ABSTRACT
Introduction: The Ebola Virus outbreak in Africa is believed to be one of the deadliest viral infections that causes severe hemorrhagic fever in human and nonhuman primates, which has resulted in increased mortality rates in the affected African countries. Thus, the current study mapped and quantified global research output and trends in the EBOV vaccine publications via a bibliometric analysis.
Methods: Publications about the Ebola virus vaccine were extracted from the Web of Science and Scopus databases. HistCite, Bibliometrix, an R package, and VOSviewer.Var1.6.6 were used for data mapping and analysis.
Results: A total of 541 (WoS) and 511 (Scopus) documents were included, with a cumulation of 24,611 citations in both databases. These documents were published in 141 journals in the Wos and 185 in Scopus. The USA was the most productive country with 206 (38.08%) publications in the Wos. Although the top-cited authors are from the USA, the United Kingdom, and Canada, only one author from Africa “Samai M” from the University of Sierra Leone contributed 13 publications. Meanwhile, the Journal of Infectious Diseases was the most productive (45, 8.32%) in this field.
Conclusion: The study provides insight for researchers and health policy on the trends and progress of the EBOV vaccine research and development, focusing on the hot topics, research collaboration, and research dearth that requires urgent redress to fast-track an all-inclusive EBOV vaccine development.
Introduction
The enormity of the Ebola Virus (EBOV) epidemic in Africa caused global panic as it was feared to escalate into a pandemic. The transmission and treatment option mode remains a global burden that consistently requires urgent policy and research mediation.Citation1 Thus, global unity was evident in the international collaboration to put the outbreak under control, which produced a remarkable result as evidenced by the World Health Organization’s intervention (WHO), healthcare practitioners, and extensive research.Citation2 While global intervention has entailed combating EBOV, the investigation conducted on the genomes of EBOV revealed that its species exist in five forms with geographical peculiarities, such as Sudan ebolavirus (SEBOV) and Zaire ebolavirus (ZEBOV).Citation3 Others are the Bundibugyo ebolavirus (BEBOV), Cote d’Ivoire ebolavirus (CEBOV), and Reston ebolavirus (REBOV). Although no substantial evidence has been reported on the airborne potentiality of the human body, physical contact with carrier animals, an infected person alive or diseased has been a significant threat. The interaction of the virus with the human body and transmission mode can cause death to its host.Citation4 It becomes imperative that continuous effort be made to find a lasting solution to EBOV.
More recently, clusters of EBOV disease cases were reported in Guinea, with four infected persons declared dead, which raised significant concern if another species have just reemerged.Citation5 With the total global death rate that has been attributed to Ebola, the virus still has serious potential to increase global mortality. The international evidence of the average case fatality rate of EBOV is currently estimated at 50%, fluctuating from 25% to 90% over series of outbreaks.Citation6,Citation7 Over the EBOV outbreak, there were 28,639 cases and 11,316 deaths reported between 2014 and 2016,Citation4 and the currently reported outbreak in the Democratic Republic of Congo (DRC) is the second-largest outbreak since the outbreak of Sierra Leone, and the present DRC outbreak has a record of 3317 cases and 2268 deaths as of 31 May 2021.Citation8
While the world tries to bring COVID-19 infection to a halt, the world cannot afford a global outbreak of another deadly virus that EBOV can be, given that there is a risk of reemergence in already plagued countries.Citation9 Although there are clinical treatment options available for EBOV,Citation10 the rapidity of its impact on the host body requires that the human body be resistant through the development of the vaccine. Hence, it is vital to ensure that the most susceptible population is vaccinated against the virus.
The development of the EBOV vaccine is crucial to the progress of fighting the virus. There is, however, remarkable clinical progress in the development of a recently approved vaccine. The EBOV (Zaire ebolavirus) vaccine is a replication-competent approved in December 2019, known as rVSVΔG-ZEBOV-GP Ebola vaccine (brand name Ervebo®) and manufactured by Merck is the only known vaccine and does not protect against other Ebola species.Citation11 The limitation of not having an EBOV vaccine for all species and the viruses’ mutation ability is a global and public health concern. Therefore, while acknowledging that there is a worldwide effort to produce EBOV vaccine to safeguard people from infection, it is essential to examine various research efforts, thematic analysis of popular keywords, and top country contributors to EBOV vaccine research and identify potential research shortage and global representation.
