ABSTRACT
Worldwide, rotavirus (RVA) and norovirus are considered major etiological agents of acute gastroenteritis (AGE) in pediatric population admitted to hospitals. This study describes the investigation of nosocomial infections caused by emergent RVA and norovirus strains reported at a pediatric hospital in southern Brazil in May 2019. This outbreak affected 30 people among children and adults. Nine stool samples (eight children and one nurse) were obtained and analyzed by RT-qPCR to detect and quantify RVA and norovirus. Positive samples were genotyped by sequencing and subjected to phylogenetic analysis. We detected RVA in 44.4% (4/9) and norovirus in 55.5% (5/9) at high viral loads, ranging from 3.5 × 107 to 6.1 × 107 and 3.2 × 102 to 3.2 × 109 genome copies/g of stool, respectively. Co-infections were not observed. RVA VP4 and VP7 gene sequencing in combination with polyacrylamide gel electrophoresis identified the circulation of equine-like G3P[8] DS-1-like, and the partial sequencing of the other nine genes revealed that strains possessed I2-R2-C2-M2-A2-N1-T2-E2-H2 genotype background. The emergent recombinant norovirus variant, GII.4 Sydney[P16], was identified by ORF1-2 sequencing. Active surveillance and effective prevention measures should be constantly reinforced to avoid the spread of nosocomial viral infections into hospitals, which could severely affect pediatric patients admitted with underlying health conditions.
Viruses are the main cause of nosocomial enteritis in infants,Citation1 and rotavirus (RVA) and norovirus are considered major etiological agents responsible for acute gastroenteritis (AGE) in children admitted to hospital.Citation2 The role in nosocomial infections of RVA and norovirus has been demonstrated, but remains poorly investigated.Citation3 In Brazil, childhood diarrhea deaths and hospital admissions have declined in 52.5% until 2018 since the introduction of RotarixTM vaccine in 2006.Citation4 Unusual combinations of RVA and norovirus have been detected in several countries. More recently, the equine-like G3P[8] DS-1-like rotavirus strain,Citation5–8 and the recombinant GII.4 Sydney[P16] norovirus have been associated with an increased number of AGE cases and outbreaks worldwide, especially in closed and semi-closed settings, such as cruise ships, schools and hospitals.Citation9–11
Nosocomial infections are of critical relevance once affects inpatients with underlying conditions affecting their vulnerable condition. In Brazil, the investigation of AGE in hospital settings is rare, especially linked to enteric viruses’ pathogens.Citation12 Here, our goal was to describe a nosocomial AGE outbreak simultaneously caused by two emergent enteric viruses that occurred among hospitalized children and adults in a pediatric hospital in southern Brazil. We identified and genetically characterized RVA and norovirus strains detected in the stool specimens of symptomatic inpatient children.
On May 6th, 2019, the Surveillance Epidemiology System of a pediatric hospital located in Rio de Janeiro, Brazil, was notified of an ongoing AGE outbreak. Stool samples with clinical medical records were sent to the Laboratory of Comparative and Environmental Virology, Fiocruz that houses the Regional Rotavirus Reference Laboratory (RRRL), Brazilian Ministry of Health (MoH). Samples were manipulated anonymously. Patient identifiers including personal information (name and address) and hospitalization number were removed to protect patient confidentiality. This study was conducted within the scope of the RRRL/MoH, as part of a federal public health policy for Rotavirus control and prevention in Brazil. For the reasons, Fiocruz Ethical Committee approval (CAAE: 94144918.3.0000.5248) was obtained without the requirement of an informed consent.
Viral RNA was extracted from 140 µl of the supernatant using QIAamp® Viral RNA Mini kit (QIAGEN, Valencia, CA, USA). A TaqMan®-based quantitative one step RT-PCR (RT-qPCR) was used for viral detection and quantification. Primers and probes targeting the conserved NSP3 gene segment for RVACitation13 and targeting the norovirus conserved genome ORF1/2 junction regionCitation14 were used as previously described.
