ABSTRACT
Vaccination has emerged as the primar approach for managing the COVID-19 pandemic. Despite certain clinical trials reporting the safety and immunogenicity of CoronaVac, additional multicenter real-world studies are still necessary. In this study, we recruited 506 healthy volunteers who were not infected with COVID-19 or vaccinated. Each participant provided peripheral blood samples three times: prior to the first dose of vaccine, prior to the second dose, and 8 weeks following the second dose. Ultimately, 388 participants completed the entire follow-up process. No serious adverse events were observed among any of the participants. Within 1 week of vaccination, 13.4% of participants experienced systemic adverse reactions, with fatigue (5.93%) and dizziness (3.35%) being the most frequent. Although some clinical indicators, including creatinine, significantly changed after vaccination (p < 0.05), the mean of all altered indicators remained within the normal range. The positive rates of neutralizing antibodies (NAb), IgG, and IgM were 12.3%, 18.85%, and 5.24% prior to the second dose, respectively; and 57.99%, 86.34%, and 2.32% at 8 weeks following the second dose, respectively. Additionally, seven indicators, such as sex, age, and BMI, were significantly correlated with NAb (p < 0.05). Finally, a prediction model was developed based on age, monocytes, and alanine aminotransferase (ALT) with an AUC value of 87.56% in the train set and 80.71% in the test set. This study demonstrated that safety and immunogenicity of CoronaVac were good. The prediction model based on the baseline clinical characteristics prior to vaccination can help to develop more suitable vaccination strategies.
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory infection caused by the highly contagious and universally susceptible Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which emerged in winter 2019 in Wuhan, China [Citation1,Citation2]. As of 1 March 2023, the world has confirmed over 700 million cases and experienced over 6.85 million deaths due to COVID-19 [Citation3]. These data suggest that COVID-19 has become a significant economic burden and public health problem worldwide.
Due to the lack of effective drugs for the treatment of COVID-19, vaccination has become the primary strategy to control the pandemic. Considering the scenario, the World Health Organization (WHO) has approved several COVID-19 vaccines, including inactivated, adenoviral vector, and nucleic acid vaccines [Citation4–6]. One of the inactivated SARS-CoV-2 vaccines is CoronaVac, developed by Sinovac Life Sciences Co., Ltd. and approved for conditional marketing in China in accordance with the law on 5 February 2021 [Citation7]. Specific receptor-binding domain (S-RBD) was the main antigenic epitope of neutralizing antibodies (NAb). Specific antibodies produced against S-RBD could block the binding of SARS-CoV-2 to angiotensin-converting enzyme 2 receptor (ACE2) to prevent the virus from invading host cells [Citation8]. Therefore, evaluating the effectiveness and immunogenicity of the COVID-19 vaccine required determining the concentration and duration of neutralizing antibodies in the blood.
A randomized, phase 1/2 clinical trial in China published the safety and immunogenicity of CoronaVac [Citation4]. By further studying the details of this clinical trial, we found that it was a single-centre study with very strict exclusion criteria (>25) and many study endpoints conducted in Jiangsu Province, China. Randomized controlled clinical trials had very strict inclusion and exclusion criteria (ideal world population) to ensure research efficacy, while real-world studies have relatively broad inclusion and exclusion criteria (real world population) [Citation9,Citation10]. So, some vaccines may be not so good in real-world clinical practice after they were marketed because of the large heterogeneity. Thus, we conducted this multicenter real-world study in China.
In this study, a total of 388 healthy volunteers received 2 doses of CoronaVac and completed the entire follow-up process (from the first dose of vaccine to 8 weeks following the second dose of vaccine). The safety and immunogenicity characteristics were evaluated. Subsequently, factors affecting the concentration of NAb were screened. At last, we were the first to construct and validate a prediction model for the concentration of NAb at 8 weeks following the second dose of vaccine based on the baseline clinical characteristics prior to vaccination in China.
Methods
Study profile
This study was a prospective observational study with a CoronaVac-vaccinated population. Ethical clearance was obtained from the Ethics Committee of the First Affiliated Hospital of Zhengzhou University with an Ethical Clearance Certificate 2021-KY-0580-002. Each participant signed informed consent.
During the period of January to February 2021, three cohorts from Henan Province, China (cohort 1: Guangshan County People’s Hospital n = 203, cohort 2: The First Affiliated Hospital of Zhengzhou University n = 201, and cohort 3: Henan Provincial Chest Hospital n = 102) enrolled a total of 506 participants. Each participant provided a peripheral blood sample three times, respectively, prior to the first dose of vaccine, prior to the second dose, and 8 weeks following the second dose. Eventually, a total of 388 participants completed the entire follow-up process (). Peripheral blood samples were used for routine blood examination, liver function, kidney function and anti-SARS-CoV-2 antibodies. For the first 7 days after each dose, participants provided the systemic adverse events (e.g. dizziness, nausea, fever, fatigue) by the daily spontaneous report. In addition, safety data were collected again at 8 weeks following the second dose by the questionnaire.
