ABSTRACT
In India, although incidence of Herpes zoster has not been assessed, regional cases have been reported. We revisited the peer-reviewed literature on clinical cases of HZ to depict the trends in population characteristics, disease presentation, and predisposing factors for the disease in India. Systematically conducted literature search yielded 27 studies, published between January 2011 and May 2020, reporting 3124 HZ clinical cases, with high proportions in older adults (>50 years of age: 15.0–81.3%). Thoracic dermatome was consistently reported as the most frequent site affected by HZ (38.9–71.0%). Post-herpetic neuralgia and secondary bacterial infections were the two most frequent complications (10.2–54.7% and 3.5–21.0%, respectively). Despite the paucity of data and gaps in the reporting of HZ cases, available evidence indicate that the disease causes an important burden to older adults in India, suggesting that preventive strategies, along with recommendations to healthcare practitioners, can help mitigate the burden of HZ.
PLAIN LANGUAGE SUMMARY
What is the context?
Herpes zoster is caused by reactivation of the varicella virus in sensory gangila usually during older age.
The most common complication involve skin and neurological disorders, such as post herpetic neuralgia.
Herpes zoster can impact quality of life for months or even years (especially for those >50 years of age).
In India, there is a lack of population-based studies on herpes zoster to reflect burden of disease.
What is new?
We reviewed the literature on clinical cases of herpes zoster in India from last decade and found that:
High propotions of older adults (>50 years of age) are reported to have the disease.
Thoracic dermatomes and post herpetic neuralgia are common clinical presentation and complication.
Post herpetic neuralgia is observed more frequently in older patients.
What is the impact?
Our review highlights sigificant number of cases of herpes zoster are reported and the disease causes a substantial burden to older adults in India.
In view of the growing elderly population in India, the finding of greater proportion of cases in >50 years of age holds importance.
Implementation of preventive strategies along with guidance to healthcare pracitioners can help prevent the disease and complications in vulnerable population.
Introduction
Herpes zoster (HZ) disease, also known as ‘Shingles,’ usually presents as a painful, vesicular dermatomal rash.Citation1 It results from the reactivation of the latent varicella-zoster virus in sensory ganglia.Citation2 The factors that trigger reactivation of dormant virus are not fully elucidated, though decline in cell-mediated immunity with age or immunosuppressive conditions or treatments play an important role.
HZ episodes may lead to complications and sequelae, including, neurological disorders like post-herpetic neuralgia (PHN), limb paralysis, cerebrovascular disorders like stroke, cardiovascular disease such as myocarditis, and serious skin alterations such as intense scarring.Citation1,Citation3,Citation4 The most common complication encountered is PHN, when the pain accompanying the rash persists for months or even years after the initial episode. The pain can be debilitating and lead to physical disability, emotional distress, and sleep disorder.Citation5 Worldwide, estimates for the risk of PHN following HZ episode range between 5% and 30%.Citation6 Herpes zoster ophthalmicus (HZO) is another presentation of the disease that can lead to ocular complications, often causing decreased visual acuity or even blindness.Citation3,Citation7
Treatment of acute HZ is primarily designed to manage pain, and hasten recovery.Citation3 Pain is usually managed with analgesics but may require strong opioids.Citation8 Antiviral agents such as acyclovir, famciclovir, and valaciclovir are commonly used in the treatment of the disease.Citation3,Citation8 Local management of skin lesions may help in alleviating the discomfort and preventing the development of long-lasting skin lesions. For patients suffering from PHN, treatment comprises anticonvulsants, tricyclic antidepressants, topical therapies, and opioids.Citation4,Citation9
Many studies have been conducted across different parts of the world to study the incidence rates of HZ.Citation4,Citation6,Citation10 In Asia, the overall HZ incidence has been estimated at 5.0 per 1000 person-years (PY).Citation4 Stratification by age groups yielded median values of 2.0–3.1, 4.3–5.2, and 7.4–13.8 per 1000 PY in the 0–20, 20–50 and in >50 years old populations, respectively.Citation4 To the best of our knowledge, there are no population-based studies with potential to provide incidence data specific to India. While there is study-to-study variation due to differences in methodology, location appears to have little impact on the incidence of HZ, which is similar in different regions of the world.Citation6,Citation11–13
The aim of this work was to better understand the clinical profile of HZ disease reported in India. To achieve this, literature was collected through searches carried out systematically in the PubMed and Embase databases for the period of Jan 2011 to May 2020. An additional search was performed in Google Scholar which is described in the literature search section. We have described the trends in demographic characteristics of HZ patients, clinical presentation of the disease, complications and predisposing factors reported in Indian studies published between 2011 and 2020.