For genetic characterization by nucleotide (nt) sequencing, RVA- and norovirus-positive samples were subjected to conventional RT-PCR followed by Sanger sequencing of purified amplicons. RVA VP7 and VP4 genes were amplified using consensus primers of 9Con1L/VP7R-DEG and 4Con3/4Con2 generating amplicons of 896 base pairs (bp) and 889 bp, respectively.Citation15 In addition, two samples were subjected to Sanger sequencing to amplify the other nine gene segments. The primers used for partial amplification of NSP1-5 and VP6 genes were GEN_NSP1F/GEN_NSP1R, GEN_NSP2F/GEN_NSP2R, GEN_NSP3F/GEN_NSP3R, GEN_NSP4F/GEN_NSP4R, GEN_NSP5F/GEN_NSP5R and GEN_VP6F/GEN_VP6R, respectively,Citation16 generating amplicons of 1581 bp for NSP1, 1059 bp for NSP2, 1104 bp for NSP3, 750 bp for NSP4, 659 bp for NSP5 and 1356 bp for VP6. The partial characterization of VP1-3 genes was performed using primers according to Varghese et al.Citation17 generating amplicons of 686 bp for VP1, VP2 and 702 bp for VP3.
In addition, RVA dsRNA segment migration profiles were analyzed by polyacrylamide gel electrophoresis (PAGE).Citation7,Citation18 For norovirus dual genotyping, amplification was performed targeting the ORF1/ORF2 overlap as previously described by Cannon et al.Citation19
PCR amplicons were purified using the ExoSAP-IT PCR Product Clean-up kit (ThermoFisher Scientific), and sequencing reactions were performed using both forward and reverse primers with the ABI Prism Big DyeTM Terminator V. 3.1 Cycle Sequencing Ready Reaction Kit™ on an ABI Prism 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) by Genomic Platform of DNA sequencing PDTIS/Fiocruz. Chromatogram analysis and consensus sequences were obtained using Geneious Prime 2020.1.2 (Biomatters Ltd, Auckland, New Zealand), and phylogenetic trees were constructed in MEGA X v. 10.1.7,Citation20 with reference sequences obtained from the National Center for Biotechnology Information (NCBI) database. The nt sequences in this study were deposited in the GenBank database under the accession numbers: MT062416 to MT062419; MT063060 to MT063067 and MZ361321 to MZ361337.
From May 3rd to 12th 2019, an AGE outbreak occurred in a hospital located in Rio de Janeiro. In total, the outbreak involved 30 people: 21 inpatient children less than 5-y old from the same ward (pediatric and infectious unit), seven hospital staff members and two accompanying mothers. From the total cases, nine stool samples were obtained; eight samples from inpatient children and one from a staff nurse. RVA was detected in 44.4% (4/9) of stool samples. From these, three samples were from inpatient children and one from the staff nurse (). Norovirus was detected in 55.5% (5/9), all from hospitalized children (). Patients enrolled in the study were aged between 3 months and 1 y, and were admitted to the Pediatric and Infectious Unit for a diagnosis other than gastroenteritis, where diarrhea (liquid stool, with or without fever) and vomit were the major symptoms observed (). Evaluating RVA and norovirus RNA shedding, we detected a broad range of viral loads, varying from 1.3 × 107 to 4 × 108 GC/g and from 3.2 × 102 to 3.2 × 109 GC/g of stool, respectively ().