Figure 1. Study design and flow diagram. A total of 506 healthy volunteers who were not infected with COVID-19 nor received any COVID-19 vaccine were enrolled. All participants received two doses of CoronaVac via intramuscular injection in the deltoid muscle, with a 4-week interval between each dose. Blood samples were collected from each participant three times: before the first dose of vaccine (time 1), before the second dose (time 2), and 8 weeks after the second dose (time 3). Ultimately, 388 participants completed the entire follow-up procedure. The blood samples were utilized for routine blood examination, as well as for assessing liver and kidney function and anti-SARS-CoV-2 antibodies. Time 1, prior to the first dose of vaccine; time 2, prior to the second dose; time 3, 8 weeks following the second dose; IgG, immunoglobulin G; IgM, immunoglobulin M.
The inclusion criteria for this study were between 18 and 59 years old. In addition, participants who have previously been infected with the COVID-19 virus or received the COVID-19 vaccine were excluded. Moreover, all participants who had comorbidities were excluded.
COVID-19 vaccination
All participants received two doses of CoronaVac (Sinovac Life Sciences Co., Ltd. Beijing, China) via intramuscular injection in the deltoid muscle, with a 28-day interval between each dose. Each injection contained 0.5 ml and included 600SU of inactivated SARS-CoV-2 antigen.
Immunoassay of SARS-CoV-2 NAb, immunoglobulin G (IgG) and immunoglobulin M (IgM)
The concentration of neutralizing antibody against SARS-CoV-2 in serum were tested through the ELISA using kits (GenScript Biotech, L00847) [Citation11]. The neutralization reactions were performed according to our previous study [Citation12], as follows: In separate tubes, we mixed the diluted Positive Control, diluted Negative Control, and the samples with the diluted HRP-RBD solution with a volume ratio of 1:1. Then, the mixture was incubated at 37°C for 15 minutes and washed four times. We added the TMB Solution and incubated the plate in the dark for 15 minutes. Stop Solution was added to each well to quench the reaction. Moreover, we read the absorbance in the microtiter plate reader at 450 nm immediately. Furthermore, quality control was as follows: The absorbance of negative control was not allowed ≤ 1.0, and the absorbance of positive control was not allowed ≥ 0.3. The neutralizing antibody inhibition rate = (1 − OD value of Sample/OD value of Negative Control) × 100%. The cut-off value of the kit was 30% (a rate < 30% was defined as negative, and a rate ≥ 30% was defined as positive).
The concentration of IgG and IgM against SARS-CoV-2 in serum were tested through the direct chemiluminometric microparticle technology using kits (YHLO Biotech, C86095M and C86095G) [Citation13]. The iFlash 3000-C chemiluminescence immunoassay analyser (Shenzhen YHLO Biotech Co., Ltd., China) was used for the test. Ten U/ml was the positive judgement value of the kit (a value >10 U/ml was defined as positive, and a value <10 U/ml was defined as negative).
Statistical analysis
T-test or Wilcoxon rank-sum test was used for comparing continuous variables between groups. The categorical variables were compared by the Chi-square test (χ2) or Fisher’s test. Univariate analysis and multivariate logistic regression analysis were used to select prediction markers and construct the prediction model. A p-value <0.05 (two-sided) was considered to indicate a significant difference.
Result
Study design and characteristics of participants
This study was a prospective observational study. A total of 506 healthy volunteers who were not infected with COVID-19 or received COVID-19 vaccine were enrolled in Henan Province, China. Each participant provided peripheral blood samples three times, respectively, prior to the first dose of vaccine (Time 1, T1), prior to the second dose (Time 2, T2), and 8 weeks following the second dose (Time 3, T3). Finally, 388 participants completed the entire follow-up process (), their data were used for subsequent analysis. The participants consisted of 270 females and 118 males, with an average age of 31.90 ± 9.16 years old and an average body mass index (BMI) of 22.31 ± 2.97 kg/m2 ().
Table 1. Alterations in routine blood examination, liver function, kidney function and anti-SARS-CoV-2 antibodies.