In effect, a bibliometric analysis, an approach that explored metrics and information was adopted for mapping to provide accountability for research trends and themes in EBOV vaccine research. As a bibliometric analytical tool is an emerging research approach, numerous researchers have adopted the method of adjudicating different topics.Citation11–15 The current study on the EBOV vaccine’s bibliometric analysis is to peruse research advances, account for progress and achievements, and provide policymakers and researchers research evidence and potential policy redress. The analytical output in this study may also expose the geographical non-representation toward EBOV vaccine research contribution. While EBOV remains a global threat, adopting different research methods is essential to support current evidence and usher in future policy frameworks and research.
Methods
Study design and data sources
The study adopted a statistical bibliometric analysis method to map the global research output of the EBOV vaccine. The current study focused on publications on the Web of Science (WoS) and Scopus databases. The WoS and Scopus are scholarly accessible platforms that host international publishing journals for researchers and scientists, hence no ethical approval was required for data extraction, analysis, and research presentation.
Search strategy and data extraction
The document retrieval was restricted to the terms and keywords like: “Ebola Vaccine,” “Ebola Virus Disease Vaccine,” “Ebola Vaccine,” and “ebolavirus Vaccine,” from the web of Science on 03 March, 2021. The documents’ screening was based on the “Title” occurrence of the keywords to improve the search quality. In addition, articles that are “Original Research Article or Review Articles” were considered for data analysis.
Data analysis
Bibliometric analysis software includes HistCite,Citation16 Bibliometrix, an R package,Citation17 and VOSviewerVar1.6.6 (Leiden University, Leiden, The Netherlands) tool (https://www.vosviewer.com) was used for constructing and visualizing bibliometric networks to understand citation relationships and analyze the trend of publication over time.Citation18 Bibliometric analysis of qualitative analysis was presented as median and quartile range, and analysis was performed using GraphPad Prism 5.0 software. The correlation between the citations and study variables were calculated using the Spearman correlation coefficient. P-values less than 0.05 were considered statistically significant.
Results
Characteristic of the metadata
Approximately 541 (WoS) and 511 (Scopus) documents were retrieved. Papers in the Wos were published in 141 journal sources, and Scopus 185 journal sources contributed by 50 (Wos) and 55 (Scopus) countries, and 528 (Wos) and (160 Scopus) organization-enhanced research in the Ebola vaccine. The research collaboration index shows that Wos had 5.57 and Scopus 5.5 ().
Table 1. Characteristics of the metadata of Ebola vaccine
The annual global trend of publications
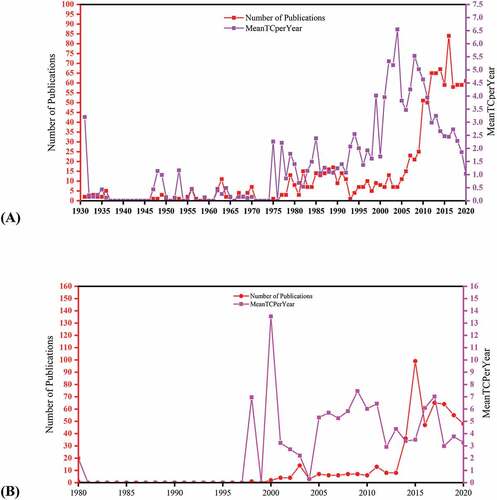
The search outcomes of annual global trends and citation scores are shown in (). About 541 (Wos) and 511 (Scopus) documents met the search criteria. The trends in EBOV vaccine research indicate that it is rapidly gaining prominence based on the recent outbreaks. The publication resources in both databases begin to rise from around 2012 and peaked in 2015.
