ABSTRACT
Subacute thyroiditis is the most common cause of painful thyroid gland diseases. It is characterized by inflammation of the thyroid gland and usually occurs after viral upper respiratory tract infections. Coronavirus disease 2019 (COVID-19) can lead to subacute thyroiditis. There are also vaccine-related subacute thyroiditis cases in the literature. Here, we describe a 67-year-old male patient developing subacute thyroiditis following COVID-19 vaccination.
Introduction
Coronavirus disease 2019 (COVID-19) caused by the virus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), declared as a pandemic by the World Health Organization (WHO) on March 11, 2020, is a systemic disease that primarily involves the upper respiratory tract and lungs. Shortly after the disease was first revealed, it was understood that the SARS-CoV-2 virus causes thromboinflammation, leading to a systemic and mortal course, and in this respect, it is much more than a simple respiratory tract infectious agent. Although vaccination for COVID-19 has been carried out in many countries, it still has a devastating effect all over the world and continues to maintain its place in the global agenda.
Since COVID-19 is a multisystemic disease, the endocrine system may also be affected. Pancreatic, pituitary, gonadal, and thyroid-related pathologies may occur. Subacute thyroiditis (SAT) cases caused by SARS-CoV-2 have been reported.Citation1 SAT is characterized by inflammation of the thyroid gland, and it usually occurs after viral upper respiratory tract infections and is a self-limiting disease.Citation2 Similar to viral infectious agents, vaccines can also trigger inflammation in the thyroid gland. Here, we describe a 67-year-old male patient developing subacute thyroiditis following COVID-19 vaccination.
Case presentation
A 67-year-old male patient was admitted to our outpatient clinic with hypertension complaints. He was hypertensive for about 10 years and was under treatment with valsartan 320 mg and metoprolol 12.5 mg due to frequent atrial extra beats for 5 years. He was using his medicine regularly, he was careful about salt consumption, and there was no medication he started to use recently. Lercanidipine was added to the treatment. After 1 week, on April 1, he applied again with a fever; he told that he measured his body temperature at 37.8°C and 38.3°C on two different days at home and his fever regressed with paracetamol. There were no infectious disease symptoms or signs in the patient’s history and physical examination. He had lost 4 kg in the last month, and his appetite was good. He described mild pain in the anterior neck and the left ear, which lasted for a day and resolved spontaneously, in the last week. Oropharyngeal and otologic examinations were normal, and there was minimal tenderness in the thyroid palpation. Blood pressure was 145/90 mmHg, and pulse was irregular at 72 beats/min. Normal sinus rhythm and atrial extra beats were detected in ECG, and heart rate was 83/min. Past examination records of the patient showed that his basal heart rate was around 45–50 beats/min.
Laboratory investigation revealed a suppressed thyroid-stimulating hormone (TSH) level, with elevated free thyroxine (FT4) and free triiodothyronine (FT3) levels (). Past medical history was unremarkable for thyroid disease, and there was no recent exposure to iodinated radiocontrast. Thyroid function tests performed 3 months ago were showing euthyroidism (). Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were high. Anti-thyroglobulin (anti-Tg), anti-thyroid peroxidase (anti-TPO), and thyrotropin receptor antibodies (TRAbs) were all negative. Ultrasound evaluation of thyroid showed reduced echogenicity and diffusely heterogeneous texture with pseudonodular areas consistent with thyroiditis ().
Figure 1. Thyroid ultrasonography shows increased dimensions of the gland and heterogeneous echotexture, with poorly defined regions of decreased echogenicity and pseudonodules consistent with thyroiditis. There are also multiple hyperechoic nodules in both the right (a) and the left (b) sides.