Safety evaluation of CoronaVac
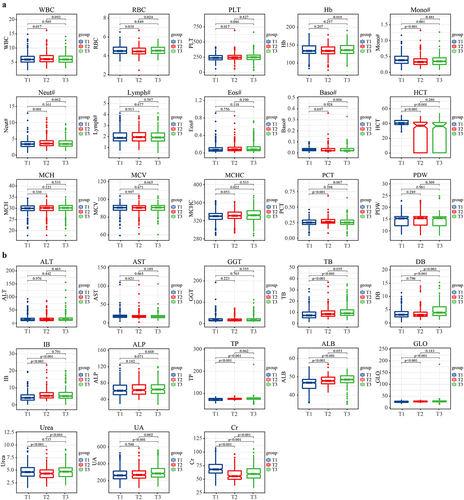
Above all, we analysed the dynamic alterations of clinical indicators such as routine blood examination, liver function and kidney function (, , Table S1) following vaccination. After the first vaccination, white blood cell (WBC, p = 0.017), platelet (PLT, p = 0.017), the absolute value of neutrophil cells (Neut, p = 0.001), total bilirubin (TB, p < 0.001), indirect bilirubin (IB, p < 0.001), total protein (TP, p < 0.001), albumin (ALB, p < 0.001), globulin (GLO, p < 0.001) significantly increased, and red blood cell (RBC, p = 0.038), the absolute value of monocytes cells (Mono, p < 0.001), haematocrit (HCT, p < 0.001), Urea (p < 0.001), creatinine (Cr, p = 0.017) significantly decreased. After the second vaccination, RBC (p = 0.024), haemoglobin (Hb, p = 0.018), total bilirubin (TB, p = 0.035), direct bilirubin (DB, p < 0.001), Urea (p < 0.001), uric acid (UA, p = 0.002), Cr (p < 0.001) were significantly increased. During the follow-up process, RBC, urea, and Cr exhibited a pattern of first decreasing and then increasing, while plateletcrit (PCT) demonstrated a pattern of first increasing and then decreasing, and TB continuously increased. Overall, PLT (p = 0.046), HCT (p < 0.001), TB (p < 0.001), DB (p < 0.001), IB (p < 0.001), TP (p < 0.001), ALB (p < 0.001), GLO (p < 0.001), UA (p < 0.001) were significantly increased, and Mono (p = 0.001), Cr (p < 0.001) were significantly decreased after vaccination. Comprehensively, although some clinical indicators have changed significantly, the mean of all the above-mentioned changed indicators were within the normal range.
Figure 2. Alterations in routine blood examination, liver function and kidney function. All participants received two doses of CoronaVac with a 4-week interval, dynamic alterations in (a) routine blood examination, (b) liver function and kidney function from the first dose of vaccine to 8 weeks following the second dose were analyzed. Overall, PLT (p = 0.046), HCT (p < 0.001), TB (p < 0.001), DB (p < 0.001), IB (p < 0.001), TP (p < 0.001), ALB (p < 0.001), GLO (p < 0.001), UA (p < 0.001) were significantly increased, and mono (p = 0.001), Cr (p < 0.001) were significantly decreased after vaccination. Comprehensively, although some clinical indicators have changed significantly, the mean of all the above-mentioned changed indicators were within the normal range. T1, prior to the first dose of vaccine; T2, prior to the second dose; T3, 8 weeks following the second dose; WBC, white blood cell; RBC, red blood cell; PLT, platelet; hb, hemoglobin; mono, the absolute value of monocytes cells; neut, the absolute value of neutrophil cells; lymph, the absolute value of lymphocyte cells; eos, the absolute value of eosinophils cells; Baso, the absolute value of basophils cells; HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; PCT, plateletcrit; PDW, platelet distribution width; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; TB, total bilirubin; DB, direct bilirubin; IB, indirect bilirubin; ALP, alkaline phosphatase; TP, total protein; ALB, albumin; GLO, globulin; UA, uric acid; Cr, creatinine.
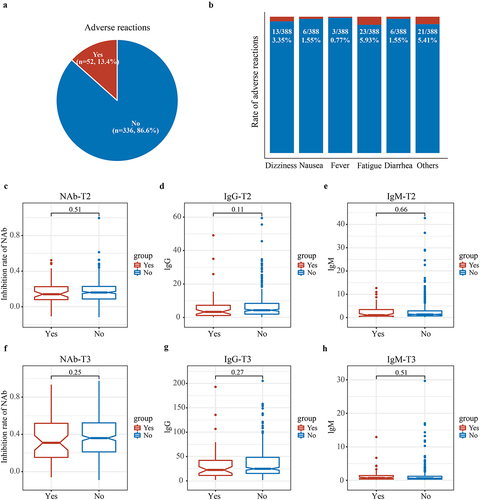
We further analysed the characteristics of systemic adverse reactions within 1 week of vaccination. The data of systemic adverse reactions including dizziness, nausea, fever, fatigue and diarrhoea were recorded and analysed. The results showed that no participants experienced serious adverse events. Fifty-two participants (13.4%) reported systemic adverse reactions within 1 week of vaccination (). Fatigue (n = 23, 5.93%) and dizziness (n = 13, 3.35%) were the most common systemic adverse reactions (). Finally, we analysed the impact of adverse reactions on anti-SARS-CoV-2 antibodies and found no significant difference in NAb, IgG, or IgM concentrations between participants who experienced adverse reactions and those who did not (p > 0.05) (). In conclusion, these findings indicated that the vaccine’s safety was good.