Top 10 most cited documents
The top 10 most cited documents on the Ebola vaccine are presented in . This exposition supports the understanding of EBOV Vaccine research that gained massive attention from researchers. There is consistency in both databases on the top-cited articles on EBOV vaccine research as evident in the first four articles. The first document was published in Nature by Sullivan NJ, (2000) under the title “Development of a preventive vaccine for Ebola virus infection in primates,”Citation19 received a score of 509 citations. The second and third most cited documents in both databases were articles focusing on the efficacy and effectiveness of an rVSV-vectored vaccineCitation20 and “Live attenuated recombinant vaccine that protects nonhuman primates against Ebola and Marburg viruses.”Citation21
Table 2. Top 10 most cited documents on the EBOV vaccine
Top 10 most active authors
The top 10 authors with Author Information and Institutions, h_index, Total citation, and the number of publications are presented in . Feldmann H from the National Institute of Allergy and Infectious Diseases (NIAID), Laboratory Virology, Division of Intramural Research (NIH), Hamilton, USA, was a top ranking author with 35 articles and total citations of 2283 in Wos and 2749 Scopus. This author has 23 articles in the Wos and 26 in Scopus databases. Among the top 10 authors, only Samai M., an African author from the University of Sierra Leone, Faculty of Pharmaceut SCII, College of Medical & Allied Health Science, Sierra Leone, with 13 documents and total citations 134 times in the Wos.
Table 3. The 10 top most prolific authors on the Ebola vaccine
Most productive corresponding author’s countries
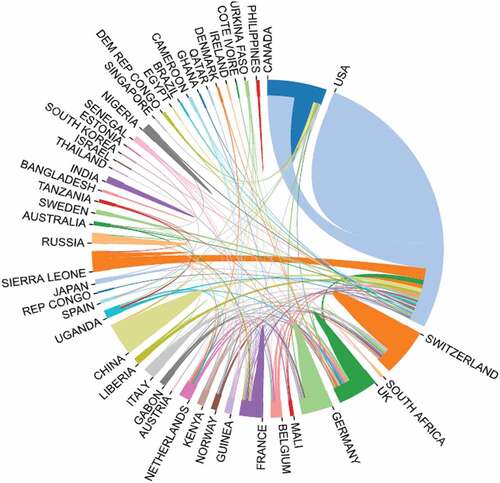
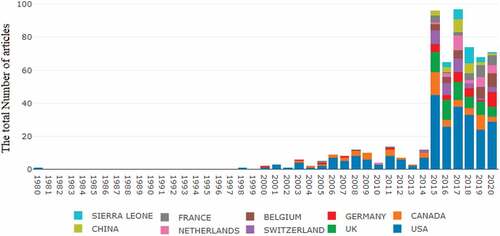
The 541 articles on Ebola vaccine research published in the WoS database were contributed by 50 corresponding author’s countries or regions and 55 countries in Scopus. Findings of the number of publications, single country publications, and multiple-country publications are presented in . In the top corresponding author country category, the leading role was maintained by authors from the United States in both Wos and Scopus databases, with 382 publications representing 65.05% Wos EBOV vaccine-related publication and 68.18% in Scopus. The United Kingdom authors had 29 publications in the Wos and ranked second while ranking third in the Scopus database with 26 publications. The representation of Nigeria in both Wos and Scopus with a combination of nine publications is of significance given the nonoccurrence of any other African country as corresponding authors. Other corresponding author’s countries notable in the top 13 categories are China, India, and Bangladesh. The map of Interstate relations of the EBOV vaccine between the countries’ cooperation indexed in the Wos is presented in . The global distribution of Ebola vaccine research per year and countries of origins contributed to EBOV vaccine-related research in the Wos is depicted in .
