 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The exposure risk to the highly infectious hepatitis B virus (HBV) is an established and recognizable hazard to healthcare professionals (HCPs). In the United States, implementing preemptive vaccination programs and safety procedures resulted in drastic reductions in HBV infections among HCPs; however, many HCPs remain unprotected and risk of exposure persists, especially among those first entering a healthcare system and undergoing professional training. First-generation HBV vaccines require completion of a 3-dose schedule over a 6-month interval for maximum immunogenicity. By comparison, HepB-CpG (HEPLISAV-B®) is a 2-dose HBV vaccine licensed in the United States in 2017, inducing rapid seroprotection over a 1-month interval and may represent a more effective strategy for combating HBV infection in US healthcare systems. In this modeling study, the health and economic impact of implementing an HBV vaccination strategy with HepB-CpG versus the 3-dose HBV vaccine (Engerix-B®) was evaluated among HCPs newly entering a healthcare system. The model used effective seroprotection rate, a real-world metric accounting for HCP vaccine compliance and seroprotection rates for different dosing regimens and considered current pricing for postexposure prophylaxis treatment. Compared with the 3-dose vaccine, HepB-CpG was anticipated to provide faster, increased protection against HBV infection among newly entered HCPs. In protecting a greater percentage of HCPs, HepB-CpG was also projected to substantially reduce the risk of HBV exposure. Accordingly, an economic analysis showed HepB-CpG vaccination would reduce costs of postexposure prophylaxis treatment compared with the 3-dose vaccine. Overall, HepB-CpG represents an effective therapeutic strategy against HBV infection for US healthcare systems.
Introduction
Hepatitis B virus (HBV) infection can cause an incurable disease of the liver, leading to complications such as hepatocellular carcinoma and inflammation.Citation1,Citation2 There are an estimated 248 million chronic HBV carriers worldwide, with more than 840 thousand of these people living in the United States.Citation3 HBV is highly infectious and primarily transmitted by percutaneous or mucosal contact with infectious blood or bodily fluids, therefore increasing the risk of exposure for healthcare professionals (HCPs) who routinely interact with patients and patient-derived material.Citation1,Citation4,Citation5 Beyond inadvertent exposure through overt percutaneous injuries (eg, contaminated needlestick exposure) or via bloodwork-related procedures, contact with environmental surfaces harboring infectious HBV can also cause disease, as the virus is environmentally stable for at least 1 week.Citation4–7 The risk of acquiring HBV infection can vary throughout the career of an HCP, but is typically higher when the HCP is first entering a medical environment and undergoing professional training.Citation5 In addition, although rare, HCPs can act as a source of HBV transmission to patients.Citation8 Thus, ensuring that vulnerable HCPs are protected against HBV infection and transmission is paramount for occupational safety at healthcare institutions.
The primary interventional strategy for preventing HBV infection is preemptive vaccination.Citation9 In the United States, the Advisory Committee on Immunization Practices (ACIP) has recommended since 1982 that HCPs receive vaccination against HBV.Citation5,Citation10 In addition, the Bloodborne Pathogen Standard by the Occupational Health and Safety Act (OSHA) mandates that employers provide HBV vaccinations for any HCPs occupationally exposed to blood or other potentially infectious materials.Citation5,Citation11 Establishing these preemptive vaccination programs and safety procedures for HCPs in the United States has led to remarkable reductions in HBV infections among this population.Citation5 However, the exposure risk persists as 30,945 blood and bodily fluid exposures among US HCPs were reported between 1995 and 2007, with the actual number of exposures likely approaching ≥62,000 because of underreporting.Citation12 Further, many HCPs remain unprotected, as HBV vaccination coverage among HCPs is reportedly between 62% and 81%.Citation13–16
In the event of exposure, hospital systems are required by OSHA to provide postexposure evaluation and prophylaxis to any occupationally exposed workers.Citation11 According to guidelines from the Centers for Disease Control and Prevention, postexposure prophylaxis differs based on the vaccination status of the HCP and the hepatitis B status of the exposure source.Citation17 If an HCP has completed HBV vaccination and postexposure testing shows sufficient anti–hepatitis B surface antibody (anti-HBsAb, ≥10 mIU/mL), no prophylaxis is necessary regardless of whether a source patient is considered infectious (positive HBsAb status).Citation17 However, HCPs who were vaccinated and have insufficient or unknown anti-HBsAb, unvaccinated, or not fully vaccinated (i.e., did not receive all required doses) and are exposed to patients with either HBsAb-positive or -unknown statuses require HBV vaccination and a single dose of hepatitis B immune globulin (HBIG).Citation17 Hospitals are thus required to cover both the direct costs of testing and treatment as well as indirect costs,Citation18,Citation19 including managing the loss of time caused by HBV exposure.