Figure 3. Characteristics of systemic adverse reactions within 1 week of vaccination. All participants received two doses of CoronaVac with a 4-week interval, the characteristics of systemic adverse reactions within 1 week of vaccination were analysed. (a) Fifty-two participants (13.4%) experienced systemic adverse reactions within 1 week of vaccination. (b) The most common systemic adverse reactions were fatigue (5.93%) and dizziness (3.35%). (c–h) there was no difference in inhibition rate of NAb, IgG and IgM concentrations in T2 and T3 between participants who experienced adverse reactions and those who did not (p > 0.05). T2, prior to the second dose; T3, 8 weeks following the second dose; NAb, neutralizing antibodies; IgG, immunoglobulin G; IgM, immunoglobulin M.
Immunogenicity evaluation of CoronaVac
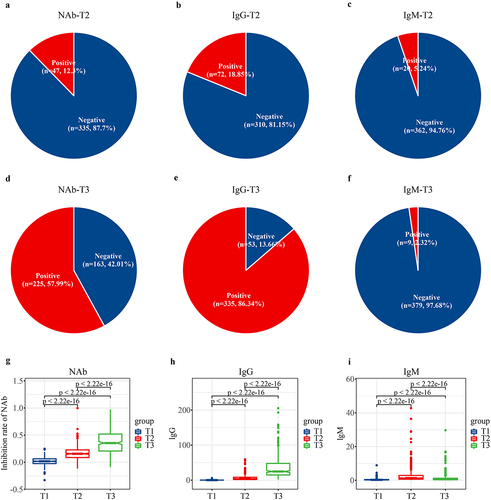
The positive rates of NAb, IgG, and IgM were 12.3% (n = 47), 18.85% (n = 72), and 5.24% (n = 20) at T2, respectively; and 57.99% (n = 225), 86.34% (n = 335), and 2.32% (n = 9) at T3, respectively (). Over time, the mean NAb inhibition rate significantly increased from 0.0156 ± 0.0692 (T1) to 0.1722 ± 0.1245 (T2) and eventually reached 0.3802 ± 0.2144 at T3 (p < 2.22e − 16) (). Moreover, the mean value of IgG significantly increased gradually from 0.3190 ± 1.3298 (T1) to 6.7665 ± 8.0270 (T2) and further to 35.2991 ± 30.7827 at T3 (p < 2.22e − 16) (). In contrast, the mean value of IgM showed an initial increase followed by a decrease ().
Figure 4. Alterations in anti-SARS-CoV-2 antibodies. All participants received two doses of CoronaVac with a 4-week interval, the dynamic alterations in anti-SARS-CoV-2 antibodies were analysed. (a–f) the positive rates of NAb, IgG, and IgM were 12.3%, 18.85%, and 5.24% at T2, respectively; and 57.99%, 86.34%, and 2.32% at T3, respectively. Alterations in the mean value of (g) inhibition rate of NAb, (h) IgG and (i) IgM following vaccination. T1, prior to the first dose of vaccine; T2, prior to the second dose; T3, 8 weeks following the second dose; NAb, neutralizing antibodies; IgG, immunoglobulin G; IgM, immunoglobulin M.
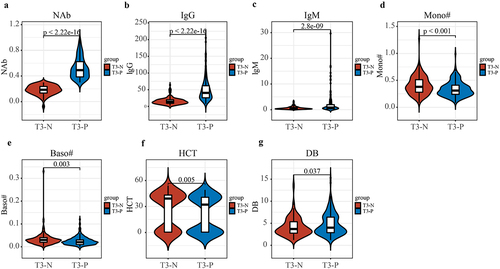
Then, the participants were divided into two groups based on their T3 neutralizing antibody concentrations: neutralizing antibody positive group (T3-P) (n = 225) and negative group (T3-N) (n = 163) (). At the same time, the concentrations of IgG (p < 2.22e − 16) and IgM (p = 2.8e − 9) significantly increased in the T3-P group compared with T3-N group (). Moreover, the concentrations of Mono (p < 0.001), the absolute value of basophils cells (Baso) (p = 0.003) and HCT (p = 0.005) were significantly decreased, and DB (p = 0.037) significantly increased in the T3-P group compared with T3-N group ().