Table 1. Epidemiological and clinical features of patients involved in diarrhea outbreak at a pediatric care hospital in Rio de Janeiro, Brazil, 2019
Table 2. Viral load and genotyping of the patients involved in an outbreak of RVA and norovirus associated with gastroenteritis at an infirmary of pediatrics and intensive care unit in Rio de Janeiro, Brazil, 2019
RVA VP4 and VP7 genes sequencing demonstrated that the isolates clustered into P[8] lineage 3 and G3 lineage 1, respectively, that comprises the equine-like G3P[8] human strains.Citation5–8,Citation21,Citation22 The sequences share 99.5% to 99.7% of nucleotide (nt) identity with previously reported G3P[8] strains from Brazil (KX469407, KX469400, MH569776 and MH569778), Spain (KU550297), Italy (MK158256), Hungary (KU870415), Australia (KU059782), Germany (KX880414) and Indonesia (LC260217) (). The phylogenetic analysis of the other genes from two samples revealed that the strains exhibited an I2-R2-C2-M2-A2-N1-T2-E2-H2 genotype background (). The atypical equine-like G3P[8] DS-1-like reassortment origin was further confirmed by PAGE, where the four samples showed a short electropherotype pattern.
Figure 1. Phylogenetic analyses based on VP7 (a) and VP4 (b) nucleotide (nt) sequences of circulating Brazilian rotavirus strains. The trees were generated in MEGA X using the best fit model, with the Maximum likelihood method based on the Kimura 2-parameter model and the bootstrap tests (2,000 replicates). Strains obtained in this study are marked with a black filled circle. Reference strains were downloaded from GenBank and all strains were labeled with RVA group, species of origin followed by country, common name, year, G and P genotype, number access. Bootstrap values above 70% are given at branch nodes.
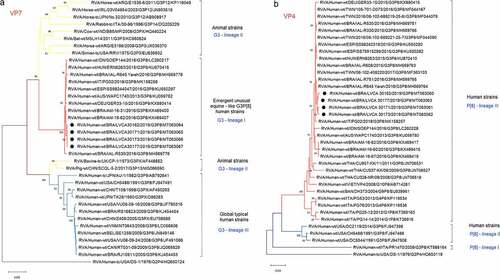
Figure 2. Phylogenetic analyses based on NSP1–NSP5, VP1-VP3, VP6 nucleotide (nt) sequences of circulating Brazilian human RVA strains. The strains obtained in this study that are marked with a filled black circle such as the reference strains that were downloaded from GenBank were both labeled as follows: RVA group, species of origin, country, common name, year, G and P genotype, number access and functions of the proteins (blue letter). Neighbor-joining phylogenetic trees were constructed with MEGA X software and bootstrap tests (2000 replicates) based on the Tamura 3-parameter models T92 + G (NSP3 – NSP5, VP3, VP6), T92 + I (NSP1, NSP2) and Tamura-Nei TN93 + G (VP2, VP1). Bootstrap values above 70% are given at branch nodes.

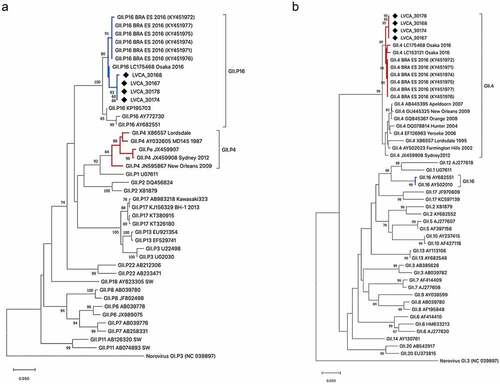
The GII.4 Sydney[P16] norovirus was detected in 100% (n = 5) of samples. The sequences from our study showed high nucleotide similarity (varying from 98.8% to 99.2%) with sequences from this same recombinant strain detected in Espírito Santo state, southeast Brazil, as well as from sequences from Australia, United States and Japan isolated in the years of 2016–2018 ().
Figure 3. Phylogenetic trees based on polymerase (a) and capsid (b) regions of GII norovirus. Norovirus GII strains (n = 4) isolated from human samples in this study are shown in the phylogenetic analysis and are marked with a back filled diamond. Reference strains were downloaded from GenBank and all strains were labeled with their genotype followed by accession number. Maximum likelihood phylogenetic trees were constructed with MEGA X software and bootstrap tests (2,000 replicates) based on the Kimura 2 – parameter model. The bootstrap percentage values above 70% are shown at each branch nodes.