Although first-generation HBV vaccines are effective (eg, Engerix-B®; GlaxoSmithKline, Research Triangle Park, NC; and Recombivax HB®; Merck Sharp & Dohme, Whitehouse Station, NJ), the recommended immunization strategy is a 3-dose series administered over a 6-month interval.Citation5,Citation20,Citation21 This immunization schedule poses particular challenges for unvaccinated HCPs newly entering a health system who remain at risk of HBV exposure during this period.Citation5 More immediate protection against HBV is provided by HepB-CpG (HEPLISAV-B®; Dynavax Technologies, Emeryville, CA), a 2-dose HBV vaccine licensed in 2017 that can be administered over a 1-month period.Citation22 In comparison to the 3-dose vaccine, HepB-CpG vaccination has been shown to induce earlier and significantly higher seroprotection rates (SPRs) among healthy adults aged 18 to 70 years.Citation23–25 Thus, HepB-CpG may represent a potent tool for health systems by offering rapid HBV protection for HCPs, especially among those newly entering the system and at high risk of infection. The objective of this study is to investigate the potential occupational health and economic impact of implementing HepB-CpG (2-dose) versus 3-dose (Engerix-B) HBV vaccination for HCPs entering health systems.
Methods
Model description
A model was developed to assess the health and economic impact of HepB-CpG versus 3-dose (Engerix-B) vaccination over a 1-year period among HCPs newly entering a health system (). Engerix-B was selected as the 3-dose comparator vaccine due to the availability of head-to-head efficacy data against HepB-CpG. Of note, other currently available 3-dose vaccines not selected as comparators have similar costsCitation26 and show generally similar efficacy to Engerix-B, including among HCPs.Citation17,Citation27 The main outcomes included the number of HCPs protected against HBV, number of HCPs potentially exposed to HBV, and cost of postexposure prophylaxis among HCPs requiring intervention (i.e., serologic monitoring, vaccine cost and administration, and HBIG).
Figure 1. Model schematic. HCPs entered the model at month 0 and those unprotected HCPs were vaccinated with either the 3-dose vaccine or HepB-CpG. The model assumed that HBV vaccination occurred at the beginning of each month. At each month, the model calculated the number of HCPs protected against HBV, the number of potential HBV exposures among HCPs who required intervention, and the cost of prophylaxis treatment. Protection against HBV was based on effective SPRs, not serologic testing. EHCPP = Protected HCP exposed to HBV. EHCPU = Unprotected HCP exposed to HBV. eSPR = effective SPR; HBIG = hepatitis B immunoglobulin; HBV = hepatitis B virus; HCP = healthcare professional; HCPP = Protected HCP. HCPU = Unprotected HCP. SPR = seroprotection rate.
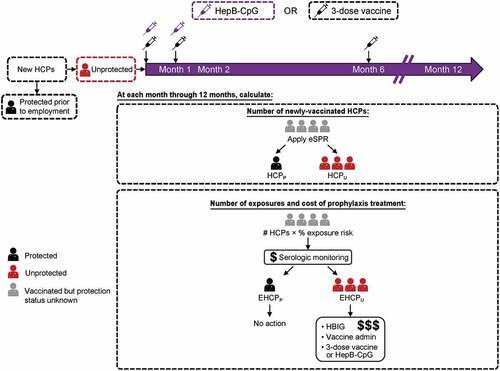
The model considered HCPs as anyone employed in the health services industry as defined by the US Bureau of Labor Statistics, including those working at public and private hospitals; nursing and residential care facilities; offices of physicians, dentists, and other practitioners; outpatient care centers; other ambulatory care services; and medical laboratories. Four different incoming populations of HCPs were accounted for in the model: clinical HCPs (i.e., those with direct patient contact) and nonclinical HCPs; clinical and nonclinical HCPs were further categorized as transferring from another health system or were newly entering a health system from the general population (Supplementary Table 1).
The model begins with HCPs entering the healthcare system at month 0. HCPs entering the system were assumed to be either (i) unprotected against HBV and thus eligible for vaccination or (ii) previously successfully immunized with a 3-dose HBV vaccine (i.e., received HBV immunization at a prior healthcare system or vaccinated through an immunization program for the general population) and thus already protected against infection. At month 0, 38.1% of the incoming HCP population was considered already protected against HBV infection based on the incoming population demographics as well as known HBV vaccine compliance and SPRs (Supplementary Table 1). The remaining unprotected HCP population was then vaccinated with either the 3-dose vaccine (3 doses at months 0, 1, and 6) or HepB-CpG vaccine (2 doses at months 0 and 1).
Population assumptions
The model assumed that a full healthcare system consisted of 10,000 HCPs with a 19.1% annual hospital turnover rate from the 2020 Nursing Solutions Inc National Health Care Retention and RN Staffing Report ().Citation28 New HCPs were assumed to enter at month 0, and the net number of HCPs remained unchanged during the 1-year evaluation period. The model also assumed that the health system tested incoming HCPs for anti-HBsAb to determine whether they were sufficiently protected against HBV before entry (no challenge dose was given).