Table 4. Top 13 productive corresponding author’s countries, scientific impact, and international collaboration on Ebola vaccine research
Distribution of most productive and active Journals
The top 10 journals according to the number of publications, h_index, Total Citations (TC), Impact factors (IF) and 5 years impact factors (5 years IF) are presented in . The total number of published articles in the top 20 academic journals were examined to account for their contribution to publishing EBOV vaccine research Journal of Infectious Diseases was the most productive journal (45, 8.32%), followed by Lancet (38, 7.02%), vaccine (36,6.65%), and Human Vaccines & Immunotherapeutics (30,5.54%) were the top-publishing journals indexed in the Wos. The Journal of Infectious Diseases was the most productive (47, 9.2%), followed by Vaccine (36, 7.05%) in the Scopus database.
Table 5. Top 10 most productive and active journals published research on the Ebola vaccine
In the top 10 most productive journals, the Lancet had the highest impact factor (59.345) and the highest number of citations (1319) in the Wos and (1454) in Scopus. The Journal of Infectious Diseases with the highest h-index and the top most productive journal has a total citation of 961 in Wos and 1241 in Scopus. Human Vaccines & Immunotherapeutics have an h_index of 8 and 276 citations, making it the fourth top productive journal in EBOV vaccine-related publications. All publishing journals in the top 10 categories in the Wos and Scopus are publishers from the United States and England, except for “Nature” from Germany.
Subject categories & organizations-enhanced Ebola vaccine research
We classified the 541 (Wos) and 511 (Scopus) documents published in EBOV vaccine research articles into 528 (Wos), and Scopus (160) organizations enhanced the research and Twenty-three (Wos) and 25 (Scopus) subject categories in . Immunology was the subject category with the most significant number of published articles (172,31.79%) in Wos, followed by Internal General Medicine (99,18.29%) and infectious diseases (98,18.11%) among the reported subjects. National Institute of Health (NIH), USA was the top-rank organization (82,15.15%), followed by NIH National Institute of Allergy Infectious Diseases (NIAID) with (75,13.86%). Medicine is top of the list with 327 (63.99%) publications in the Scopus database.
Table 6. Top 10 categories of Wos & organizations-enhanced Ebola vaccine research
Keywords plus analysis
The occurrence of the analysis of the keywords shown the terms infection (71), hemorrhagic-fever (64), nonhuman-primates (60), double-blind (51), immunogenicity (46), protects nonhuman-primates (41), safety (40), disease (36), and immunization (35) ()). The top 10 keywords in Scopus in : Ebola vaccine (616), followed by hemorrhagic fever ebola (486), ebolavirus (456), ebola hemorrhagic fever (423), human (366), ebola vaccines (338), vaccination (329), humans (314), priority journal (273), and (272)
The conceptual structure of keywords analysis
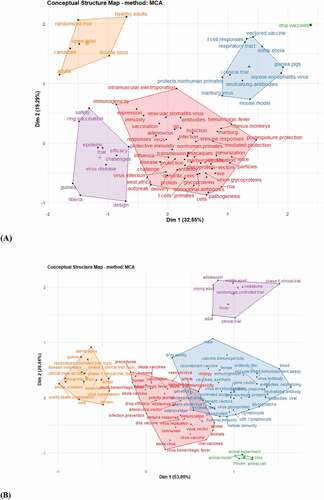
The analysis of the 100 keywords plus is distributed into 5 clusters as Cluster 1(DNA. Vaccines), cluster 2 (safety, ring.vaccination, epidemic, efficacy, challenges, Guinea, Liberia, design, and trial). Cluster 3 (t-cell responses, vectored.vaccine, lethal, Ebola, i.clinical. trial, equine encephalitis virus, neutralized antibodies, mouse model, Marburg virus, protects.nonhuman.primates), and cluster 4 include (cell, vaccination, expression vectors among others) as shown in . shows the extension of the conceptual structure of the top 100 keywords in the Scopus database.