Figure 5. Difference in clinical indicators between T3-P and T3-N group. (a–c) NAb (p < 2.22e − 16), IgG (p < 2.22e − 16) and IgM (p = 2.8e − 9) were significantly increased in the T3-P group compared with T3-N group. (d–g) mono (p < 0.001), Baso (p = 0.003) and HCT (p = 0.005) were significantly decreased, and DB (p = 0.037) was significantly increased in the T3-P group compared with T3-N group. T3-P, people with positive neutralizing antibody 8 weeks following the second dose; T3-N, people with negative neutralizing antibody 8 weeks following the second dose; NAb, neutralizing antibodies; IgG, immunoglobulin G; IgM, immunoglobulin M; mono, the absolute value of monocytes cells; Baso, the absolute value of basophils cells; HCT, haematocrit; DB, direct bilirubin.
Screening for factors affecting the concentration of NAb
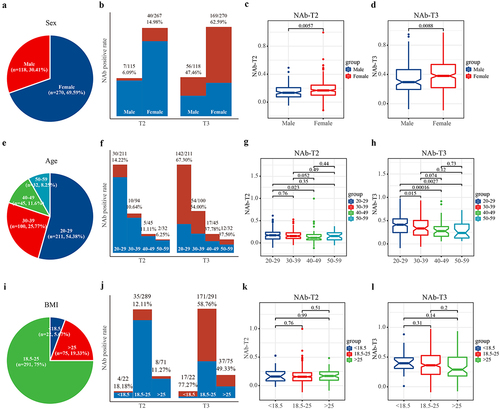
The study initially screened for demographic factors that may affect the concentration of NAb. This study included 118 males (30.41%) and 270 females (69.59%) (, Table S2). The data showed that both the positive rates and mean values of NAb in females were significantly higher than those in males at both T2 and T3 (p < 0.01) (). The analysis of the age composition of participants showed that there were 211 people aged 20–29, 100 people aged 30–39, 45 people aged 40–49, and 32 people aged 50–59 (). The youngest group, 20–29 years old, had the highest positive rate of NAb, and the NAb declined gradually with age (). The impact of BMI on NAb was evaluated next, and participants were divided into normal body weight group (BMI 18.5–25 kg/m2), high body weight group (BMI >25 kg/m2), and low body weight group (BMI <18.5 kg/m2) based on their BMI (). Results showed that the low BMI group had the highest NAb positive rate, but no significant difference in mean NAb values was observed among the three groups ().
Figure 6. The effect of sex, age and BMI on the concentration of NAb. All participants received two doses of CoronaVac with a 4-week interval, the effect of sex, age and BMI on the concentration of NAb were analysed. (a) The sex structure characteristics of this study. (b) The positive rates and (c–d) the mean of NAb in females were significantly higher than those in males (p < 0.01). I the age structure characteristics of this study. (f–h) the youngest group, 20–29 years old, had the highest positive rate of NAb, and the NAb declined gradually with age. (i) The BMI structure characteristics of this study. (j–l) the low body weight group had the highest NAb antibody rate, but there was no significant difference in the mean NAb values among the three groups. BMI, body mass index; T2, prior to the second dose; T3, 8 weeks following the second dose; NAb, neutralizing antibodies; IgG, immunoglobulin G; IgM, immunoglobulin M.
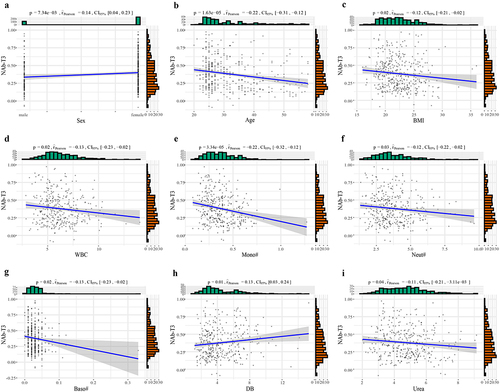
Pearson correlation analysis was performed to identify correlations of demographic and clinical indicators with neutralizing antibodies of 8 weeks following the second dose (NAb-T3). The results revealed that sex was closely associated with NAb-T3, with females showing higher NAb levels (). Age, BMI, WBC, Mono, Neut, Baso, and Urea were significantly negatively correlated with NAb-T3 (r2 < 0, p < 0.05), while DB (p = 0.01) was significantly positively correlated with NAb-T3 ().