Table 1. Model inputs
Effective seroprotection rate and outcome measurements
Over the course of 1 year after entering the healthcare system, the potential real-world efficacy of either the 3-dose vaccine or HepB-CpG (2-dose) immunization was measured by taking into account the effective SPR (eSPR). The eSPR accounts for both vaccine compliance and SPRs among different dosing schedules to provide clinically relevant analyses of HBV vaccine effectiveness against disease.Citation32,Citation33 The model used eSPRs specific to each dose in a particular vaccine series that were calculated by multiplying vaccine-specific compliance rates by SPR for each dose in the series (); the total eSPR was applied after completing the regimen (eg, dose 2 for HepB-CpG or dose 3 for the 3-dose vaccine) and was determined by summing eSPR values for each dose in the series (Supplementary Table 2).
The model calculated the number of HCPs protected (HCPP) after each dose of either HBV vaccine by summing (i) the number of HCPs already protected upon model entry (month 0) based on prior successful vaccination, (ii) the number of HCPs protected by any previous dose(s) after model entry, and (iii) the incremental number of HCPs protected by that particular dose. The incremental number of HCPs protected by a particular dose was calculated by multiplying the dose-specific eSPR by the number of HCPs unprotected at month 0 (). The number of HCPs unprotected (HCPU) after a specific dose was then determined by subtracting the number of HCPs protected after that dose from the initial number of HCPs entering the hospital system.
Beginning at 1 month after dose 1 of either vaccine, the model calculated the monthly number of potentially HBV exposed protected (EHCPp) and unprotected HCPs (EHCPU). For month 1 (i.e., within 30 days after receiving 1 dose of either the 3-dose vaccine or HepB-CpG, but before receiving dose 2; ), EHCPP and EHCPU were determined by multiplying a monthly HBV exposure rate by the number of already protected (previously vaccinated) or unprotected HCPs, respectively, entering the model at month 0. For each subsequent month (i.e., months 2–12), the model determined EHCPP and EHCPU using the following methodology. For EHCPP, the model first calculated HCPP after each vaccine dose (see above paragraph) except any HCPs exposed during prior months were removed, since the model assumed that these HCPs would require serologic monitoring (EHCPP) and prophylaxis treatment (EHCPU) on their first exposure only. The model next multiplied this modified HCPP number by the exposure rate to determine EHCPP for that month. For EHCPU, the model subtracted both the HCPP for that particular month and any EHCPU in each prior month from the initial number of HCPs entering the hospital system at month 0, and then multiplied by the exposure rate. Of note, while previously exposed HCPs could be re-exposed in subsequent months, the model excluded these individuals as it was assumed no action would be necessary since they were either already known to be protected or were already receiving testing and/or treatment for a previous exposure. As such, the exposure rate calculated in the model includes initial exposures only, as re-exposures were not expected to result in additional prophylaxis costs. If re-exposures were included, the total exposure rate would have been higher. At all timepoints examined, the model only considered exposures that were anticipated to result in prophylaxis treatment costs.
For each month after vaccination, the model also determined the cost of prophylaxis treatment among exposed HCPs requiring intervention. The EHCPU and EHCPP over months 1–6 and months 1–12 were first determined as described above; the EHCPU was then multiplied by the sum of prophylaxis costs (see below; i.e., serologic monitoring, vaccine cost and administration, and HBIG), while the EHCPP was multiplied by the costs of serologic monitoring only. The costs for both EHCPU and EHCPP were then summed to determine the total cost of prophylaxis treatment over that time period.
Model inputs: compliance rates
The model assumed 100% compliance to dose 1 and derived remaining rates from a previous publication of HBV vaccine compliance rates among HCPs.Citation13 Compliance rate inputs for each dose in the series include those HCPs receiving only that dose as well as those who continue to receive subsequent doses in the vaccine series. The percentage of HCPs receiving all 3 doses of the 3-dose vaccine (63.4%) was based on reported 3-dose HBV compliance rates among HCPsCitation13 (). As compliance rates for HCPs receiving remaining doses of the 3-dose vaccine series were not available in the literature, these percentages were calculated based on the following equations:
The multiplication factor of 0.578 was derived from 1- and 2-dose HBV vaccine compliance rates among the general populationCitation34 (Supplementary Table 3):
The percentage of HCPs receiving 2 doses of the 3-dose vaccine (both those who received 2 doses without the 3rd dose and those who went on to receive the 3rd dose) was then calculated by subtracting the 1st dose only rate (eg, percentage receiving only dose 1 and then discontinuing the series) from 100%. For HepB-CpG, the percentage of HCPs receiving all 2 doses was assumed identical to the entirety of HCPs receiving 2 doses of the 3-dose vaccine (84.6%). The 3-dose vaccine and HepB-CpG were assumed to take 1 month after each dose to provide protection.
