ABSTRACT
The evidence that BCG (bacille Calmette-Guerin) vaccine may increase the ability of the immune system to fight off pathogens other than tuberculosis has been studied in the past. This nonspecific immunity gained our interest, especially after initial reports of less cases in countries with universal BCG vaccination. In hopes of possible protective immunity, all staff of the Emirates International Hospital (United Arab Emirates) were offered a booster BCG vaccine in early March 2020. All the hospital staff were then tested for Covid-19 infection by the end of June 2020. We divided the subjects into two groups: booster vaccinated versus unvaccinated. The rate of Covid-19 infection was compared between the groups. Criteria included all staff who were offered the vaccine. Seventy-one subjects received the booster vaccination. This group had zero cases of positive COVID 19 infection. Two hundred nine subjects did not receive the vaccination, with 18 positive PCR confirmed COVID 19 cases. The infection rate in the unvaccinated group was 8.6% versus zero in the booster vaccinated group (Fisher’s exact test p-value = .004). Our findings demonstrated the potential effectiveness of the booster BCG vaccine, specifically the booster in preventing Covid-19 infections in an elevated-risk healthcare population.
BCG (bacille Calmette-Guerin) is a vaccine for M. tuberculosis infection (TB) both pulmonary and more so extra-pulmonary forms. It contains a live, attenuated form of Mycobacterium bovis strain and only used in countries where prevalence of TB is high. With initial reports of less cases and a lower death toll of Covid-19 in countries with universal childhood BCG vaccination, attention was turned to the nonspecific effects (NSE) of this vaccine and how it may boost the immune response against Covid-19.Citation1,Citation2 Booster BCG vaccines have been shown to have benefit against respiratory tract infections particularly in the elderly.Citation3 These NSE have also been reported in other vaccines such as MMR.Citation4,Citation5 The Emirates International Hospital (EIH) functions as one of the centers for Covid-19 testing in the region as well as treating infected patients, putting the hospital staff at an elevated risk. In the first half of March 2020, at the upslope of the Covid-19 curve in the UAE, a decision was made by the EIH Covid-19 safety committee to offer a booster BCG (Serum Institute of India PVT. LTD. Hadaspar India) vaccination to the hospital staff. The prospect of increasing community infection, elevated risk to the hospital staff, and the available evidence about the BCG vaccine and potential NSE against Covid-19 was taken into consideration. After thorough information about the vaccine and its prospects were provided to the hospital staff, voluntary vaccination was started. Out of 280 total staff, 71 received the vaccine in the span of 2 weeks. Compulsory government-mandated Covid-19 testing with PCR (CITOSWAB® nasopharyngeal swab) in the months of April, May, and June was done on all hospital staff. Testing was also was done in the setting of contact with positive patients and staff symptoms. The results of BCG immunity against Covid-19 infections were studied retrospectively.
After approval by the hospital review board and ethics committee, a retrospective cohort study of the entire hospital personnel was done and they were divided into two groups: those who received the BCG booster vaccine in March (group A), and those who did not (group B). Using a two-tailed Fisher’s exact test, the probability of testing positive in each group was compared. Live, attenuated BCG vaccine (Serum Institute of India PVT., LTD.) was used for all booster vaccinations.
Hospital staff were of Arabic, Indian, East Asian, African, and European origin. They were aged between 21 and 80 years and included clinical and non-clinical staff such as the receptionists, nurses, and physicians. In those who chose to receive the vaccine, 19% were physicians, 17.7% nurses, 3.8% nursing assistants, 7.6% receptionist, and 41.8% administration staff. In all, the staff of EIH is composed of 15.4% physicians, 12.6% nurses, and 72% other staff. By June 24th, 2020, 18 cases of SARS-cov-2 infection were documented in the hospital staff (). Group A included 71 individuals who received the BCG booster, and none tested positive for SARS-cov-2 infection. Group B, which included 209 non-booster vaccinated staff, had 18 positive PCR COVID-19 cases (), 13 of which were symptomatic and 5 had no symptoms. The infection rate was 18/209 or 8.6% in Group B, and zero in Group A. No reports of local or systemic complications was noted with the BCG booster vaccine group. The difference was statistically significant, with a p-value of .004.
Table 1. Outcomes in study cohort, P = .005
Figure 1. BCG booster vaccine was given to 71 subjects (Group A) in the first half of March 2020. 209 subjects opted out of the vaccine (Group B). Group A continued to have no positive cases through June 17, roughly 3 months after receiving the BCG booster. Group B’s first positive case was April 18. *Dates where two subjects tested positive included May 12, May 22, May 27, May 30, and June 11.