Methods
Research articles in English language published since 2011 were searched for “zoster” and “India” in all fields on May 20th and 26th, 2020 in PubMed and Embase, respectively. An additional search was performed in Google Scholar for “study” and “herpes zoster” (all in title, including citations) on January 8th, 2021 to identify additional literature not indexed in PubMed nor Embase. Screening of titles, abstracts, and full texts was performed by two independent reviewers. Any discordance about the final selection of articles was collegially resolved according to the pre-specified inclusion/exclusion criteria. Data were collated for descriptive purpose.
Inclusion criteria
Articles reporting analytical, prospective observational, cross sectional, retrospective reviews, and time bound studies conducted in either dermatology, medicine and/or ophthalmology departments of various medical colleges and tertiary care centers across India were included. Only studies reporting data from multiple HZ patients, providing quantitative data about demographics, various disease presentations, complications or risk factors in India were selected.
Exclusion criteria
Individual case reports, case series not providing quantitative analysis, scholar theses, editorials and letters to editors were excluded from the review. Articles reporting interim analyses of studies for which the final analysis was already included were excluded from the review to avoid inclusion of redundant datasets. Articles published before 2011 and after May 2020 were excluded from the Google scholar search to match the settings of the Embase and PubMed searches.
Literature search
There were 242 and 528 records retrieved from PubMed and Embase respectively (Supplementary Figure 1). After elimination of 163 duplicate records, titles and abstracts of 607 unique records were screened. A total of 14 articles were included from this initial search.Citation14–27 A search for additional literature in Google Scholar, was carried out due to limited number of articles retrieved in the initial search. Post Google scholar search, 13 additional articles were further added.Citation28–40 Also the 2013 paper from Nithyanandam et al.Citation25 was a post-hoc analysis, so we retrieved the data from the primary publication.Citation41 Data were extracted from the 27 relevant articles for review.
Results
Characteristics of included studies
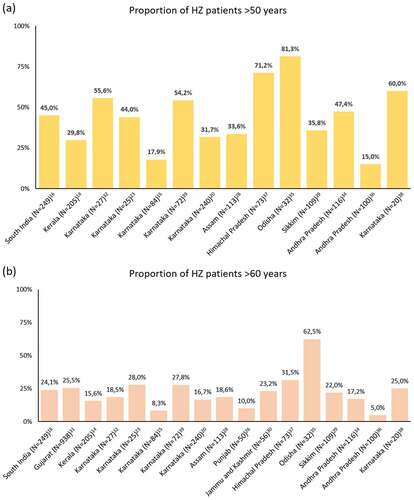
A total of 27 studies collectively reporting 3124 HZ patients (range 18 to 938) conducted between 2003 and 2019 were retrieved. Location and period of these studies along with demographic characteristics of the HZ patients are reported in . Key outputs extracted from all studies are reported in Supplementary Table 1.
Figure 1. Study location, number of cases (including frequency among outpatients department visits) and their characteristics (age descriptors and sex ratio) from the retrieved peer-reviewed literature on herpes zoster in India (January 2011 – May 2020). Interactive version of the figure available on Figshare Supplemental data for this article can be accessed on the publisher’s website at [https://doi.org/10.6084/m9.figshare.15156114.v1].