Model inputs: seroprotection rates
Seroprotection rates for the 3-dose and HepB-CpG vaccines were derived from 3 different clinical trials in adult subjectsCitation23–25 (). For doses 1 and 2 of the 3-dose vaccine, SPRs were pooled from week 4 or week 8 from 2 trials.Citation23,Citation24 For dose 3 of the 3-dose vaccine, SPRs were pooled from week 28 from all 3 trials.Citation23–25 For HepB-CpG, dose 1 SPRs were pooled from week 4 from 2 trials,Citation23,Citation24 whereas dose 2 SPRs were pooled from week 24 in 3 trials.Citation23–25
Model inputs: HBV exposure rates
A 3% monthly risk of exposure to HBV was estimated from available data in the literature, based on (i) the percentage of patients (hospital and community based) who are tested for HBV (tested: 68%; nontested: 32%),Citation29 (ii) the percentage of the US population with HBV (0.3%),Citation30 and (iii) the percentage of HCPs exposed to HBV through blood and/or sharps injuries in 1-month period (9.45%).
To calculate the number of exposures that would involve a patient who was either HBV positive or of unknown HBV status, it was assumed that the source patient would be tested for HBV in 68% of exposure cases (hospital and community based) and not tested in 32% of cases. Of those 68% of exposures tested, it was assumed that 0.3% would involve an HBV-positive patient. Therefore, the model assumed that 32.2% of exposures would involve a patient who either tested positive for HBV or were of unknown HBV status and would thus be considered as an HBV-positive exposure. The percentage of exposed HCPs (9.45%) was derived from a previous publicationCitation31 that reported the rate of blood exposures and sharps injuries among HCPs over a 3-month period, with a conversion to a monthly rate for this study using the following equation:
In the above equation, y represents the probability of not being exposed to HBV as derived from the literature.Citation31 Of note, this calculation accounts for the percentages of exposures that would not be reported by HCPs. Thus, assuming that 9.45% of HCPs were exposed monthly to HBV with only 32.2% of these incidents involving a potentially HBV-positive patient, the final monthly exposure rate among HCPs used in the model was 3%. The model assumed that all HCPs would have an equal chance of HBV exposure; that a single exposure does not affect the likelihood of an HCP’s future exposure to HBV; and that the risk of HBV exposure is not seasonally influenced.
Model inputs: prophylaxis costing
The analysis considered only the direct costs of prophylaxis treatment and incorporated the costs (2020; $USD) of each vaccine series and its administration, serologic monitoring, and HBIG (). The analysis excluded any future costs related to subsequent diagnosis of HBV. Vaccine costs included the 2020 RED BOOK drug pricing for either the 3-dose vaccine regimen ($185.58) or HepB-CpG regimen ($242.50) and an administration fee of $16.94 based on the Medicare Physician Fee Schedule.Citation35,Citation36 Serologic monitoring included both the cost of antibody testing and physician appointment ($33.81) based on the Medicare Clinical Laboratory Fee Schedule and Physician Fee Schedule,Citation36,Citation37 whereas HBIG costs $117.83 based on the 2020 Medicare Drug Pricing Files.Citation38
Sensitivity analyses
One-way sensitivity analyses assuming 15% higher or lower values than the base-case inputs were conducted and evaluated the effects of altering the base-case value of 1 of the parameters at a time. The 1-way sensitivity analysis examined the most influential variables affecting the cost difference of prophylaxis treatment after vaccination with either the 3-dose vaccine or HepB-CpG (). A 2-way sensitivity that simultaneously altered 2 base-case inputs by assuming 15% higher or lower values of influential variables was also performed to evaluate the impact of input variability on the cost difference of prophylaxis treatment after HBV vaccination.
Table 2. Inputs for 1-way ±15% sensitivity analysis
A scenario analysis was also undertaken to evaluate the influence of compliance rates on the number of HCPs protected and prophylaxis costs by assuming HBV vaccine compliance rates among HCPs from a different data sourceCitation14 (Supplementary Table 4).
Results
Protection rates after vaccination among HCPs newly entered to a hospital system
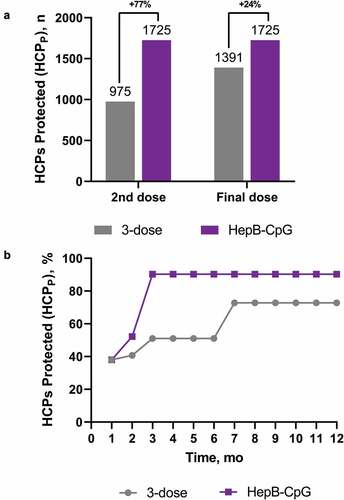
An estimated 1910 HCPs annually joined the healthcare system. The estimated number of newly entered HCPs protected (HCPP) after receiving each dose of either HepB-CpG or the 3-dose vaccine is shown in . After 2 doses of HepB-CpG, an anticipated 1725 newly entered HCPs would be protected against HBV, a 77% increase in protection compared with 2 doses of the 3-dose vaccine (). On series completion, HepB-CpG would protect an additional 24% of newly entered HCPs compared with the 3-dose vaccine. Throughout the entire 1-year period, HepB-CpG was projected to protect an increased percentage of newly entered HCPs versus the 3-dose vaccine.