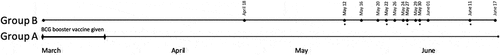
Both groups A and B had the BCG vaccine administered at birth; however, no information was available about the exact brand of the BCG vaccine that were used. None of the Covid-19 positive staff had reported any high risk activity outside the hospital, infected household, or travel prior to their positive test. The positive Covid-19 subjects did not demonstrate any particular departmental and location distribution compared to the not infected. There was no collected data on hospital staff comorbidities and other medications used in either group.
Certain vaccines such as BCG, measles, and oral polio may have beneficial NSE beyond the pathogens they had been intended for.Citation6 The epidemiological evidence that BCG may increase the ability of the immune system to fight off pathogens other than TB has been studied in the past, receiving mixed reviews initially and then a positive review by the World Health Organization.Citation7,Citation8 Such NSEs are thought to be due to not only T cell–mediated adaptive immunity but also induction and enhancement of innate immunity or “trained immunity.”Citation7,Citation9 This is in part thought to be due to epigenetic histone modification effects on macrophages and other cells of the innate immune system. It has been shown that the positive effect of the BCG vaccine given at birth or to children diminishes over time which likely indicates declining central memory immunity. It has also been shown that a booster vaccine is needed to maintain the T cell response and antigen-specific central memory to battle TB infections years after the initial vaccine.Citation10,Citation11 In adults in whom the effects of early BCG vaccination are weakened, a booster is likely to increase the effect in the role against COVID-19. A study by Kelleni et al. concluded that BCG early in life alone is highly unlikely to be an effective tool against COVID-19.Citation12 All our 280 hospital staff (Group A and B) were vaccinated with BCG at birth. The lack of Covid-19 infection in our recently vaccinated versus the high rate of infection in the non-recently vaccinated group, suggest stronger protective effect of booster BCG vaccination compared to birth vaccination.
Epidemiological studies have shown that countries that give their population BCG vaccine compared to those that do not have lower rates of COVID-19 infection and lower mortality. This can be difficult to interpret as there are certainly variables and differences between nations that cannot be controlled.
Two recent review articles provided an effective overview of current literature and understanding of BCG and COVID-19.Citation13,Citation14 They review early epidemiological studies, as well as provide an overview of ongoing clinical trials, and importantly conclude that the results of these ongoing clinical trials will be important in confirming data from ecological studies and small observational studies such as this one. Trained immunity induced by vaccines such as BCG may be an important bridge before more widespread availability of specific vaccines, possibly an effective adjunct alongside specific vaccines, and likely an important tool in future viral pandemics.
With lack of clinical data on the effectiveness of BCG vaccine in preventing Covid-19 infection, the decision to offer the vaccine to the hospital staff was based on available population based evidence at the time, elevated risk of death and morbidity with the spread of Covid-19 amongst the staff, as well as proven safety of BCG vaccine over the years. This is a relatively small population sample who all work in the same environment with elevated levels of exposure to Covid-19 compared to the general population of the Al-Ain region of the UAE. None of the hospital staff who were booster BCG vaccinated tested positive with Covid-19 versus a 8.6% infection rate amongst those who were not recently vaccinated with a booster. There was similar universal personal protective equipment policy throughout the hospital with no specific pattern of infection correlating to the location of work in the hospital. Thus, we assumed that any discrepancy of exposure level is minimized between the two groups.
Our results are not only supported by population-based findings on the protective role of BCG vaccination on Covid-19 infection,Citation1,Citation2,Citation15 but recent published data on the effect of BCG in the clinical setting. A retrospective observational study by Rivas et al. showed that in a Los Angeles hospital, BCG-vaccinated healthcare providers had a lower rate of Covid-19 diagnoses and seropositivity compared to those were who unvaccinated. They found this decrease in seroconversion effect only in BCG but not meningococcal, pneumococcal, or influenza vaccination.Citation16,Citation17
The limitations of our study are several, such as lack of clear understanding of any confounding factors between the two groups that could have influenced the transmission and infection rate as well as discrepancy between the number of subjects in the two groups. Despite the limitations of this study, we feel that our findings of 8.6% versus zero percent infection rate is significant enough to suggest the promising effectiveness of an up-to-date BCG booster vaccine in prevention of Covid-19 infection.
It is important to note that despite the statistically significant clinical evidence presented here, recommending mass BCG vaccination before prospective trials is an overreach and could create a false and dangerous sense of confidence in vaccine recipients. The health authority in many countries have recommended a booster BCG vaccine to prevent TB infection.Citation18 Some physicians may consider providing voluntary booster BCG vaccination, especially in areas of substantial Covid-19 outbreak in which tuberculosis is already prevalent. If such decision is made, it should be in accordance to the local ethical medical practice principles, and with full disclosure of treatment and preventative options, short term and long term side effects, with no misleading or deceptive promises made to patients. Adverse effects of BCG vaccine are rare local reactions that are temporary, and extremely rare serious complication of disseminated BCG infection. BCG vaccination should not be given during pregnancy, to persons who are immunosuppressed, or who are likely to become immunocompromised.Citation19
Several studies, in particular prospective controlled clinical trials, are ongoing which may or may not corroborate this study’s findings. The results of these prospective clinical trials are therefore invaluable to our understanding of the role of BCG vaccine, and its ability to elicit nonspecific trained immunity, on COVID-19 but potentially future emerging pathogens.