Network visualization map
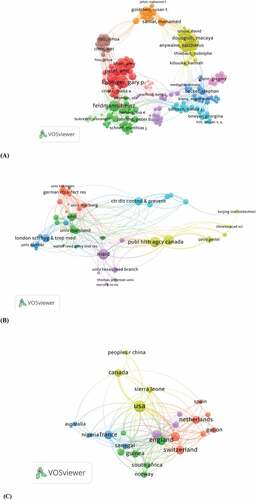
Coauthor analysis refers to establishing the relationship between items according to the number of authors based on total link strength (TLS) to establish a similar relationship between documents. The network visualization of the WoS publication is presented 6A, 6B, and 6C. Close cooperation between authors with the highest number of publications was also presented based on their links. A minimum of three documents of the author were selected, which resulted in 207 meeting the thresholds. The authors Feldmann, Heinz (TLS = 107), Patel, Ami (TLS = 75), Douoguih, Macaya (TLS = 64), among others (). A minimum of 5 documents of an organization. A total of 50 organizations meet the thresholds and are presented in the 6 clusters with (TLS = 277). Public health agency Canada (TLS = 77), World Health Organization WHO (TLS = 42), University of Penn (TLS = 31), University of Oxford (TLS = 16), among others (). Papers identified in the 50 countries were analyzed using VOSviewer. A minimum of five documents of countries were selected, and it results in 26 (TLS = 615). The USA was reported with (TLS = 229), England (TLS = 123), France (TLS = 81), Australia (TLS = 11), Canada (TLS = 59), Sierra Leone (TLS = 36), South Africa (TLS = 28), Gabon (TLS = 22), Nigeria (TLS = 14), and Spain (TLS = 9) among others ().
Figure 6. Network visualization map of country coauthorships (6A), organizations (6B), and countries (6C) indexed in WoS.
The close cooperation between authors with the highest number of publications was also presented based on their links in the Scopus database. A minimum of three documents of the author were selected, which resulted in 218 meeting the thresholds. The authors Feldmann, Heinz (TLS = 129), Koup RA (TLS = 65), and Russell JBW (TLS = 41), among others ().
Figure 7. Network visualization map of country coauthorships (6A1), organizations (6B1), and countries (6C1) indexed in Scopus database.
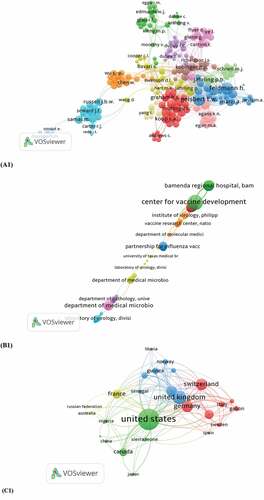
A minimum of two documents per organization and 148 organizations meets the thresholds and are presented in 11 clusters (TLS = 616). The Center for Vaccine Development (TLS = 46), followed by the Department of Medical Microbiology (TLS = 23), among others ().
Papers identified in the 50 countries were analyzed using VOSviewer. A minimum of 5 countries was selected, and it results in 26 meet the thresholds and presented into 5 clusters with (TLS = 592). The USA was reported with (TLS = 227), United Kingdom (TLS = 118), Switzerland (TLS = 96), Netherlands (TLS = 67), France (TLS = 65), Canada (TLS = 56), Sierra Leone (TLS = 21), and Nigeria (TLS = 18), among others ().
Discussion
The 20th century saw an explosion in research output across all medical and scientific fields.Citation23 EBOV, being highly pathogenic for humans and nonhuman primates and the subject of former weapons programs is now one of the most feared pathogens worldwide.Citation22 The development of the EBOV vaccine is crucial to the eradication of the virus. There is, however, remarkable clinical progress in the development of an approved vaccine. The present study provides a comprehensive overview of the global research productivity of the Ebola vaccine indexed in the WoS and Scopus databases. The results indicated that the research trend on the EBOV vaccine has increased over the years.
The top most cited document on the Ebola vaccine was published in Nature by Sullivan NJ (2000) under the title Development of a Preventive Vaccine for EBOV Infections in primates. Meanwhile, the second paper titled “Efficacy and effectiveness of an RVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomized trial,” was published by Henao-Restrepo AM (2015) in The Lancet. These two articles were the top two most cited in the two databases explored in the study.