Table 3. Estimated number of HCPs protected (HCPP) with HepB-CpG vs the 3-dose vaccine
Figure 2. Protection rates among newly entered HCPs after vaccination with either HepB-CpG or 3-dose vaccine. (A) Number of HCPs newly entered into a healthcare system who are protected after 2 doses or all doses of either HepB-CpG or the 3-dose vaccine. (B) Percentage of HCPs protected over the 1-year period. HepB-CpG vaccination was assumed to occur at months 0 and 1; 3-dose vaccination occurred at months 0, 1, and 6. The model assumed that vaccination occurred at the beginning of the month and that either vaccine dose would take 1 month to provide protection. HCP = healthcare professional. HCPP = Protected HCP.
Risk of exposure and costs of prophylaxis among HCPs newly entered to a hospital system
In a hospital system that vaccinates newly entered HCPs with the 3-dose vaccine, a total of 173 unprotected HCPs would be potentially exposed to HBV (EHCPU) and require intervention within 1 to 6 months after entry (i.e., before series completion; , ). Throughout the entire 1-year period after entry, an estimated 249 unprotected HCPs would be potentially exposed to HBV. By comparison, in a hospital system vaccinating newly entered HCPs with HepB-CpG, 84 unprotected HCPs would be potentially exposed to HBV within 1 to 6 months and 115 unprotected HCPs would be exposed through 12 months after entry. Thus, compared with the 3-dose vaccine, a hospital system vaccinating HCPs with HepB-CpG was estimated to reduce the potential number of exposed HCPs from 1 to 6 months after entry by a reduction of 51%. For each month, compared with the 3-dose vaccine, HepB-CpG was anticipated to reduce the number of newly entered, unprotected HCPs exposed to HBV and at 12 months after entry, HepB-CpG was projected to reduce the risk of exposure among unprotected HCPs by a reduction of 54% compared with that of the 3-dose vaccine ().
Table 4. Estimated number of unprotected HCPs Exposed to HBV (EHCPU) and cost of prophylaxis for exposed HCPs With HepB-CpG and the 3-dose vaccine
Figure 3. Risk of exposure and total prophylaxis costs for newly entered HCPs. (A) The potential number of unprotected HCPs exposed to HBV over 6 or 12 months in a healthcare system that vaccinates newly entered HCPs with HepB-CpG versus the 3-dose vaccine. (B) The total cost of prophylaxis over a 1-year period for newly entered HCPs receiving either HepB-CpG or the 3-dose vaccine. Costs include those incurring for both unprotected and protected HCPs exposed to HBV. EHCPU = Unprotected, HBV exposed HCP. HBV = hepatitis B virus; HCP = healthcare professional.
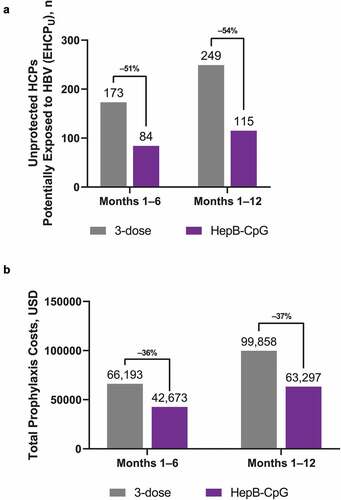
In a 1-year period, a hospital system vaccinating newly entered HCPs with the 3-dose vaccine was projected to spend a total of approximately $100,000 on prophylaxis (, ). In that same period, a hospital system that instead vaccinated HCPs with HepB-CpG would spend approximately $63,000, representing a cost savings of approximately 37% from the 3-dose vaccine.
Sensitivity analyses
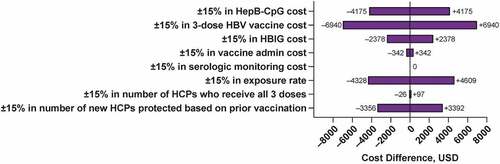
The 1-way sensitivity analysis in prophylaxis treatment between the 3-dose vaccine and HepB-CpG determined that the incremental cost was most sensitive to the cost of the 3-dose vaccine and the HBV exposure rate (). In the 2-way sensitivity analysis, HepB-CpG was cost-saving across all comparisons with the 3-dose vaccine (). The case study sensitivity analysis using higher HBV vaccine compliance rates showed minimal difference in prophylaxis costs from the base case scenario (base case: $36,560; sensitivity analysis: $36,630).