Viruses are important causes of nosocomial infection in pediatric populations.Citation1 Infections raise the costs for the public health system associated with the duration of hospital stay, re-hospitalization loss of working days for parents and staff. Moreover, nosocomial infections are associated with the worsening of symptoms and clinical conditions mostly in children affected by severe diseases.Citation23 The prolonged shedding of virus can represent a risk for spreading of the viruses, leading to hospital-acquired infections and outbreaks.Citation24
In the present study, we reported a nosocomial AGE outbreak caused by RVA and norovirus in a pediatric hospital in Rio de Janeiro, Brazil. Recently, a study of nosocomial infection described a norovirus-related outbreak at neonatal and pediatric intensive care units in southeastern Brazil, where the genotype GII.4 Sydney[P31] was detected in four from six symptomatic children.Citation12 Another study performed in Brazil investigating viral infections in hospitalized children with AGE reported the detection of RVA, norovirus and astrovirus at rates of 41.9%, 30.3% and 12.7%, respectively. RVA and norovirus were detected in 20 of the 24 reported nosocomial infection cases. RVA G1[P8] and G9[P8] genotypes were characterized, and norovirus GII.21 genotype was firstly detected in Brazil from these samples.Citation25 A study conducted in Italy showed RVA in 85.5% and norovirus in 38.2% of stool samples (n = 55) from hospitalized children with AGE symptoms in the Children’s Hospital in Rome. The predominant RVA genotype was G4P[8] followed by G1P[8], and GII.4 was the predominant norovirus genotype detected. Mixed RVA infections were also detected. This study demonstrated that young children admitted to hospitals are at risk of exposure to infections due to their condition of vulnerability and the lack of a mature microbiota.Citation3
In our study, during the outbreak, none of the children that tested positive for RVA had the full RotarixTM scheme completed (), probably due to the contraindication of being administered in children with primary and secondary immune deficiencies.Citation26 Two children, who did not receive any dose, presented severe dehydration and were rapidly transferred to the Intensive Care Unit (ICU). However, as the number of affected patients was small, it is not possible to draw any correlation with regard to the vaccination status and severity of the symptoms. Nevertheless, concerning the equine-like G3P[8] detection, it is been described its emergence and dominance in countries using RotarixTM vaccine at national level, in both vaccinated and unvaccinated patients.Citation5–7,Citation27
The index case reported was a 10-month-old child, who had been hospitalized since its birth. Initial symptoms observed were diarrhea, nausea and fever, resulting in severe dehydration that led to ICU admission. After that, subsequential patients and staff contacts showed AGE symptoms and RVA and norovirus GII were detected from symptomatic patients. Due to the high transmissibility of both viruses, demonstrated by their high viral load (>107 GC/g of stool samples), and by the fact that asymptomatic infections are well described for both viral agents, it is possible that asymptomatic carriers, such as family members or healthcare staff, could have introduced and which may favor transmission the viruses through close contact during visits or routine examination.