The Journal of Infectious Diseases, Lancet, Vaccine, Human Vaccines, Immunotherapeutics and Lancet Infectious Diseases were the most productive published journals in the EBOV vaccine-related research. Both the Lancet and Journal of Infectious Diseases had the highest impact factor and the highest number of citations. The United States of America (USA), the United Kingdom, and Canada had the highest number of documents, total citations, and average citations. The USA is the leading country because the top-ranking organizations that fund EBOV vaccine research are dominated by the National Institutes of Health (NIH) and the National Institute of Allergy Infectious Diseases (NIAID) from the USA.
The highest-ranking researchers,’ analysis showed that Feldmann H from the USA was the top ranking author in WoS and Scopus publications explored. Some of his early papers highlight reviews about virus vaccine discovery,Citation24 and Lessons on Vaccine Development.Citation25 At the same time, the recent review paper was about virus, epidemiology, disease, and pathogenesis, diagnosis, patient care, treatment, vaccine, outbreak management, perspective on the future.Citation26 Simultaneously, Samai M was the only African author among the top 10 who contributed to EBOV vaccine research. In his study concerning the Sierra Leone Trial to Introduce a Vaccine Against EBOV, he studied the recombinant vesicular stomatitis virus Ebola vaccine (rVSV∆G-ZEBOV-GP) safety and efficacy.Citation27
In the present study, the published articles are indexed in many research categories in the WoS, including immunology, general internal medicine, and infectious diseases, and the Scopus had “Medicine,” Immunology, and Microbiology top two categories. However, the terms' infection, hemorrhagic-fever, nonhuman-primates, double-blind, immunogenicity, protection of non-human-primates, safety, disease, and immunization were the most common keywords in the Wos. On the other hand, in Scopus, the terms such as ebola vaccine, hemorrhagic fever ebola, and vaccination were some of the most occurring keywords. These reported keywords indicate the significant areas of interest in the EBOV vaccine and future research directions.
The bibliometric analysis highlights the global effort to speed up progress on the EBOV vaccine, which will benefit the population currently at risk of contracting the virus. Although only one EBOV vaccine has been approved in the United States, there are still several species variations and the futuristic potentiality of mutation. Thus, research efforts must continue to ensure that an effective vaccine is developed. Also, the population most vulnerable to EBOV infection is in sub-Sahara Africa, and there is significant infection reported from the region from time to time based on the ecological uncertainty of the virus.Citation28 An exposition from the current study analysis shows limited research contribution, authorship, collaboration, and funding for the EBOV vaccine from Africa. This evidence highlights a significant disadvantage from the region based on the premise that the areas most impacted by the virus outbreak.Citation29 The lack of proactiveness from clinical researchers from Africa, collaboration, and lack of available research funds may hamper efforts to abate the rate of infection and vaccine progress. Therefore, policy interventions for research and vaccine development should be encouraged in the region most affected by EBOV.
Strength and limitation
The current EBOV vaccine research analysis gives a comprehensive mapping and ‘snapshot’ of the research trends and production for documents indexed in the WoS and Scopus databases. Although it provides the reader with complete information on the research productivity and insight into EBOV vaccine research characteristics, there are few limitations to be considered. For example, the presence of false positive and false-negative results must be regarded as in any bibliometric study. Also, only two databases (Web of Science and Scopus) were used for analysis and focused only on English published documents. Other databases, such as PubMed, Google Scholar, and some foreign databases were not included in the investigation to present an all-inclusive bibliometric analysis. We also assessed the top-cited articles based on the total citation score. However, authors have self-citations that can have an impact on the overall number of citations and h-index. Besides these limitations, the study also shows that the EBOV vaccine citation increased in 2015 and provides essential insights on countries with the highest contribution to the EBOV vaccine research and African researcher’s contribution to scientific research production. The study also proposes the importance of building and sustaining research collaborations between African countries in developing the Ebola vaccine.