Table 5. Two-way sensitivity analysis on ±15% variation on influential variables
Figure 4. One-way ±15% sensitivity analysis on influential variables. The cost difference in prophylaxis treatment between HBV vaccines (the 3-dose vaccine, HepB-CpG) is shown for each changed factor. HBIG = hepatitis B immune globulin; HBV = hepatitis B virus; HCP = healthcare professional; USD = United States dollars.
Discussion
Hepatitis B virus is highly infectious and primarily affects the liver, resulting in either acute or chronic infection of varying severities. Vaccination is an effective interventional strategy that is essential for preventing HBV infection in populations at high risk of exposure.Citation9 HCPs are particularly susceptible to HBV infection, and although overall vaccination rates among this population are higher compared with the general population,Citation5,Citation13–15 rates are still below the Healthy People 2020 target of 90%.Citation17 Insufficient vaccination rates leave a substantial number of HCPs without protective immunity, creating several challenges within the healthcare industry as an estimated 385,000 percutaneous injuries occur annually in US hospitals.Citation39 As HBV vaccination adherence rates among the general and certain at-risk patient populations remain lowCitation14 and 67% of HBV infected individuals are unaware of their infection,Citation40 it is essential that effective vaccination strategies exist for HCPs at high risk for potential HBV exposure from the patient population.
HepB-CpG is an HBV vaccine approved in 2017 that offers a favorable alternative over commonly used 3-dose HBV vaccines (i.e., Engerix-B or Recombivax) as it has a dosing schedule that requires only 1 month for series completion.Citation22 HepB-CpG induces greater and earlier seroprotection than 3-dose HBV vaccinesCitation23–25 and thereby could be a more effective strategy for protecting at-risk HCPs against HBV infection, especially those high-risk HCPs newly entering a healthcare system. Our analysis showed that, within 3 months of implementing HepB-CpG vaccination at US healthcare systems, the vaccine was anticipated to protect an additional 77% of HCPs newly entering the system compared with the 3-dose vaccine. For each month throughout the course of 1 year, HepB-CpG was predicted to protect an increased percentage of HCPs than with the 3-dose vaccine, with an added 24% of HCPs protected against infection after 12 months. These results are in line with a previous modeling analysis that projected HepB-CpG could prevent 71 and 84 cases of acute and chronic HBV infections, respectively, in the HCP population.Citation14 Taken together, the shorter 2-dose vaccination schedule of HepB-CpG is likely to provide earlier and more substantial HBV protection among susceptible HCPs; thus, HepB-CpG may represent a superior interventional strategy for US healthcare systems required to provide occupational HBV vaccination.
Rapid, increased protection against HBV is likely to translate into reduced risk of infection after HBV exposure. Our analysis showed that HepB-CpG was anticipated to further reduce the number of unprotected HCPs potentially exposed to HBV by 105% within 6 months after entry (ie, after 2 doses of either vaccine) compared with the 3-dose vaccine. For HCPs newly entered into the hospital system, the increased efficiency and seroprotection provided from HepB-CpG is an undeniable benefit as HCPs are most vulnerable when first entering a hospital environment.Citation1
When considering downstream medical costs resulting from exposure, vaccination with HepB-CpG is also less costly compared with the 3-dose vaccine, providing a 37% reduction in money spent on prophylaxis for newly entered HCPs during a 1-year period. A 2-way sensitivity analysis further supported the base-case findings, showing that HepB-CpG is cost-saving among newly-entered HCPs when compared to the 3-dose vaccine. These findings are important given the annual downstream medical costs resulting from HCP exposure, including $827 and $1824 for acute and chronic HBV infection, respectively.Citation14 The cost of the 3-dose vaccine was the most influential driver of the cost difference in prophylaxis treatment between the 2 vaccines, which likely reflects the need for administering a vaccine multiple times because of its conferring lower levels of protection. Another key factor was the monthly exposure rate, which underlies the percentage of HCPs potentially exposed to an HBV–positive source patient. Our results are supported by a previous study showing that HepB-CpG was a cost-effective interventional strategy among multiple at-risk populations, including HCPs, compared with the 3-dose vaccine.Citation14
The findings presented here are applicable to a wide population of HCPs in the United States. Our model based HBV vaccination compliance rates on a previous study reporting a 3-dose, self-reported adherence rate of 63.4% among HCPs working or volunteering in a healthcare facility.Citation13 Notably, this compliance rate is similar to that in other reportsCitation15,Citation16 and is also applicable to specialized groups of HCPs. For example, HCPs in correctional facilities, who are at substantial risk of HBV infection because of the higher rates of HBV seen in inmate populations,Citation41,Citation42 have similar 3-doseCitation43** vaccination compliance rates (64%Citation41) to that used in our model. Thus, HepB-CpG is likely to also provide increased protection among HCPs working in a variety of occupational settings where time to protection is critical. Further, a 1-way sensitivity analysis showed that increasing the 3-dose compliance rate to 81.0%, based on a separate report on HCP vaccination rates,Citation14 had minimal effect on the cost differential of prophylaxis strategies, supporting our findings that HepB-CpG enhances protection to thereby reduce exposure risk and associated prophylaxis costs.