Figure 7. The Pearson correlation of demographic and clinical indicators with NAb-T3. All participants received two doses of CoronaVac with a 4-week interval, the Pearson correlation analysis were used to assess the correlation between demographic, clinical indicators and NAb-T3. (a) Sex was closely related to NAb, with females indicating higher NAb. (b–i) age, BMI, WBC, Mono, Neut, Baso and Urea were significantly negatively correlated with NAb-T3 (r2 < 0, p < 0.05), and DB (p = 0.01) was significantly positively correlated with NAb-T3. NAb, neutralizing antibodies; T3, 8 weeks following the second dose; BMI, body mass index; WBC, white blood cell; Mono, the absolute value of monocytes cells; Neut, the absolute value of neutrophil cells; Baso, the absolute value of basophils cells; DB, direct bilirubin.
Prediction model for NAb-T3 based on the baseline clinical characteristics
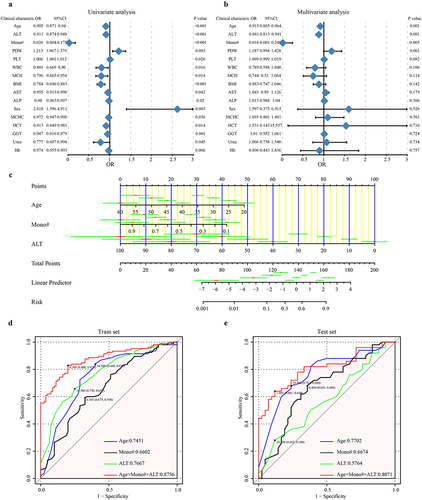
To demonstrate the predictive value of baseline clinical characteristics for NAb-T3, a logistic regression prediction model was developed in this study. After excluding 11 participants with incomplete data, the remaining 377 participants were randomly assigned to the train set (n = 185) and test set (n = 92) in a 2:1 ratio. We evaluated NAb-T3- associated factors by the univariate and multivariate cox regression analysis based on the train set (). A total of 16 factors including age, alanine aminotransferase (ALT), Mono, PLT and PLT were selected through univariate analysis (p < 0.05). After further multivariate analysis, age, ALT and Mono were finally identified as key markers to construct the prediction model (p < 0.05). The obtained model formula was as follows: NAb-T3 = − 0.11451725 * Age − 4.37138389 * Mono − 0.09409663 * ALT + 7.62116340. The nomogram based on the prediction model was presented in . In the train set, the prediction model, combining the three markers, achieved an AUC value of 87.56%, with the independent predictive potential of age, Mono, and ALT at 74.51%, 66.02%, and 76.67%, respectively (, Table S3). Furthermore, to verify the diagnostic predictive potential of the baseline clinical characteristics for NAb-T3, the test set was used for further analysis. An AUC value of 80.71% was identified based on the test set (, Table S4). Both the train set and test set achieved high AUC values which indicates a high predictive efficiency. These findings demonstrated that baseline clinical characteristics could effectively predict NAb in individuals at 8 weeks following the second dose of CoronaVac.
Figure 8. Prediction model for NAb-T3 based on the baseline clinical characteristics. All participants received two doses of CoronaVac with a 4-week interval, to demonstrate the predictive value of baseline clinical characteristics for NAb-T3, a logistic regression prediction model was developed in this study. (a) Univariate and (b) multivariate cox regression analysis based on the train set. (c) The nomogram based on the prediction model. (d) In the train set, the prediction model achieved an AUC value of 87.56%. (e) An AUC value of 80.71% was identified based on the test set. T3, 8 weeks following the second dose; NAb, neutralizing antibodies; ALT, alanine aminotransferase; Mono, the absolute value of monocytes cells; PDW, platelet distribution width; PLT, platelet; WBC, white blood cell; MCH, mean corpuscular haemoglobin; BMI, body mass index; AST, aspartate aminotransferase; ALP, alkaline phosphatase; MCHC, mean corpuscular haemoglobin concentration; HCT, haematocrit; GGT, gamma-glutamyltransferase; hb, haemoglobin.
Discussion
The development of vaccines for newly emerged pathogens often takes more than a decade. However, ongoing outbreaks and strong support from drug regulatory authorities have greatly accelerated the process of vaccine development. Currently, most COVID-19 vaccines were injected through emergency use authorizations. In China, widespread use of CoronaVac began shortly after the release of phase III clinical trial data. Therefore, although previous clinical trials have confirmed the safety and immunogenicity of CoronaVac [Citation4,Citation14–16], additional multicenter real-world studies are still needed in China.