![Figure 1. Study location, number of cases (including frequency among outpatients department visits) and their characteristics (age descriptors and sex ratio) from the retrieved peer-reviewed literature on herpes zoster in India (January 2011 – May 2020). Interactive version of the figure available on Figshare Supplemental data for this article can be accessed on the publisher’s website at [https://doi.org/10.6084/m9.figshare.15156114.v1].](/cms/asset/18e587f7-4bb3-462f-9a38-b54d37a026fe/khvi_a_1968737_f0001_c.jpg)
Of the retrieved studies, 24 were prospective cross-sectional and three were retrospective (Supplementary Table 1). Nine studies either did not mention or failed to report the study period. Clinical diagnosis and Tzanck smear tests were mostly used as case definition for HZ, though this information was not systematically reported. Most studies (n = 21) included patients of all ages, while four studies reported HZ cases in the pediatric population only.Citation18,Citation19,Citation21,Citation33 Eight studies reported exclusively on HZO cases.Citation16,Citation17,Citation23,Citation32,Citation35,Citation37,Citation38,Citation41
Of the 27 publications, sevenCitation21,Citation22,Citation24,Citation27,Citation28,Citation31,Citation40 provided proportions of HZ cases among all patients who attended outpatient visits in the considered departments, ranging between 0.28 and 2.36% (). For example in the study with the largest sample size conducted in a rural hospital in Gujarat, 938 HZ cases were reported between June 2008 and December 2016 among all dermatology outpatients, yielding a proportion of 0.38%.Citation31
Demographics
Age range
As seen in Supplementary Table 1, eleven studies that included patients of all ages reported their mean age ranging between 29.6 and 57.3 years.Citation15,Citation16,Citation22–24,Citation28,Citation31,Citation35,Citation37,Citation38,Citation41 Roughly half of the studies reported proportions of patients by age groups. Proportions of patients with HZ in >50 and >60 years ranged from 15.0%Citation36 to 81.3%Citation35 and from 5.0%Citation36 to 62.5%,Citation35 respectively. The very low proportions observed in the studies conducted by Usha et al.Citation36 and Aggarwal et al.Citation15 is at odd with other studies. It is noteworthy that the latter was conducted in a military hospital, explaining the high proportion of young adult male patients.Citation15 This selection bias is however not discussed by the authors.Citation15 Little details are provided by Usha et al. regarding the selection of the 100 patients included in the study, presenting a high risk of bias for the presented population.Citation36 The distribution across studies for both age groups is depicted in . The largest proportions were reported in a study conducted on 32 HZO patients in Odisha during 2016–2018, where 81.3% and 62.5% of the patients were older than 50 years and 60 years of age, respectively.Citation35 In the retrieved study having the largest number of patients (N = 938), conducted between 2008 and 2016 in rural Gujarat, 25.5% of the cases occurred in individuals older than 60 years.Citation31
Sex ratio
Male:female ratio ranged between 1.1:1 and 1.9:1 in 15 studies ().Citation14,Citation16,Citation19,Citation20,Citation22,Citation24,Citation28–31,Citation33,Citation35,Citation37,Citation38,Citation41 Six studies found the ratios to be in the order of 2:1,Citation15,Citation17,Citation23,Citation26,Citation36,Citation40 Five studies, however, reported more women patients with HZ than men, in the range of 0.6:1 to 0.9:1.Citation18,Citation21,Citation32,Citation34,Citation39
History of varicella infection and vaccination
Eleven studies reported the proportion of HZ patients with a known history of varicella infection (chickenpox).Citation14,Citation17–22,Citation24,Citation28,Citation31,Citation33 Proportion of patients who had chickenpox ranged between 22.7%Citation31 and 79.6% (Supplementary Table 1).Citation28 Varicella vaccination history was documented in only four studies,Citation18,Citation19,Citation21,Citation33 and proportions were in the range of 0.0%Citation33 to 30.8%Citation18,Citation21 of cases.
Predisposing factors for HZ disease
Besides advanced age, human immunodeficiency virus (HIV) seropositivity, diabetes mellitus, prolonged steroid use, and malignancies with therapy were the frequently associated predisposing/co-morbid conditions in the retrieved studies (Supplementary Table 1). HIV status of the enrolled population was clearly stated in 23 studies.Citation14,Citation15,Citation17–24,Citation26–28,Citation31,Citation32,Citation34–41 For other studies, HIV status was either not assessed or mentioned. The reported frequency of HIV seropositivity among the studied patients varied between 0%Citation22,Citation27,Citation38 and 44.4%.Citation17 Diabetes mellitus was reported in 15 studiesCitation14,Citation16,Citation20,Citation22,Citation23,Citation27–29,Citation32,Citation34–39 with a percentage range of 0%Citation27 to 56.3%.Citation35 The highest proportion of diabetes (56.3%) was reported in a study conducted in the Ophthalmology department of a tertiary hospital in Odisha where 20/32 patients (62.5%) were older than 60 years of age.Citation35
Immunocompetence level is not assessed or clearly reported in all studies. Moreover, some authors considered diabetes as an immunocompromising condition while others did not. According to authors’ assessments, proportion of immunocompromised patients varied between 0%Citation27 and 44.4%.Citation17 However, given the high uncertainty of the data and possible selection bias, data on immunocompromised population should be interpreted with caution.