A potential limitation of this analysis is that this model assumed that all newly hired HCPs entered the hospital at one point in time and therefore does not account for the dynamics of hiring and entry of a hospital setting. Secondly, the model assumed that the percentage of patients with HBV infection was equal to the infection rate among the general population. Third, the model did not consider the costs of lost productivity or long-term medical costs associated with HBV infection among HCPs. Finally, because 1-dose and 2-dose HBV vaccine compliance rates among HCPs were not available from prior publications, these rates were calculated by incorporating compliance rates among the general US population and thus may not be entirely reflective of compliance rates among HCPs in US healthcare systems.
Conclusion
Vaccinating the HCP population, particularly those newly entering a hospital system, with the 2-dose HepB-CpG vaccine compared with the 3-dose vaccine has the potential to provide earlier and more substantial protection that could reduce the risk of occupational exposure to HBV and confer economic savings within a hospital system. Overall, HepB-CpG thus has the potential to dramatically reduce the risk of HBV infection among HCPs newly-entering a hospital system and represents a cost-effective option for US hospitals that are required to provide HBV vaccination and postexposure prophylaxis.
Disclosure of potential conflicts of interest
CS and CD are employees of Dynavax Technologies. J-HY is an employee of ICON plc, which received funding from Dynavax Technologies to conduct model validation. MSH has received research funding from Dynavax Technologies.
Supplemental Material
Download MS Word (71.5 KB)Acknowledgments
Editorial/medical writing support was provided by Emily Stackpole, PhD, of ICON plc (North Wales, PA) and was funded by Dynavax Technologies, Emeryville, California.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1965807
Additional information
Funding
References
- World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B Infection. World Health Organization. [accessed 2020 December 23] https://apps.who.int/iris/bitstream/handle/10665/154590/9789241549059_eng.pdf;jsessionid=774FE3696FDF60D945FC39AAB691E916?sequence=1 .
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi:10.1046/j.1365-2893.2003.00487.x.
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. doi:10.1016/S0140-6736(15)61412-X.
- Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR–11):1–52.
- Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(RR–7):1–45.
- Rosenberg JL, Jones DP, Lipitz LR, Kirsner JB. Viral hepatitis: an occupational hazard to surgeons. JAMA. 1973;223(4):395–400. doi:10.1001/jama.1973.03220040013003.
- Trinkoff AM, Le R, Geiger-Brown J, Lipscomb J. Work schedule, needle use, and needlestick injuries among registered nurses. Infect Control Hosp Epidemiol. 2007;28(2):156–64. doi:10.1086/510785.
- Sydnor E, Perl TM. Healthcare providers as sources of vaccine-preventable diseases. Vaccine. 2014;32(38):4814–22. doi:10.1016/j.vaccine.2014.03.097.
- Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, Nelson N. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67(15):455–58. doi:10.15585/mmwr.mm6715a5.
- Centers for Disease Control and Prevention. Recommendation of the Immunization Practices Advisory Committee (ACIP). Inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31(24):317–322, 327–318.
- Occupational Safety and Health Administration. OSHA’s bloodborne pathogens standard fact sheet. OSHA. [accessed 2020 June 11] https://www.osha.gov/OshDoc/data_BloodborneFacts/bbfact01.pdf .
- The National Surveillance System for Healthcare Workers (NaSH). Summary report for blood and body fluid exposure data collected from participating healthcare facilities (June 1995 through December 2007). CDC; 2011.
- Byrd KK, Lu PJ, Murphy TV. Hepatitis B vaccination coverage among health-care personnel in the United States. Public Health Rep. 2013;128(6):498–509. doi:10.1177/003335491312800609.
- Kuan RK, Janssen R, Heyward W, Bennett S, Nordyke R. Cost-effectiveness of hepatitis B vaccination using HEPLISAV in selected adult populations compared to Engerix-B® vaccine. Vaccine. 2013;31(37):4024–32. doi:10.1016/j.vaccine.2013.05.014.
- Lu PJ, Euler GL. Influenza, hepatitis B, and tetanus vaccination coverage among health care personnel in the United States. Am J Infect Control. 2011;39(6):488–94. doi:10.1016/j.ajic.2010.10.009.
- Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ. 2017;66(11):1–28. doi:10.15585/mmwr.ss6611a1.
- Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67(1):1–31. doi:10.15585/mmwr.rr6701a1.
- Kirkman-Liff B, Dandoy S. Cost of hepatitis B prevention in hospital employees: post-exposure prophylaxis. Infect Control. 1984;5(8):385–89. doi:10.1017/s0195941700062226.