We did not detect any co-infections among the symptomatic patients or staff tested, and it was not investigated any asymptomatic case. This is a limitation of our study, since asymptomatic testing could indicate or help to track the source of infection. Also, insights on genetic susceptibility of RVA and norovirus infections could be explored with the inclusion of asymptomatic patients. Concerning the origin of the nosocomial infection, the main vectors of transmission are contaminated (mostly asymptomatic) healthcare workers and family member visitors.Citation28
In our study, it is worth mentioning that it was not possible to track the source of viruses’ introduction in the hospital. As the outbreak was not widespread among many patients, it is unlikely that it was transmitted via contaminated food or water. An interesting aspect of our study is that the nosocomial infections were caused by two different viral agents, probably introduced into the hospital by different carriers. Hand washing, glove discharge (changed between patients), isolation of children with diarrhea and limitation of “traffic” around patients (short visits, patient transport) are preventive measures that should be routinely used to minimize the impact of nosocomial infections. Recent studies have demonstrated the emergence and spread of equine-like G3P[8] DS-1-like strains worldwide, including Brazil.Citation7,Citation8,Citation21,Citation22 A recent study from our group demonstrated the dominant circulation of the G3P[8] genotype in eleven states Brazilians, with detection rates of 83.7% in 2018 and 65.5% in 2019.Citation15
In the present study, RVA sequences showed an I2-R2-C2-M2-A2-N1-T2-E2-H2 genotype background, belonging to genogroup 2 with an additional reassortment event in the NSP2 gene which exhibited genotype 1 (N1) of Wa-like origin. Similar findings, with RVA sequences exhibiting the same genetic profile, were already reported in BrazilCitation6 and in Germany.Citation29 The global emergence of equine-like G3P[8] DS-1-like strain, predominantly in countries using the Rotarix vaccine, raises the question of whether vaccine could induce selective pressures on zoonotic strains.Citation30 It has also been hypothesized that protective immunity elicited against the fully heterotypic genotype, which belongs to the DS-1-like genotype constellation, might be less robust than homotypic protection stimulated for G1P[8] strains (Wa-like genotype constellation).Citation31,Citation32
The GII.4 Sydney[P16] norovirus detected in our study is an emergent and widespread genotype that has caused AGE outbreaks in several countries worldwide.Citation9–11 In Brazil, it was firstly detected in 2016 from inpatients and outpatients with AGE.Citation33 More recently, it was reported a new variant (GII.4 Hong Kong), but with limited circulation in Eurasia since mid-2017.Citation34 In addition, uncommon genotypes, such as GII.17 and GII.2, have recently emerged linked with a rapid increase of cases and outbreaks in different countries worldwide.Citation35,Citation36
Currently, norovirus vaccines are in clinical trials (phase I and II); however, these vaccine candidates face the challenge of norovirus genetic diversity and still an unclear definition of cross-protection immunity among the different genotypes.Citation37,Citation38 A very successful norovirus vaccine blocking onward transmission events will benefit children and unvaccinated persons across all age groups.Citation39
RVA and norovirus demonstrate a high genetic diversity and evolution which reinforces the recommendation for ongoing monitoring of emergent strains. The availability of rotavirus vaccines in addition to the development of an effective anti-norovirus vaccine may have a major impact in reducing both community-acquired infections and as a consequence in nosocomial infections in children. Moreover, emergent strain surveillance aids in the evaluation of RVA vaccine efficacy, and in relation to norovirus is essential to design and adapt future vaccine candidates.
In summary, we described the simultaneous detection of the emergent equine-like G3P[8] RVA and GII.4 Sydney[P16] norovirus during a nosocomial AGE outbreak at a pediatric hospital in Brazil. Fortunately, prevention measures were implemented, and the outbreak was rapidly controlled. Continuous viruses’ surveillance and diagnostics should be constantly reinforced to early detect outbreaks to rapidly initiate control protocols in order to prevent the spread of the disease among inpatient children susceptible, especially those admitted for other underlying conditions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical approval
This study is currently approved by the Ethics Committee of the Oswaldo Cruz Foundation (FIOCRUZ), number CAAE: 94144918.3.0000.5248. Samples were manipulated anonymously and patient’s information was maintained securely in compliance with the Ethical Protocol Statement ISO 15189. This study is part of the ongoing national viral AGE surveillance program performed by the Laboratory of Comparative and Environmental Virology, Oswaldo Cruz Institute, that houses the Rotavirus Regional Reference Laboratory (RRRL) coordinated by the General Coordination of Public Health Laboratories, Brazilian Ministry of Health.
Acknowledgments
The authors thanks to LVCA technical staff members Juliana da Silva Ribeiro de Andrade, Rosane Maria Santos de Assis, Sérgio da Silva e Mouta for help with sample processing and technical assistance. We would like to thank the Brazilian Rotavirus Surveillance network, coordinated by the Coordenação Geral de Laboratórios de Saúde Pública (CGLab), Brazilian Ministry of Health, and the Instituto Nacional de Saúde da Mulher, da Criança e do Adolescente Fernandes Figueira (IFF/Fiocruz) involved in the study.