Disease presentation
Prodromal symptoms
Twenty studies described the prodromal symptoms experienced by the patients (Supplementary Table 1).Citation14,Citation16,Citation18–24,Citation26,Citation28,Citation31,Citation32,Citation34–39,Citation41 Reported prodromal symptoms included segmental pain (58.9%–100%),Citation24,Citation39 fever (2.5%–44.4%),Citation22,Citation31 headache (3.4%–10.0%),Citation20,Citation31 itching (8.0%–45.5%),Citation22,Citation28 burning sensation (13.3%–68.9%),Citation19,Citation34 and paresthesia (6.2%–50.0%).Citation26,Citation28 In studies focused on HZO patients,Citation16,Citation17,Citation23,Citation32,Citation35,Citation37,Citation38,Citation41 the reported prodromal symptoms were eye watering (33.3%–58.9%),Citation32,Citation37 lid swelling (18.5%–87.5%),Citation32,Citation35 and diminution of vision (25.0%–37.0%).Citation32,Citation38
Rash characteristics
Eighteen studies reported dermatomal distribution as per site/location in HZ patients (). Thoracic dermatome was consistently reported as the most frequent dermatome involved (38.9%–71.0%).Citation27,Citation39 Other commonly affected sites were the cranial (3.3%–28.3%),Citation14,Citation19,Citation28 cervical (4.0%–23.8%),Citation27,Citation36 and lumbar (5.5%–35.0%) regions.Citation29,Citation33 Three studies reported cases of multi-dermatomal involvement.Citation29,Citation31,Citation36 Sharma and Sharma observed multi-dermatomal presentation in 8.25% of the 109 reported cases.Citation29 Among the 100 patients reported by Usha et al., 42% had multi-dermatomal involvement, among which 87.5% were living with HIV.Citation36 Vora et al. noticed that among 938 HZ patients, 43 cases (4.6%) had multiple dermatomes involved.Citation31
Table 1. Proportions of cervical, cranial, lumbar, and thoracic dermatomes among HZ patients across the retrieved studies
Pain characteristics
Pain was a common symptom in many studies.Citation14,Citation18–22,Citation24,Citation26,Citation28,Citation30,Citation31,Citation34,Citation37–39,Citation41 Pain preceded vesicles onset in 60.4% of the patients recruited in Karnataka by Malkud et al.Citation20 In the same study, 15 out of 240 patients (6.3%) experienced intermittent radicular pain and 10 patients (4.2%) suffered continuous pain.Citation20
In the study by Vora et al., burning pain affected 720/938 HZ patients (76.8%), which was by far the most common type of pain encountered; other types of pain were itching pain (8.0%), pricking pain (7.4%) and throbbing pain (5.2%).Citation31 Burning pain (18.3%) and pricking pain (54.2%) were also described in the study by Rachana et al. on 72 HZ patients.Citation39 Burning pain was also denoted as a common finding in the three studies reported by Adhicari and Agarwal, Gupta and Sareen, and Mitra et al.Citation21,Citation28,Citation37
Complications
Among the retrieved studies, 21 publications provided data on associated complications (Supplementary Table 1).Citation14–18,Citation20–24,Citation26,Citation28,Citation30,Citation32–38,Citation41 PHN (10.2%-54.7%)Citation14,Citation41 and secondary bacterial infections (3.5%–21.0%)Citation28,Citation36 were the most frequently reported complications. Other complications included scarring (including keloids),Citation14,Citation20,Citation24,Citation28,Citation34,Citation36 paresthesia with motor involvement,Citation14,Citation15,Citation20,Citation30 sensory and hearing loss,Citation16,Citation32,Citation34,Citation35,Citation37,Citation41 Ramsay Hunt syndrome,Citation26,Citation30 and pigmentary changes.Citation14,Citation20,Citation22,Citation28,Citation36 Among HZO patients, corneal lesions were common.Citation16,Citation17,Citation23,Citation41 HZO patients also suffered a wide range of complications including visual impairment,Citation23,Citation32,Citation35,Citation37,Citation41 and secondary glaucoma (detailed in Supplementary Table 1).Citation17,Citation32,Citation35,Citation37,Citation38
Post-herpetic neuralgia
PHN accounted for 10.2% to 54.7% of the reported complication among HZ patients (Supplementary Table 1),Citation14,Citation41 and its incidence was found to be higher among the older individuals (>50 years old) in the study of Puri.Citation26 Also, as per Adhicari et al., maximum cases of PHN were concentrated in the age group 41–70 years old.Citation28 Similarly, Malkud et al.Citation20 reported that overall proportion of PHN among the 240 HZ patients was 10.4%, and that half of HZ patients 60 years or older developed this complication.Citation20