- Hicks RA, Cullen JW, Jackson MA, Burry VF. Hepatitis B virus vaccine. Cost-benefit analysis of its use in a children’s hospital. Clin Pediatr (Phila). 1989;28(8):359–65. doi:10.1177/000992288902800805.
- ENGERIX-B® (hepatitis B vaccine [recombinant]). Full Prescribing Information, GlaxoSmithKline Biologicals, 2019.
- RECOMBIVAX HB® (hepatitis B vaccine [recombinant]). Full Prescribing Information, Merck, Sharp & Dohme Corp. 2018.
- HEPLISAV-B® (hepatitis B vaccine [recombinant], adjuvanted). Full Prescribing Information, Dynavax Technologies Corporation, 2020.
- Heyward WL, Kyle M, Blumenau J, Davis M, Reisinger K, Kabongo ML, Bennett S, Janssen RS, Namini H, Martin JT. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine. 2013;31(46):5300–05. doi:10.1016/j.vaccine.2013.05.068.
- Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, Embree J, McGeer A, Zickler P, Moltz KH, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine. 2012;30(15):2556–63. doi:10.1016/j.vaccine.2012.01.087.
- Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, Shockey G, Akella L, Erby K, Heyward WL, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a Toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. 2018;36(5):668–74. doi:10.1016/j.vaccine.2017.12.038.
- Centers for Disease Control and Prevention. CDC Vaccine Price List. [accessed 2021 April 1] https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/ .
- Coates T, Wilson R, Patrick G, Andre F, Watson V. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23(3):392–403. doi:10.1016/s0149-2918(01)80044-8.
- NSI Nursing Solutions Inc. 2020 NSI national health care retention & RN staffing report. [accessed 2020 June 11] https://www.nsinursingsolutions.com/Documents/Library/NSI_National_Health_Care_Retention_Report.pdf.
- Leigh JP, Gillen M, Franks P, Sutherland S, Nguyen HH, Steenland K, Xing G. Costs of needlestick injuries and subsequent hepatitis and HIV infection. Curr Med Res Opin. 2007;23(9):2093–105. doi:10.1185/030079907X219517.
- Chang MS, Nguyen MH. Epidemiology of hepatitis B and the role of vaccination. Best Pract Res Clin Gastroenterol. 2017;31(3):239–47. doi:10.1016/j.bpg.2017.05.008.
- Doebbeling BN, Vaughn TE, McCoy KD, Beekmann SE, Woolson RF, Ferguson KJ, Torner JC. Percutaneous injury, blood exposure, and adherence to standard precautions: are hospital-based health care providers still at risk? Clin Infect Dis. 2003;37(8):1006–13. doi:10.1086/377535.
- David C, Hirst A, Hyer R Consideration of effective seroprotection rate (eSPR) and cost per protected patient (CPP) as estimates of real-world outcomes in adult hepatitis B virus (HBV) vaccination. Paper presented at: National Foundation for Infectious Diseases 2020 Annual Conference on Vaccinology Research; 2020 March 23–25 [Virtual].
- Data on File. Dynavax Technologies, Emeryville (CA). 2020.
- Nelson JC, Bittner RC, Bounds L, Zhao S, Baggs J, Donahue JG, Hambidge SJ, Jacobsen SJ, Klein NP, Naleway AL, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(suppl 2):S389–397. doi:10.2105/AJPH.2008.151332.
- Truven Health Analytics. RED BOOK Online®. Micromedex healthcare series [database online]. [accessed 2020 January].
- Centers for Medicare & Medicaid Services. Medicare physician fee schedule. [accessed 2020 June 11] https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-National-Payment-Amount-File .
- Centers for Medicare & Medicaid Services. Medicare clinical laboratory fee schedule. [accessed 2020 June 11] https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files .
- Centers for Medicare & Medicaid Services. Medicare part B drug average sales price. [accessed 2020 June 11]. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2020-asp-drug-pricing-files.
- Centers for Disease Control and Prevention. Blood/body fluid exposure option. CDC. [accessed 2020 June 09]. https://www.cdc.gov/nhsn/pdfs/hps-manual/exposure/3-hps-exposure-options.pdf.
- US Department of Health and Human Services. Viral hepatitis in the United States: data and trends. [accessed 2020 October 16]. https://www.hhs.gov/hepatitis/learn-about-viral-hepatitis/data-and-trends/index.html .
- Gershon RR, Mitchell C, Sherman MF, Vlahov D, Lears MK, Felknor S, Lubelczyk RA. Hepatitis B vaccination in correctional health care workers. Am J Infect Control. 2005;33(9):510–18. doi:10.1016/j.ajic.2005.04.245.
- Bick JA. Infection control in jails and prisons. Clin Infect Dis. 2007;45(8):1047–55. doi:10.1086/521910.
- Frogner BK. The health care job engine: where do they come from and what do they say about our future? Med Care Res Rev. 2018;75(2):219–31. doi:10.1177/1077558716688156.