Our findings are the first and only one to date to demonstrate possible induced NSE immunity to SARS-cov-2 infection using a booster BCG vaccine in a clinical setting, supporting previous such small studies. Much is still unknown about the current COVID-19 vaccines, and substantial evidence suggests that the BCG vaccine inversely relates to COVID-19 severity and possibly infection rates. The ongoing clinical trials being conducted in several nations will provide further clarity to the role of how BCG can be used in this pandemic, as well as how this and other such live attenuated vaccines could play in a role in future pandemics especially in low- and middle-income countries where the infrastructure and access to BCG already exists.
Contributorship statement
Dr. Bardia Amirlak, as the guarantor, accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosure of potential conflicts of interest
All authors have no competing interests or conflicts of interests to report.
Data sharing statement
Data are available upon reasonable request, including deidentified participant data available from the authors.
Additional information
Funding
References
- Berg MK, Yu Q, Salvador CE, Melani I, Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci Adv. 2020 Aug 5;6(32):eabc1463. doi:10.1126/sciadv.abc1463. PMID:32923613; PMCID:PMC7457335.
- Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020;2020.03.24.20042937.
- Wardhana DEA, Sultana A, Mandang VV, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43:185–90.
- Lopez-Martin I, Andres Esteban E, Garcia-Martinez FJ. Relationship between MMR vaccination and severity of Covid-19 infection. Survey among primary care physicians. Med Clin (Engl Ed). 2021;156:140–41.
- Marakasova E, Baranova A. MMR vaccine and COVID-19: measles protein homology may contribute to cross-reactivity or to complement activation protection. mBio. 2021;12. doi:10.1128/mBio.03447-20.
- Higgins J, Soares-Weiser K, Reingold A. Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. WHO Rt, ed. Report to WHO Report to WHO 2014.
- Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, Van Crevel R, Rimmelzwaan GF, Pickkers P, Netea MG. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J Infect Dis. 2015;212:1930–38. doi:10.1093/infdis/jiv332.
- Higgins JP, Soares-Weiser K, Lopez-Lopez JA, Kakourou A, Chaplin K, Christensen H, Martin NK, Sterne JAC, Reingold AL. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi:10.1136/bmj.i5170.
- Netea MG, Van Crevel R. BCG-induced protection: effects on innate immune memory. Semin Immunol. 2014;26:512–17. doi:10.1016/j.smim.2014.09.006.
- Gupta N, Garg S, Vedi S, Kunimoto DY, Kumar R, Agrawal B. Future path toward TB vaccine development: boosting BCG or re-educating by a new subunit vaccine. Front Immunol. 2018;9:2371. doi:10.3389/fimmu.2018.02371.
- Whittaker E, Nicol MP, Zar HJ, Tena-Coki NG, Kampmann B. Age-related waning of immune responses to BCG in healthy children supports the need for a booster dose of BCG in TB endemic countries. Sci Rep. 2018;8:15309. doi:10.1038/s41598-018-33499-4.
- Kelleni MT. BCG vaccination potential for COVID-19: an analytical approach. Hum Vaccin Immunother. 2021;1–3. doi:10.1080/21645515.2021.1885281.
- Gonzalez-Perez M, Sanchez-Tarjuelo R, Shor B, Nistal-Villan E, Ochando J. The BCG vaccine for COVID-19: first verdict and future directions. Front Immunol. 2021;12:632478. doi:10.3389/fimmu.2021.632478.
- Koneru G, Batiha GE, Algammal AM, Mabrok M, Magdy S, Sayed S, AbuElmagd ME, Elnemr R, Saad MM, Abd Ellah NH, et al. BCG vaccine-induced trained immunity and COVID-19: protective or bystander? Infect Drug Resist. 2021;14:1169–84. doi:10.2147/IDR.S300162.
- Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci. 2020;117:17720–17726.
- Netea MG, van der Meer JW, Van Crevel R. BCG vaccination in health care providers and the protection against COVID-19. J Clin Invest. 2021;131:e145545. doi:10.1172/JCI145545
- Rivas MN, Ebinger JE, Wu M, Sun N, Braun J, Sobhani K, Van Eyk JE, Cheng S, Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021;131:e145157. doi:10.1172/JCI145157.
- Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012. doi:10.1371/journal.pmed.1001012.
- BCG vaccines: WHO position.