PHN was also observed with higher frequency in immunocompromised patients. In a study conducted by Gupta et al.,Citation17 proportion of PHN was higher among patients living with HIV (n = 6/8: 75%) when compared to the rest of HZ patients (n = 1/10: 10.0%). Usha et al. also reported a greater proportion of PHN cases in HIV seropositive patients (n = 8/32: 25%) relative to HIV seronegative patients (n = 7/68: 12.9%).Citation36
Herpes zoster ophthalmicus
HZO, the form of HZ affecting the first branch of the trigeminal nerve, i.e. the ophthalmic nerve, may include additional ocular symptoms and complications. Eight studies specifically described HZO and its associated complications in detail (Supplementary Table 1).Citation16,Citation17,Citation23,Citation32,Citation35,Citation37,Citation38,Citation41 Acute corneal epithelial lesions (64.1%), reduced corneal sensation (67.2%) and uveitis (48.4%) were some of the serious complications reported in a study of 64 patients.Citation41 In another study (n = 25 HZO patients), 76.0% suffered lid edema while 52% had decreased corneal sensitivity.Citation23 Also, impaired visual acuity was reported in 40% of the patients in the same study.Citation23 Lid (52.6%) and corneal (56.6%) involvement, along with PHN (30.9%) were also reported in a retrospective study (n = 249 HZO cases) between 2006 and 2016 in two tertiary referral eye centers.Citation16 Anterior uveitis was the most frequent presenting symptom (>50% of cases) seen.Citation16 In a separate study of 18 HZO, 83.3% of the patients manifested corneal involvement.Citation17 In the same study it was mentioned that visual acuity was better in patients with HIV-negative status than in those living with HIV.
Discussion
This is the first large scale review of the published literature to ascertain the occurrence of HZ and its complications in India, as well as gaps in available data about the disease in India. Acknowledging the fact that HZ is not notifiable and in the absence of population-based epidemiological studies, along with poor surveillance system, it is important to fall back on a literature review. Though studies in various outpatient settings of tertiary care centers differ in terms of design, methodology and other various aspects, the data are of significance as it gives a fair estimate of the course of disease, its characteristics, and complications. Nonetheless, possible recruitment bias and gaps in data regarding patients’ characteristics, disease presentation, as well as complication in the retrieved literature, impaired us from performing quantitative analyses and may be responsible for the differences in proportions of HZ in older adults, as well as rates of complications, PHN and bacterial infections.
This review notably indicates that the risk of HZ is significant in individuals older than 50 years of age, as observed in studies conducted in other parts of the world, and that HZ cases are seen by a variety of medical specialists (dermatology, ophthalmology, internal medicine, neurology, etc), depending on disease presentation. Proportions of HZ patients among patients with other conditions at dermatology outpatient departments, was between 0.28%Citation22 and 2.36%.Citation27 The proportions differ among studies mainly due to differences in characteristics of the population evaluated. Despite this fact, the pattern is similar to that reported in Asia-Pacific region (e.g. Australia: 1.81 HZ cases/1000 consultations, 2006–2012).Citation4 This tends to indicate that proportion of HZ in India is similar to rates reported elsewhere.Citation4 However, HZ proportions seen in this review are likely to be underestimated, mainly due to factors such as community and patient seeking behavior, physician practice, HZ severity, etc. It was moreover observed that HZ incidence rates are increasing in countries across Asia-Pacific (Korea 1994–2003: 3.0/1000 PY; Korea 2003–2007: 10.0/1000 PY; Taiwan 2000: 4.04/1000 PY; Taiwan 2009: 6.24/1000 PY),Citation4,Citation42 which is most likely attributable to rising incidence in aging populations.Citation4 Similarly, a growing number of HZ cases is expected to occur in India in the coming years as the Indian population aged 50 years and above has quadrupled over the last years, and is expected to comprise 404 million people in 2036, representing 27% of the country’s projected population.Citation43,Citation44 Indeed, while HZ cases were reported across age groups, it was predominant in individuals >50 years of age (15.0–81.3%). This is in agreement with the literature where the age-related increased incidence of HZ is thought to result from the decline in cell-mediated immunity (immunosenescence).Citation13 Besides advanced age, patients affected by chronic diseases are also fragile due to impairment of cell-mediated immunity.Citation45–47 Accordingly, HIV, diabetes mellitus, malignancies, and other chronic conditions were the frequently reported ailments in patients with HZ. It is noteworthy that India had an epidemic peak of HIV around 2000 and that the prevalence trend is now declining, reaching 0.22% among adults aged 15 to 49 years in 2019.Citation48
