinoma metastasis associating with FAK and CD44/c-MET signaling. J Funct Foods. 2018;48:542–550.
- Su N, Li J, Yang L, et al. Hypoglycemic and hypolipidemic effects of fermented milk with added roselle (Hibiscus sabdariffa L.) extract. J Funct Foods. 2018;43:234–241.
- Aziz MHA, Raduan SZ, Roslida AH, et al. Anti-pyretic activity of two varieties of Hibiscus Rosa Sinensis L. Biomed Pharmacol J. 2021;14(1):61–74.
- Hopkins AL, Lamm MG, Funk JL, et al. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: a comprehensive review of animal and human studies. Fitoterapia. 2013;85:84–94.
- Rason N, Ahmad WANW, Ramli NS, et al. Therapeutic effects of Roselle extract as anti-obesity and cholesterol lowering agent on obese- hypercholesterolemic rat. Ann Microsc. 2018;17:4–12.
- Adebisi OA, Agbaje WB, Adewale OO. Modulatory efficacy of Punica granatum L. powder ethanol extract (PLEE) on lead acetate-induced hepatic and renal toxicity. Clin Phytoscience. 2022;8(1):1–9.
- Oyagbemi AA, Omobowale TO, Asenuga ER, et al. Sodium fluoride induces hypertension and cardiac complications through the generation of reactive oxygen species and activation of nuclear factor-kappa beta. Environ Toxicol. 2017;32(4):1089–1101.
- Yuliastri WO, Diantini A, Ghozali M, et al. Immunomodulatory activity and phytochemical analysis of Hibiscus sabdariffa L. flower fractions. J Appl Pharm Sci. 2021;11(11):131–140.
- Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;105:764–775.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132.
- Miranda GHN, Gomes BAQ, Bittencourt LO, et al. Chronic exposure to sodium fluoride triggers oxidative biochemistry misbalance in mice: effects on peripheral blood circulation. Oxid Med Cell Longev. 2018;2018:1–9.
- Kaysen GA, Strecker HJ. Purification and properties of arginase of rat kidney. Biochem J. 1973;133(4):779–788.
- Jollow DJ, Mitchell JR, Zampaglione N, et al. Bromobenzene induced liver necrosis. protective role of glutathione and evidence for 3, 4-bromobenzene oxide as a hepatotoxic metabolite. Pharmacol. 1974;11:151–169.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
- Dobiásová M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004;50(7):1113–1115.
- Brehm A, Pfeiler G, Pacini G, et al. Relationship between serum lipoprotein ratios and insulin resistance in obesity. Clin Chem. 2004;50(12):2316–2322.
- Ouhaddouch H, Cheikh A, Idrissi MOB, et al. FT-IR spectroscopy applied for identification of a mineral drug substance in drug products: application to bentonite. J Spectrosc. 2019;2019:2960845.
- Song GH, Gao JP, Wang CF, et al. Sodium fluoride induces apoptosis in the kidney of rats through caspase-mediated pathways and DNA damage. J Physiol Biochem. 2014;70(3):857–868.
- Basha MP, Sujitha NS. Chronic fluoride toxicity and myocardial damage: antioxidant offered protection in second generation rats. Toxicol Inter. 2011;18(2):99–104.
- Pahlavani N, Sedaghat A, Bagheri Moghaddam A, et al. Effects of propolis and melatonin on oxidative stress, inflammation, and clinical status in patients with primary sepsis: study protocol and review on previous studies. Clin Nutr ESPEN. 2019;33:125–131.
- Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants, and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126.
- Lu Y, Luo Q, Cui H, et al. Sodium fluoride causes oxidative stress and apoptosis in the mouse liver. Aging. 2017;9(6):1623–1639.
- Ekor M, Adesanoye OA, Udo IE, et al. Hibiscus sabdariffa ethanolic extract protects against dyslipidemia and oxidative stress induced by chronic cholesterol administration in rabbits. Afr J Med Medic Sci. 2010;39:161–170.
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi:10.1006/niox.2000.0319.
- Bratt JM, Zeki AA, Last JA, et al. Competitive metabolism of L -arginine: arginase as a therapeutic target in asthma. J Biomed Res. 2011;25(5):299–308.
- Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res. 2013;98(3):334–343.
- Adetunji JB, Agbolade AO, Iyoha AE, et al. Bryophyllum pinnatum inhibits angiotensin converting enzyme and arginase activities in sodium fluoride induced hypertensive rats. J Herbs Spices Med Plants. 2023:1–14. doi:10.1080/10496475.2023.2184897.
- Oyagbemi AA, Omobowale TO, Ola-Davies OE, et al. Luteolin-mediated Kim 1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. Biofactors. 2018;44(6):518–531.
- Ajeigbe OF, Oboh G, Ademosun AO, et al. Ficus asperifolia Miq-enriched biscuit diet protects against L-NAME induced hyperlipidemia and hypertension in rats. Food Front. 2022;3:150–160.
- Hassarajani S, Souza TD, Mengi SA. Efficacy study of the bioactive fraction (F3) of Acorus calamus in hyperlipidemia. Indian J Pharmacol. 2007;39:196–200.
- Emejulu AA, Alisi CS, Asiwe ES, et al. Hypolipidemic effect of Irvingia gabonensis fruits juice on sodium fluoride-induced dyslipidemia in rats. Afr J Biochem Res. 2014;8(8):151–157.
- Omóbòwálé TO, Oyagbemi AA, Alaba BA, et al. Ameliorative effect of Azadirachta indica on sodium fluoride-induced hypertension through improvement of antioxidant defense system and upregulation of extracellular signal-regulated kinase 1/2 signaling. J Basic Clin Physiol Pharmacol. 2018;29(2):155–164.
- Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts andorganosulfur compounds: human and animal studies. J Nutr. 2001;131(3):989–993.
- Fernández-Macías JC, Ochoa-Martínez AC, Varela-Silva JA. Atherogenic index of plasma: novel predictive biomarker for cardiovascular illnesses. Arch Med Res. 2019;50(5):285–294.
- Bule M, Albelbeisi AH, Nikfar S. The antidiabetic and antilipidemic effects of Hibiscus sabdariffa: a systematic review and meta-analysis of randomized clinical trials. Food Res Intern. 2020;130:108980.
- Edwards MK, Blaha MJ, Loprinzi PD. Atherogenic index of plasma and triglyceride/high-density lipoprotein cholesterol ratio predict mortality risk better than individual cholesterol risk factors, among an older adult population. Mayo Clin Proc. 2017;92(4):680–681.
- Silva Mendes BI, Oliveira-Santos M, Vidigal Ferreira MJ. Sodium fluoride in cardiovascular disorders: a systematic review. J Nucl Cardiol. 2021;28(4):1461–1473.
- Utkina NK, Makarchenko AE, Shchelokova OV, et al. Antioxidant activity of phenolic metabolites from marine sponges. Chem Nat Compd. 2004;40:373–377.
- Ntshanka NM, Ejidike IP, Mtunzi FM, et al. Investigation into the phytochemical profile, antioxidant and antibacterial potentials of Combretum molle and Acacia mearnsii leaf parts. Biomed Pharmacol J. 2020;13(4):1683–1694.
- Jantan I, Saputri FC, Qaisar MN, et al. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evid Based Complement Altern Med eCAM. 2012;2012:438356.
- Mansoori A, Singh N, Dubey SK, et al. Phytochemical characterization and assessment of crude extracts from Lantana camara L. for antioxidant and antimicrobial activity. Front Agron. 2020;2:582268.