Additional information
Funding
References
- Cunliffe NA, Booth JA, Elliot C, Lowe SJ, Sopwith W, Kitchin N, Nakagomi O, Nakagomi T, Hart CA, Regan M, et al. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis. 2010;16(1):55–62. doi:10.3201/eid1601.090401.
- Tran A, Talmud D, Lejeune B, Jovenin N, Renois F, Payan C, Leveque N, Andreoletti L. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J Clin Microbiol. 2010;48(5):1943–46. doi:10.1128/JCM.02181-09.
- Valentini D, Ianiro G, Di Bartolo I, Di Camillo C, Boccuzzi E, Vittucci AC, Ruggeri FM, Monini M. Hospital-acquired rotavirus and norovirus acute gastroenteritis in a pediatric unit, in 2014-2015. J Med Virol. 2017;89(10):1768–74. doi:10.1002/jmv.24866.
- De Jesus MCS, Santos VS, Storti-Melo LM, De Souza CDF, Barreto ÍDDC, Paes MVC, Lima PAS, Bohland AK, Berezin EN, Machado RLD, et al. Impact of a twelve-year rotavirus vaccine program on acute diarrhea mortality and hospitalization in Brazil: 2006-2018. Expert Rev Vaccines. 2020;19(6):585–93. doi:10.1080/14760584.2020.1775081.
- Komoto S, Ide T, Negoro M, Tanaka T, Asada K, Umemoto M, Kuroki H, Ito H, Tanaka S, Ito M, et al. Characterization of unusual DS-1-like G3P[8] rotavirus strains in children with diarrhea in Japan. J Med Virol. 2018;90(5):890–98. doi:10.1002/jmv.25016.
- Guerra SFS, Soares LS, Lobo PS, Penha Júnior ET, Sousa Júnior EC, Bezerra DAM, Vaz LR, Linhares AC, Mascarenhas JDP. Detection of a novel equine-like G3 rotavirus associated with acute gastroenteritis in Brazil. J Gen Virol. 2016;97(12):3131–38. doi:10.1099/jgv.0.000626.
- Luchs A, Da Costa AC, Cilli A, Komninakis SCV, Carmona RDCC, Boen L, Morillo SG, Sabino EC, Timenetsky MDCST. Spread of the emerging equine-like G3P[8] DS-1-like genetic backbone rotavirus strain in Brazil and identification of potential genetic variants. J Gen Virol. 2019;100(1):7–25. doi:10.1099/jgv.0.001171.
- Chansaenroj J, Chuchaona W, Lestari FB, Pasittungkul S, Klinfueng S, Wanlapakorn N, Vongpunsawad S, Chirathaworn C, Poovorawan Y, et al. High prevalence of DS-1-like rotavirus infection in Thai adults between 2016 and 2019. PLOS ONE. 2020;15(6):e0235280. doi:10.1371/journal.pone.0235280.
- Medici MC, Tummolo F, Martella V, De Conto F, Arcangeletti MC, Pinardi F, Ferraglia F, Chezzi C, Calderaro A. Emergence of novel recombinant GII.P16_GII.2 and GII. P16_GII.4 Sydney 2012 norovirus strains in Italy, winter 2016/2017. New Microbiol. 2018;41(1):71–72.
- Lun JH, Hewitt J, Yan GJH, Enosi Tuipulotu D, Rawlinson WD, White PA. Recombinant GII.P16/GII.4 Sydney 2012 was the dominant norovirus identified in Australia and New Zealand in 2017. Viruses. 2018;10(10):548. doi:10.3390/v10100548.
- Ruis C, Roy S, Brown JR, Allen DJ, Goldstein RA, Breuer J, Hasenkrug KJ. The emerging GII.P16-GII.4 Sydney 2012 norovirus lineage is circulating worldwide, arose by late-2014 and contains polymerase changes that may increase virus transmission. PLoS One. 2017;12(6):e0179572. doi:10.1371/journal.pone.0179572.