PHN is the most common complication of HZ. In present review, 10.2% to 54.7%Citation14,Citation41 of the patients accounted with PHN. Though the risk of PHN may vary by study design, age or definitions used for PHN, the proportion was in accordance with the reported numbers (5% to >30%), including studies from North and South America, Europe, the Middle East, as well as the Asia-Pacific region.Citation6,Citation49 The incidence of PHN was found to be higher among the older (>50 years old) HZ patients, in agreement with the reported increase in frequency and severity of PHN with advancing age.Citation50 The pain and discomfort associated with PHN can be prolonged and disabling, diminishing the patient’s quality of life and ability to function.Citation4,Citation51
HZO is another relatively common presentation of HZ with a reported incidence of 10–15% of all herpes zoster cases.Citation6,Citation52 HZO and ocular complications were also reported in studies included in present review. As per peer-reviewed literature, wide range of eye complications, such as keratitis, uveitis and conjunctivitis have been reported in the range of 30% to 78%.Citation6
In India, HZ is not a reportable disease and despite poor surveillance system, a good number of clinical cases were reported in this review. Also, in view of the growing elderly population in India, the finding of greater proportion of cases in >50 years of age holds importance. The evidence generated needs be further strengthened and one way would be to conduct nationwide population-based study that is critical to investigate the true burden and epidemiology of HZ disease in India. However, until that time, as HZ cases are seen by a variety of medical specialists and the disease can cause substantial morbidity among older adults, prevention strategies and recommendations or guidance from healthcare professionals (HCPs) can play a critical role.
elaborates on the findings in a form that could be shared with patients by HCPs.
Abbreviations
Contributorship
All authors participated in the design or implementation or analysis, and interpretation of the study and contributed to the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Disclosure of potential conflicts of interest
RP, SK, AA and RD are employees of the GSK group of companies. SK and RP hold shares as part of their employee remuneration. RP, SK, AA and RD declare no other financial or non-financial relationships and activities. AV and AP declare no financial or non-financial relationships and activities and no conflicts of interest.
Supplemental Material
Download MS Word (126.8 KB)Supplemental Material
Download PDF (985 KB)Acknowledgments
The authors thank Business & Decision Life Sciences platform for editorial assistance, manuscript coordination and design support for the digital illustrations, on behalf of GSK. Benjamin Lemaire coordinated publication development and editorial support. Jonathan Ghesquiere provided medical writing support.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1968737.
Additional information
Funding
References
- Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211–15. doi:10.1136/bmj.39206.571042.AE.
- Hope-Simpson RE. The nature of Herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20.
- Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol. 2018;84:251–62. doi:10.4103/ijdvl.IJDVL_1021_16.
- Chen L-K, Arai H, Chen L-Y, Chou M-Y, Djauzi S, Dong B, Kojima T, Kwon KT, Leong HN, Leung EMF, et al. Looking back to move forward: a twenty-year audit of herpes zoster in Asia-Pacific. BMC Infect Dis. 2017;17:213.
- Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. doi:10.1186/1741-7015-8-37.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi:10.1136/bmjopen-2014-004833.
- Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002;66:1723–30.
- Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748–50. doi:10.1136/bmj.326.7392.748.
- Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31:235–43. doi:10.3344/kjp.2018.31.4.235.
- Araújo LQ, Macintyre CR, Vujacich C. Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America. Herpes. 2007;14:40–44.
- Lu WH, Lin CW, Wang CY, Chen LK, Hsiao FY. Epidemiology and long-term disease burden of herpes zoster and postherpetic neuralgia in Taiwan: a population-based, propensity score-matched cohort study. BMC Public Health. 2018;18:369. doi:10.1186/s12889-018-5247-6.
- Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013;13:170. doi:10.1186/1471-2334-13-170.
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81:928–30. doi:10.1212/WNL.0b013e3182a3516e.
- Abdul Latheef E, Pavithran K. Herpes zoster: a clinical study in 205 patients. Indian J Dermatol. 2011;56:529–32. doi:10.4103/0019-5154.87148.
- Aggarwal SK, Radhakrishnan S. A clinico-epidemiological study of herpes zoster. Med J Armed Forces India. 2016;72:175–77. doi:10.1016/j.mjafi.2015.05.003.
- Babu K, Mahendradas P, Sudheer B, Kawali A, Parameswarappa DC, Pal V, Philips M. Clinical profile of Herpes zoster ophthalmicus in a South Indian patient population. Ocul Immunol Inflamm. 2018;26:178–83. doi:10.1080/09273948.2017.1381272.
- Gupta N, Sachdev R, Sinha R, Titiyal JS, Tandon R. Herpes zoster ophthalmicus: disease spectrum in young adults. Middle East Afr J Ophthalmol. 2011;18:178–82. doi:10.4103/0974-9233.80710.
- Katakam BK, Kiran G, Kumar U. A prospective study of Herpes zoster in children. Indian J Dermatol. 2016;61:534–39. doi:10.4103/0019-5154.190121.
- Malkud S, Dyavannanavar V. Childhood herpes zoster: a study from tertiary center. J Pak Assoc Dermatol. 2017;27:120–23.
- Malkud S, Dyavannanavar V, Purnachandra MKS. Clinical and morphological characteristics of herpes zoster - A study from tertiary care centre. J Pak Assoc Dermatol. 2016;26:219–22.
- Mitra B, Chopra A, Talukdar K, Saraswat N, Mitra D, Das J. A clinico-epidemiological study of childhood Herpes zoster. Indian Dermatol Online J. 2018;9:383–88.
- Mondal A, Kumar P, Swathi G, Dasarathan S. A comparative study of herpes zoster: adult versus paediatric patients. J Pak Assoc Dermatol. 2019;29:278–85.
- Naveen KN, Pradeep AV, Athanker SB. A study of clinical profile and ophthalmological manifestations of herpes zoster ophthalmicus with HIV seropositivity in Northern Karnataka. J Pak Assoc Dermatol. 2016;26:21–25.
- Naveen KN, Tophakane RS, Hanumanthayya K, Pv B, Pai VV. A study of HIV seropositivity with various clinical manifestation of herpes zoster among patients from Karnataka, India. Dermatol Online J. 2011;17:3. doi:10.5070/D33N65H0C5.
- Nithyanandam S, Joseph M, Stephen J. Ocular complications and loss of vision due to herpes zoster ophthalmicus in patients with HIV infection and a comparison with HIV-negative patients. Int J STD AIDS. 2013;24:106–09. doi:10.1177/0956462412472303.
- Puri N. A study on clinical presentation of herpes zoster in a district hospital in North India. J Pak Assoc Dermatol. 2016;26:134–37.
- Singh GK, Singh Deora M, Grewal R, Kushwaha A, Minhas S. Is high altitude a risk factor in development of Herpes zoster? High Alt Med Biol. 2018;19:244–48. doi:10.1089/ham.2018.0005.
- Adhicari D, Agarwal D. A hospital-based clinical study of Herpes zoster- a report of 113 cases. IOSR JDMS. 2017; 16:33–38.
- Sharma R, Sharma R. Clinical study of herpes zoster in 109 patients in central referral hospital, Gangtok. Int J Res Dermatol. 2019;5:849–52. doi:10.18203/.2455-4529.IntJResDermatol20194680.
- Nusratnazirmakroo CAH, Peerzada G. A study based on clinical presentation and complications in Herpes zoster patients: an analytical study. Indian J Dent Adv. 2017;9:24–28.
- Vora RV, Singhal RR, Anjaneyan G, Patel TM. Clinicoepidemiological study of herpes zoster at rural based tertiary center of Gujarat. Indian J Clin Exp Dermatol. 2018;4:40–43.
- Maiya AS, Shenoy S. A clinical study of Herpes zoster ophthalmicus. IOSR JDMS. 2013;12:9–13. doi:10.9790/0853-1260913.