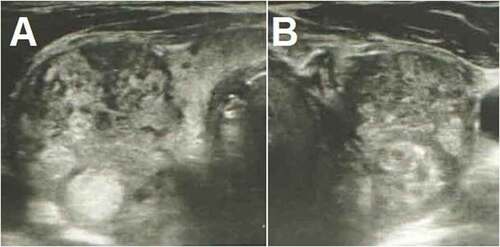
Table 1. The patient’s laboratory test results 3 months before and during the course of SAT
The patient was diagnosed with subacute thyroiditis. He did not give any viral infection history in the recent months. He also said that he complied with the social isolation precautions applied for people over the age of 65 in Turkey and did not come into contact with anyone without a mask, and there were no COVID-19 cases in his close vicinity. His nasopharyngeal swab test for SARS-CoV-2 was negative. He had had two doses of CoronaVac® (SARS-CoV-2 Vaccine (Vero Cell), Inactivated) vaccine on February 13 and March 13. The patient’s subacute thyroiditis condition was thought to be associated with the COVID-19 vaccine. When ibuprofen therapy was started (800 mg/day), fever disappeared within a few days and the patient reported marked symptomatic relief. Neck pain intermittently continued for a while, but there was not any corticosteroid requirement during follow-up. His subsequent laboratory test results are shown in . In the last evaluation on June 3, TSH was 3.15 uIU/ml, FT3 and FT4 were in the normal range, and the acute-phase reactants were completely normal. Therefore, the patient was evaluated as euthyroid and in complete remission ().
Discussion
Subacute thyroiditis, also known as De Quervain’s thyroiditis or granulomatous thyroiditis, is the most common cause of painful thyroiditis. It usually develops after upper respiratory tract infection and most often occurs at the ages of 40–50, in women. Clinically, moderate fever, diffuse myalgia, pharyngitis, and cervical pain radiating to the ears and jaw can be seen. Symptoms of thyrotoxicosis and signs of systemic inflammation may also be present. Anti-thyroglobulin and anti-thyroid peroxidase antibodies are usually negative.Citation3
In SAT, there is thyroid damage caused by the viral agent itself or the antigenic stimuli, arising from tissue damage affected by the viral infection, by binding to HLA-B35 molecules in macrophages and activating cytotoxic T lymphocytes. Unlike autoimmune thyroiditis, the immune reaction in SAT is self-limiting.Citation4 However, it has been suggested that SAT may recur in case of reexposure to the triggering agent. The recurrence rate in long-term follow-up has been reported as 4% over 6–21 years and 1.6% at 13.6 ± 5.6 years in different series.Citation5,Citation6
It is known that SAT generally develops following viral upper respiratory tract infections and cases tend to increase during virus outbreaks.Citation7 The first SAT case associated with COVID-19 was described by Brancatella et al. in March 2020.Citation8 After that, many case reports or case series continued to be reported.Citation1 With the identification of SAT cases associated with COVID-19, it has been investigated how the virus causes subacute thyroiditis. It has been shown that angiotensin-converting enzyme-2 (ACE-2) and transmembrane protease serine 2 (TMPRSS2) play a role in the entry of SARS-CoV-2 into host cells.Citation9 With the demonstration of the presence of ACE-2 and TMPRSS2 expression in thyroid gland cells, it has been shown that the thyroid may be one of the target tissues during SARS-CoV-2 viremia.Citation10,Citation11
Cases of SAT seen after influenza and hepatitis B virus vaccines have been reported in the literature.Citation12–17 It is thought that viral antigens in the vaccine may trigger inflammation in the thyroid, similar to the infectious agent.
There are various vaccines against COVID-19 that are currently being applied or under phase studies. As far as we know, only one case of SAT is reported after COVID-19 mRNA vaccine so far.Citation18 CoronaVac® is an inactive vaccine that is prepared by traditional methods and contains all the antigenic properties of the virus. SAT symptoms started in our patient after the second dose of the vaccine. The patient has complied with isolation precautions and has not had COVID-19 before. Therefore, we consider that the viral antigens in the COVID-19 vaccine triggered SAT in this case.
In our country, COVID-19 vaccination was started by healthcare professionals and then continued with the advanced age group. For this reason, it was thought that the first case of SAT associated with the COVID-19 vaccine was a male patient over 65 years of age, different from the age group and gender with classical SAT. With the widespread application of vaccination throughout the population, it is predicted that vaccine-related SAT cases may increase. Until now, only Coronavac® and Pfizer-BioNTech COVID-19 vaccine® have been used in our country. At the time our patient is vaccinated, only Coronavac® was available, so he had had no other options. We wonder if the SAT complication could occur with other types of vaccines in different patients.
We think that the incidence of SAT, associated with the SARS-CoV-2 virus, may increase with the ascending trend in COVID-19 cases and also with the increased vaccination rates. With COVID-19 reinfection or booster vaccine doses, the rate of recurrent SAT may also increase. For all these reasons, we could say that clinicians should be aware of SAT symptomatology, and it is important to have sufficient knowledge about follow-up and treatment.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the patient for allowing us to share his details.