- Volpini LPB, Barreira DMPG, da Almeida PLS, Spano LC. An outbreak due to a norovirus GII.Pe-GII.4 Sydney_2012 recombinant in neonatal and pediatric intensive care units. J Infect Public Health. 2020;13(1):89–93. doi:10.1016/j.jiph.2019.06.012.
- Zeng SQ, Halkosalo A, Salminen M, Szakal ED, Puustinen L, Vesikari T. One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. J Virol Methods. 2008;153(2):238–40. doi:10.1016/j.jviromet.2008.08.004.
- Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41(4):1548–57. doi:10.1128/jcm.41.4.1548-1557.2003.
- Gutierrez MB, Fialho AM, Maranhão AG, Malta FC, Andrade JDSRD, Assis RMSD, Mouta SDSE, Miagostovich MP, Leite JPG, Machado Fumian T, et al. Rotavirus A in Brazil: molecular epidemiology and surveillance during 2018-2019. Pathogens. 2020;9:7. doi:10.3390/pathogens9070515.
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J Virol. 2008;82(7):3204–19. doi:10.1128/JVI.02257-07.
- Varghese V, Ghosh S, Das S, Bhattacharya SK, Krishnan T, Karmakar P, Kobayashi N, Naik TN. Characterization of VP1, VP2 and VP3 gene segments of a human rotavirus closely related to porcine strains. Virus Genes. 2006;32(3):241–47. doi:10.1007/s11262-005-6908-y.
- Herring AJ, Inglis NF, Ojeh CK, Snodgrass DR, Menzies JD. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982;16(3):473–77. doi:10.1128/JCM.16.3.473-477.1982.
- Cannon JL, Barclay L, Collins NR, Wikswo ME, Castro CJ, Magaña LC, Gregoricus N, Marine RL, Chhabra P, Vinjé J, et al. Genetic and epidemiologic trends of norovirus outbreaks in the United States from 2013 to 2016 demonstrated emergence of novel GII.4 recombinant viruses. J Clin Microbiol. 2017;55(7):2208–21. doi:10.1128/JCM.00455-17.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura KMEGAX. Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–49. doi:10.1093/molbev/msy096.
- Katz EM, Esona MD, Betrapally NS, De La Cruz De Leon LA, Neira YR, Rey GJ, Bowen MD. Whole-gene analysis of inter-genogroup reassortant rotaviruses from the Dominican Republic: emergence of equine-like G3 strains and evidence of their reassortment with locally-circulating strains. Virology. 2019;534:114–31. doi:10.1016/j.virol.2019.06.007.
- Agbemabiese CA, Nakagomi T, Damanka SA, Dennis FE, Lartey BL, Armah GE, Nakagomi O. Sub-genotype phylogeny of the non-G, non-P genes of genotype 2 Rotavirus A strains. PLoS One. 2019;14(5):e0217422. doi:10.1371/journal.pone.0217422.
- Álvarez Aldeán J, Aristegui J, López-Belmonte JL, Pedrós M, Sicilia JG. Economic and psychosocial impact of rotavirus infection in Spain: a literature review. Vaccine. 2014;32(30):3740–51. doi:10.1016/j.vaccine.2014.04.058.
- Sukhrie FHA, Siebenga JJ, Beersma MFC, Koopmans M. Chronic shedders as reservoir for nosocomial transmission of norovirus. J Clin Microbiol. 2010;48(11):4303–05. doi:10.1128/JCM.01308-10.
- Ferreira MSR, de Cubel Garcia RC, da Xavier MPTP, Ribeiro RL, Assis RM, Mota MDCM, Leite JPG, Miagostovich MP, OliveiR F.ra SAD. Genotyping of gastroenteric viruses in hospitalised children: first report of norovirus GII.21 in Brazil. Mem Inst Oswaldo Cruz. 2012;107(8):1064–67. doi:10.1590/s0074-02762012000800017.