Conclusions
The progress in EBOV vaccine research is acknowledged given the total number of publications in the Wos and Scopus databases. These two databases are comprehensively the most widely accessible repository globally, and the extraction of publications from the two databases gives an apt insight into the global progress of EBOV vaccine research to measure research performance and milestones. Hence, this study is the first attempt to present bibliometric evidence on the international research output of EBOV vaccine research which consolidated the recent global research publication on EBOV.Citation2 In over 30 years of research on the EBOV vaccine, only one vaccine has been approved to counter the EBOV virus in Zaire Region.Citation11 Thus, there is a need to intensify vaccine development research efforts to produce more result-oriented evidence by ensuring that more vaccines are developed and approved for immediate remedial action in regions plagued with disease outbreaks, such as the ongoing outbreaks in DRC and Guinea.
Furthermore, there is a need for health stakeholders such as the WHO to intensify support through funding and engaging scientists to develop an EBOV vaccine urgently. The focus should be on developing a vaccine that can offer immune support for all EBOV variants from low-resourced African countries. This bibliometric analysis has exposed the shortage of research contributions from scientists from the affected African region, with the exception of Nigeria taking the leading role. Therefore, it becomes imperative to address this shortfall through policy redress and an increase in research funding for the underperforming African countries.
The study concludes that the Ebola vaccine’s research trends increased over the years, especially in non-human-primates, immunogenicity, and vaccine safety. In addition, the developed countries contributed more research on the Ebola vaccine compared with developing countries. Therefore, while groundbreaking EBOV vaccines that will take care of all species existing can be anticipated, global efforts must continue in clinical research, collaborations, and funding availability.
Data access
All data presented in this article can be retrieved from WoS and Scopus using keywords listed in the methodology.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author’s contributions
TYA and THM Conceived the idea and designed the study; TYA and THM: Searched and collected the data; TYA, EI, and HHM: Wrote the first draft of the manuscript; TYA and THM: Software and formal analysis; TYA, THM, HHM, EI, JK, SC and SM: Reviewed and edited the final draft. All the authors read and approved the final manuscript for publication.
Acknowledgments
The authors acknowledge the support of the Biomedical Research Institute, Darfur University College, Nyala, Sudan, and the Organization of African Academic Doctors (OAAD) for enhancing research collaboration and innovation in Africa.
Additional information
Funding
References
- Beeching NJ, Fenech M, Houlihan CF. Ebola virus disease. BMJ. [Internet] 2014; 349:1–15. doi:10.1136/bmj.g7348.
- Kawuki J, Yu X, Musa TH. Bibliometric analysis of ebola research indexed in web of science and Scopus (2010-2020). Biomed Res Int. 2020;2020:1–12. doi:10.1155/2020/5476567.
- Rewar S, Mirdha D. Transmission of Ebola virus disease: an overview. Ann Glob Heal. [Internet] 2014; 80:444–51. doi:10.1016/j.aogh.2015.02.005.
- CDC. History of Ebola virus disease (EVD) outbreaks (B) [Internet]. [ accessed 2021 Apr 10]. https://www.cdc.gov/vhf/ebola/history/summaries.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvhf%2Febola%2Foutbreaks%2Fhistory%2Fsummaries.html .
- WHO. WHO | ebola virus disease – guinea. WHO. MSF.org. [Internet] 2021. [accessed 2021 Apr 10]. http://www.who.int/csr/don/17-february-2021-ebola-gin/en/.
- Kawuki J, Musa TH, Yu X. Impact of recurrent outbreaks of Ebola virus disease in Africa: a meta-analysis of case fatality rates. Public Health [Internet] 2021; 195:89–97. [accessed 2021 Jun 2]. https://linkinghub.elsevier.com/retrieve/pii/S0033350621001396 .
- Ebola virus disease [Internet]. [accessed 2021 May 31]. https://www.who.int/health-topics/ebola#tab=tab_1 .
- Ebola DRC KIVU. Dashboard redone [Internet]; 2018. [accessed 2021 May 31. https://who.maps.arcgis.com/apps/opsdashboard/index.html#/e70c3804f6044652bc37cce7d8fcef6c .