The safety of CoronaVac has been reported in some previous studies. In clinical trials, an incidence of any adverse event of 18.9% was reported in Turkey, with no deaths or serious adverse events [Citation14]. The incidence of systemic adverse events was 17.7%, including fatigue (8.2%), myalgia (4.0%), chills (2.5%) and nausea (0.7%), and the most common local adverse event (2.4%) was injection site pain. Clinical trials in China and Indonesia also reported that most adverse reactions were mild and resolved within a few days of onset, and no vaccine-related serious adverse events were reported within 28 days of vaccination [Citation4,Citation15]. In real-world studies, Eda Celik Guzel et al. claimed that the most common adverse reactions were fatigue (24.4%), headache (23.9%), and myalgia (18.1%) [Citation17]. The incidence of adverse reactions after the first/second vaccination of 1673 medical staff in China were 15.6% and 14.6%, respectively [Citation18], and the most common systemic adverse reactions were fatigue (8.3%, 6.5%), muscle soreness (8.1%, 7.8%) and headache (6.0%, 3.4%), another Chinese population study also found similar results [Citation19]. In our study, no participants experienced serious adverse events. The incidence of systemic adverse reactions within 1 week of vaccination was 13.4%, and fatigue (5.93%) and dizziness (3.35%) were the most common systemic adverse reactions. Although some clinical indicators, including routine blood examination, liver function, and kidney function, changed significantly after vaccination, the mean of all the changed indicators remained within the normal range. In summary, CoronaVac was found to be very safe, with a low incidence of adverse events, most of which were systemic. The majority of adverse events were mild and resolved within a short period of time.
Several studies have assessed the immunogenicity of CoronaVac. In the clinical trials conducted in Turkey, 89.7% of participants were seropositive for RBD-specific total antibodies after receiving two doses [Citation14]. Results from a Chinese study showed that the seroconversion rate of NAb was 46% (3 μg group) and 50% (6 μg group) 14 days following the second dose of vaccine, and the seroconversion rate of IgG was 83% and 100%, respectively [Citation4]. Findings from Indonesia indicated that the positive rate of IgG was 99.74%, and the positive rate of NAb was 95.72%, 14 days after the second dose [Citation15]. In real-world studies, the positive rate of IgG was 98.9% one month following the second dose [Citation20]. The positive rate of IgM and IgG following the second dose were 3.1% and 74.2%, respectively [Citation21]. In our study, we observed positive rates of NAb, IgG, and IgM of 12.3%, 18.85%, and 5.24% at T2, respectively, and 57.99%, 86.34%, and 2.32% at T3, respectively. Overall, although other vaccines appeared to have higher antibody positive rates after vaccination [Citation22,Citation23], the immunogenicity of CoronaVac was also excellent to some extent.
Factors affecting the concentration of NAb have been a topic of interest in COVID-19 vaccine research. Several studies have shown that age was a critical influencing factor, with NAb concentration decreasing with age [Citation14,Citation21,Citation24–26]. Then, sex has also been identified as an important influencing factor [Citation24], and studies have found that prior to the second vaccination, all antibody-positive participants were female [Citation19]. In addition, Eda Çelik Güzel et al. reported that normal weight individuals had significantly higher antibody concentrations than overweight and obese groups, the absolute lymphocyte counts were positively correlated with peak NAb titres [Citation25]. In our study, we found that sex was closely related to NAb, with females having higher NAb. Furthermore, age, BMI, WBC, Mono, Neut, Baso, and Urea were significantly negatively correlated with NAb, and DB was significantly positively correlated with NAb. The factors we identified as affecting antibody concentrations were similar to those reported in multiple studies, further confirming that younger, non-obese women have higher antibodies. Finally, our predictive model, based on logistic regression, can accurately predict the concentration of NAb at 8 weeks following the second dose based on baseline clinical characteristics prior to vaccination. This finding has important implications for guiding vaccination.
This study had some limitations. Firstly, we only recorded systemic adverse reactions and didn’t account for local adverse reactions, which might underestimate the overall incidence of adverse reactions. Secondly, due to laboratory limitations, we only measured the concentration of neutralizing antibodies and didn’t perform a live virus neutralization assay to examine the effectiveness of the vaccine further. Thirdly, from the perspective of immunogenicity, we only analysed the alterations in antibodies. In addition to the antibody level, cellular immune response was also an important factor in the evaluation of immunogenicity. We plan to address these deficiencies in future studies.