- Arvas A. Vaccination in patients with immunosuppression. Turk Pediatri Ars. 2014;49(3):181–85. doi:10.5152/tpa.2014.2206.
- Roczo-Farkas S, Cowley D, Bines JE. The Australian rotavirus surveillance group. Australian rotavirus surveillance program: annual report, 2017. Commun Dis Intell (2018). 2019;43.28. doi:10.33321/cdi.2019.43.28.
- Gleizes O, Desselberger U, Tatochenko V, Rodrigo C, Salman N, Mezner Z, Giaquinto C, Grimprel E. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J. 2006;25(1 Suppl):S12–21. doi:10.1097/01.inf.0000197563.03895.91.
- Pietsch C, Liebert UG. Molecular characterization of different equine-like G3 rotavirus strains from Germany. Infect Genet Evol. 2018;57:46–50. doi:10.1016/j.meegid.2017.11.007.
- Roczo-Farkas S, Kirkwood CD, Bines JE, Enteric Virus Group. Murdoch childrens research institute, royal children’s hospital. Australian rotavirus surveillance program: annual report, 2016. Commun Dis Intell Q Rep. 2017;41(4):E455–E471.
- Roczo-Farkas S, Kirkwood CD, Cowley D, Barnes GL, Bogdanovic-Sakran N, Boniface K, Donato CM, Bines JE. The impact of rotavirus vaccines on genotype diversity: a comprehensive analysis of 2 decades of Australian surveillance data. J Infect Dis. 2018;218(4):546–54. doi:10.1093/infdis/jiy197.
- Clarke E, Desselberger U. Correlates of protection against human rotavirus disease and the factors influencing protection in low-income settings. Mucosal Immunol. 2015;8(1):1–17. doi:10.1038/mi.2014.114.
- Barreira DMPG, Fumian TM, Tonini MAL, Volpini LPB, Santos RP, Ribeiro ALC, Leite JPG, Souza MTBDME, Brasil P, da Cunha DC, et al. Detection and molecular characterization of the novel recombinant norovirus GII.P16-GII.4 Sydney in southeastern Brazil in 2016. PLoS One. 2017;12(12):e0189504. doi:10.1371/journal.pone.0189504.
- Chan MC-W, Roy S, Bonifacio J, Zhang L-Y, Chhabra P, Chan JCM, Celma C, Igoy MA, Lau S-L, Mohammad KN, et al. Detection of norovirus variant GII.4 Hong Kong in Asia and Europe, 2017-2019. Emerg Infect Dis. 2021;27(1):289–93. doi:10.3201/eid2701.203351.
- Chan MCW, Lee N, Hung T-N, Kwok K, Cheung K, Tin EKY, Lai RWM, Nelson EAS, Leung TF, Chan PKS, et al. Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat Commun. 2015;6(1):10061. doi:10.1038/ncomms10061.
- Niendorf S, Jacobsen S, Faber M, Eis-Hübinger AM, Hofmann J, Zimmermann O, Höhne M, Bock CT. Steep rise in norovirus cases and emergence of a new recombinant strain GII.P16-GII.2, Germany, winter 2016. Euro Surveill. 2017;22(4). doi:10.2807/1560-7917.ES.2017.22.4.30447.
- Ramani S, Estes MK, Atmar RL. Correlates of protection against norovirus infection and disease-where are we now, where do we go? PLoS Pathog. 2016;12(4):e1005334. doi:10.1371/journal.ppat.1005334.
- Cates JE, Vinjé J, Parashar U, Hall AJ. Recent advances in human norovirus research and implications for candidate vaccines. Expert Rev Vaccines. 2020;19(6):539–48. doi:10.1080/14760584.2020.1777860.
- Cannon JL, Bonifacio J, Bucardo F, Buesa J, Bruggink L, Chan MCW, Fumian TM, Giri S, Gonzalez MD, Hewitt J, et al. Global trends in norovirus genotype distribution among children with acute gastroenteritis. Emerg Infect Dis. 2021;27(5):1438–45. doi:10.3201/eid2705.204756.