- OMS. Technical information note: WHO recommended criteria for declaring the end of the Ebola virus disease outbreak. 2020. [accessed 2021 Apr 10]. https://www.who.int/who-documents-detail/who-recommended-criteria-for-declaring-the-end-of-the-ebola-virus-disease-outbreak .
- Bishop BM. Potential and emerging treatment options for ebola virus disease. Ann Pharmacother. 2015;49:196–206. doi:10.1177/1060028014561227.
- Ebola vaccine: information about Ervebo® | clinicians | ebola (Ebola virus disease) | CDC [Internet]. [accessed 2021 Apr 10]. https://www.cdc.gov/vhf/ebola/clinicians/vaccine/index.html .
- Yi F, Yang P, Sheng H. Tracing the scientific outputs in the field of Ebola research based on publications in the web of science. BMC Res Notes. 2016. doi:10.1186/s13104-016-2026-2.
- Chahrour M, Assi S, Bejjani M, Nasrallah AA, Salhab H, Fares MY, Khachfe HH, Bibliometric A. Analysis of COVID-19 research activity: a call for increased output. Cureus. 2020;12(3):e7357.
- Musa TH, Akintunde TY, Musa HH, Ghimire U, Gatasi G. Malnutrition research output: a bibliometric analysis for articles index in web of science between 1900 and 2020. Electron J Gen Med. 2021;18(3):em293.
- Musa TH, Akintunde TY. Global scientific research output on sickle cell disease : a comprehensive bibliometric analysis of web of science publication. Sci African. [Internet] 2021; 12:e00774. doi:10.1016/j.sciaf.2021.e00774.
- Garfield E, Paris SW, Stock WG. HistCiteTM: a software tool for informetric analysis of citation linkage. Information-wiss und Prax. 2006;5:1143–1153.
- Dervis H. Bibliometric analysis using bibliometrix an R package. J Scientometr Res. 2019;8(3):156–160.
- Van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. doi:10.1007/s11192-009-0146-3.
- Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–09. doi:10.1038/35046108.
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. [Internet] 2017; 389:505–18. [accessed 2021 May 31].
- Jones SM, Feldmann H, Ströher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi:10.1038/nm1258.
- Feldmann H, Jones S, Klenk H, Schnittler H. Ebola virus: from discovery to vaccine. Nature Reviews. Immunology. 2003;3(8):677–85. doi:10.1038/nri1154.
- Musa TH, Musa IH, Osman W, Campbell MC, Musa HH. A bibliometric analysis of global scientific research output on Gum Arabic. Bioact Carbohydrates Diet Fibre. 2021;25:100254. doi:10.1016/j.bcdf.2020.100254.
- Traf U, Sullivan NJ, Sanchez A, Rollin PE, Yang Z, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–609.
- Feldmann H, Feldmann F, Marzi A. Ebola: lessons on vaccine development. Annu Rev Microbiol. 2018;72:423–46. doi:10.1146/annurev-micro-090817-062414.
- Feldmann H, Sprecher A, Geisbert TW. Ebola. N Engl J Med. 2020;382:1832–42. doi:10.1056/NEJMra1901594.
- Schuchat A, Seward JF, Goldstein ST, Mahon BE. Comment: the Sierra Leone trial to introduce a vaccine against Ebola (STRIVE). J Infect Dis 2018;217:S1–5. doi:10.1093/infdis/jix665.
- Jacob ST, Crozier I, Fischer WA, Hewlett A, Kraft CS, De la Vega MA, Soka MJ, Wahl V, Griffiths A, Bollinger L, et al. Ebola virus disease [Internet]. Nature Rev Dis Primers. 2020;6:13. doi:10.1038/s41572-020-0147-3.
- Coltart CEM, Lindsey B, Ghinai I, Johnson AM, Heymann DL. The Ebola outbreak, 2013–2016: old lessons for new epidemics. Philos Trans R Soc B Biol Sci. 2017;372(1721):2013–16. doi:10.1098/rstb.2016.0297.