Conclusion
In conclusion, this multicenter real-world study demonstrated that safety and immunogenicity of CoronaVac was good. The prediction model based on the baseline clinical characteristics prior to vaccination for NAb at 8 weeks following the second dose could help to develop more suitable vaccination strategies.
Ethics statement
Ethical clearance was obtained from the Ethics Committee of the First Affiliated Hospital of Zhengzhou University with Ethical Clearance Certificate 2021-KY-0580-002. Each participant signed informed consent.
Author contributions
ZGR and ZJY designed the study. BCR, LW, JYS, SSL, HYW, XMW, LL and GYC collected clinical samples. LW and CYY performed the test of routine blood examination, liver and kidney function and anti-SARS-CoV-2 antibodies. ZGR, BCR and MZY analysed the data. BCR and MZY wrote the manuscript. All authors read and approved the final manuscript.
revised supplmentary table.xlsx
Download MS Excel (301.5 KB)Acknowledgements
We thank all the generous volunteer subjects who enrolled in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original data presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2310450
Additional information
Funding
References
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–13. doi: 10.1056/NEJMoa2001017
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
- Organization WH. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update, 2020. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. 2020
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021 Feb;21(2):181–192.
- Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020 Aug 15;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6
- Polack F, Thomas S, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577
- Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020 Jul 3;369(6499):77–81. doi: 10.1126/science.abc1932
- Benton DJ, Wrobel AG, Xu P, et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020 Dec;588(7837):327–330.
- Royce TJ, Zhao Y, Ryals CA. Improving diversity in clinical trials by using real-world data to define eligibility criteria. JAMA Oncol. 2023 Apr 1;9(4):455–456. doi: 10.1001/jamaoncol.2022.7170
- Brouillette M. The FDA brings real-world data head to head with clinical trials. Nature Med. 2020 Mar;26(3):302–303. doi: 10.1038/s41591-020-0772-0
- Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020 Sep;38(9):1073–1078.
- Cui GY, Rao BC, Zeng ZH, et al. Characterization of oral and gut microbiome and plasma metabolomics in COVID-19 patients after 1-year follow-up. Mil Med Res. 2022 Jun 17;9(1):32. doi: 10.1186/s40779-022-00387-y
- Qian C, Zhou M, Cheng F, et al. Development and multicenter performance evaluation of fully automated SARS-CoV-2 IgM and IgG immunoassays. Clin Chem Lab Med. 2020 Aug 27;58(9):1601–1607. doi: 10.1515/cclm-2020-0548
- Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021 Jul 17;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X
- Fadlyana E, Rusmil K, Tarigan R, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: an interim analysis in Indonesia. Vaccine. 2021 Oct 22;39(44):6520–6528. doi: 10.1016/j.vaccine.2021.09.052
- Jara A, Undurraga EA, Gonzalez C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021 Sep 2;385(10):875–884. doi: 10.1056/NEJMoa2107715
- Guzel EC, Yildiz T, Buyukkiyici O, et al. Safety and adverse effects of inactive SARS-Cov-2 vaccine (CoronaVac) in health care professionals. J Pak Med Assoc. 2022 Sep;72(9):1792–1796.
- Zhang MX, Zhang TT, Shi GF, et al. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021 Jul;20(7):891–898.
- Zhang J, Xing S, Liang D, et al. Differential antibody response to inactivated COVID-19 vaccines in healthy subjects. Front Cell Infect Microbiol. 2021;11:791660. doi: 10.3389/fcimb.2021.791660
- Caglayan D, Suner AF, Siyve N, et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J Med Virol. 2022 May;94(5):2212–2221.
- Banga Ndzouboukou JL, Zhang YD, Lei Q, et al. Human IgM and IgG responses to an inactivated SARS-CoV-2 vaccine. Curr Med Sci. 2021 Dec;41(6):1081–1086.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577
- Halperin SA, Ye L, MacKinnon-Cameron D, et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022 Jan 15;399(10321):237–248. doi: 10.1016/S0140-6736(21)02753-7
- Cowling BJ, Wong IOL, Shiu EYC, et al. Strength and durability of antibody responses to BNT162b2 and CoronaVac. Vaccine. 2022 Jul 30;40(32):4312–4317. doi: 10.1016/j.vaccine.2022.05.033
- Guzel EC, Celikkol A, Erdal B, et al. Immunogenicity after CoronaVac vaccination. Rev Assoc Med Bras. 2021 Oct;67(10):1403–1408.
- Yildiz Y, Ozger HS, Senol E, et al. Evaluation of long-term antibody kinetics in healthcare workers vaccinated with inactivated COVID-19 Vero cell vaccine (CoronaVac), a propensity score-matched observational study. Int J Infect Dis. 2022 Sep;122:99–106.