Additional information
Funding
References
- Caron P. Thyroiditis and SARS-CoV-2 pandemic: a review. Endocrine. 2021 Mar 27;1–6. Epub ahead of print. PMID: 33774779; PMCID: PMC8000691. doi:10.1007/s12020-021-02689-y.
- Lazarus JH. Silent thyroiditis and subacute thyroiditis. In: Braverman LE, Utiger RD, editors. The thyroid: a fundamental and clinical text. 7th ed. Philadelphia (Pennsylvania): Lippincott Williams & Wilkins; 1996. p. 577.
- Rouland A, Buffier P, Petit JM, Vergès B, Bouillet B. Thyroïdites: où en est-on en 2019 ? [Thyroiditis: what’s new in 2019?]. Rev Med Interne. 2020 Jun;41(6):390–95. French. Epub 2020 Feb 24. PMID: 32107053. doi:10.1016/j.revmed.2020.02.003.
- Burman KD. Subacute thyroiditis. In: Post TW, editor. UpToDate. Waltham (MA): UpToDate. [accessed 2021 May 7].
- Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003 May;88(5):2100–05. PMID: 12727961. doi:10.1210/jc.2002-021799.
- Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, Ito M, Kubota S, Fukata S, Miyauchi A. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47(8):725–29. Epub 2008 Apr 16. PMID: 18421188. doi:10.2169/internalmedicine.47.0740.
- Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009 Jan 12;6:5. PMID: 19138419; PMCID: PMC2654877. doi:10.1186/1743-422X-6-5.
- Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after SARS-CoV-2 infection. J Clin Endocrinol Metab. 2020 Jul 1;105(7):dgaa276. PMID: 32436948; PMCID: PMC7314004. doi:10.1210/clinem/dgaa276.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr 16;181(2):271–280.e8. Epub 2020 Mar 5. PMID: 32142651; PMCID: PMC7102627. doi:10.1016/j.cell.2020.02.052.
- Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, Villani L, Magri F, Latrofa F, Chiovato L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest. 2021 May;44(5):1085–90. Epub 2020 Oct 6. PMID: 33025553; PMCID: PMC7538193. doi:10.1007/s40618-020-01436-w.
- Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020 Apr 28;9(1):45. PMID: 32345362; PMCID: PMC7186534. doi:10.1186/s40249-020-00662-x.
- Passah A, Arora S, Damle NA, Reddy KS, Khandelwal D, Aggarwal S. Occurrence of subacute thyroiditis following influenza vaccination. Indian J Endocrinol Metab. 2018 Sep-Oct;22(5):713–14. Erratum in: Indian J Endocrinol Metab. 2018 Nov-Dec;22(6):867. PMID: 30294587; PMCID: PMC6166570. doi:10.4103/ijem.IJEM_237_18.
- Altay FA, Güz G, Altay M. Subacute thyroiditis following seasonal influenza vaccination. Hum Vaccin Immunother. 2016 Apr 2;12(4):1033–34. Epub 2016 Jan 25. PMID: 26809709; PMCID: PMC4962945. doi:10.1080/21645515.2015.1117716.
- Hernán Martinez J, Corder E, Uzcategui M, Garcia M, Sostre S, Garcia A. Subacute thyroiditis and dyserythropoiesis after influenza vaccination suggesting immune dysregulation. Bol Asoc Med P R. 2011 Apr-Jun;103(2):48–52. PMID: 22111471.
- Girgis CM, Russo RR, Benson K. Subacute thyroiditis following the H1N1 vaccine. J Endocrinol Invest. 2010 Jul-Aug;33(7):506. PMID: 20671410. doi:10.1007/BF03346633.
- Hsiao JY, Hsin SC, Hsieh MC, Hsia PJ, Shin SJ. Subacute thyroiditis following influenza vaccine (Vaxigrip) in a young female. Kaohsiung J Med Sci. 2006 Jun;22(6):297–300. PMID: 16793568. doi:10.1016/s1607-551x(09)70315-8.
- Toft J, Larsen S, Toft H. Subacute thyroiditis after hepatitis B vaccination. Endocr J. 1998 Feb;45(1):135. PMID: 9625459.
- Franquemont S, Galvez J. Subacute thyroiditis after mRNA vaccine for COVID-19. Journal of the Endocrine Society. 2021;5(1 Suppl):A956–A957. doi:10.1210/jendso/bvab048.1954.