- Lanker AM, Jeelani S, Jeelani N. Herpes zoster in the pediatric age group: study from a tertiary care hospital. Int J Contemp Pediatr. 2015;2:321–24. doi:10.18203/2349-3291.ijcp20150779.
- Naik B. Herpes zoster, shingles - A clinicoepidemiological study and its complications among immunocompetent and immunocompromised patients. Int J Sci Res. 2019;8:69–71.
- Behera S, Swain LM, Dora J, Pandey S. Herpes zoster ophthalmicus: clinical profile and management, a prospective study at a tertiary health care centre, Western Odisha. Int J Sci Res. 2019;8:61–63.
- Usha G, Srinivasulu P, Bharathi G. Clinico epidemiological study of Herpes zoster in HIV era in a tertiary care hospital in South India. IOSR JDMS. 2015;14:32–35.
- Gupta M, Sareen A. A prospective study to observe ocular outcome and clinicoepidemiological pattern in Herpes zoster ophthalmicus at district hospital in hilly area in India. SJAMS. 2017;5:2607–14.
- Shanthaveerappa P, Parappallil RJ. The clinical profile and ocular manifestations of Herpes zoster ophthalmicus - a hospital based study. IJOVS. 2019;4:19–23. doi:10.11648/j.ijovs.20190401.14.
- Rachana R, Shivaswamy KN, Anuradha HV. A study on clinical characteristics of herpes zoster in a tertiary care center. Int J Res Dermatol. 2017;3:79–82. doi:10.18203/.2455-4529.IntJResDermatol20170081.
- Sundaram M, Adikrishnan S, Krishnakanth M, Sudha R, Mahalakshmi V, Shobana S, Anandan S. Hospital based cross sectional study of herpes zoster with reference to HIV seropositivity. BMC Infect Dis. 2012;12:P58. doi:10.1186/1471-2334-12-S1-P58.
- Nithyanandam S, Stephen J, Joseph M, Dabir S. Factors affecting visual outcome in herpes zoster ophthalmicus: a prospective study. Clin Exp Ophthalmol. 2010;38:845–50. doi:10.1111/j.1442-9071.2010.02352.x.
- Wu PY, Wu HD, Chou TC, Sung FC. Varicella vaccination alters the chronological trends of herpes zoster and varicella. PLoS One. 2013;8:e77709. doi:10.1371/journal.pone.0077709.
- National Commission on Population - Ministry of Health & Family Welfare - Goverment of India. Census of India. Population projections for India and States 2011-2036. Report of the Technical Group on Population Projections. 2011 [accessed 22 Mar 2021]. https://nhm.gov.in/New_Updates_2018/Report_Population_Projection_2019.pdf .
- United Nations - Department of Economic and Social Affairs - Population Division. World population prospects 2019. [accessed 22 Mar 2021]. https://population.un.org/wpp/Download/Standard/Population/.and./https://population.un.org/wpp/Publications/Files/WPP2019_10KeyFindings.pdf .
- Kawai K, Yawn BP. Risk factors for Herpes zoster: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4:S313–S4. doi:10.1093/ofid/ofx163.733.
- Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for Herpes zoster infection: a meta-analysis. Open Forum Infect Dis. 2020;7:ofaa005. doi:10.1093/ofid/ofaa005.
- Wu MY, Hsu YH, Su CL, Lin YF, Lin HW. Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am J Kidney Dis. 2012;60:548–52. doi:10.1053/j.ajkd.2012.03.018.
- Government of India - Ministry of Health and Family Welfare. HIV facts & figures. [accessed 2 July 2021]. http://naco.gov.in/hiv-facts-figures .
- Yang F, Yu S, Fan B, Liu Y, Chen YX, Kudel I, Concialdi K, DiBonaventura M, Hopps M, Hlavacek P, et al. The epidemiology of Herpes zoster and postherpetic neuralgia in China: results from a cross-sectional study. Pain Ther. 2019;8:249–59. doi:10.1007/s40122-019-0127-z.
- Johnson RW, McElhaney J. Postherpetic neuralgia in the elderly. Int J Clin Pract. 2009;63:1386–91. doi:10.1111/j.1742-1241.2009.02089.x.
- Watson CPN, Gershon AA, Oxman MN, Herpes zoster: postherpetic neuralgia and other complications. Cham: Adis; 2017. DOI:10.1007/978-3-319-44348-5
- Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982;61:310–16. doi:10.1097/00005792-198209000-00003.