ABSTRACT
Japanese encephalitis (JE) is an endemic disease dominantly in the Asia-Pacific region with mortality rate varying between 3% and 30%. Long-term neuropsychiatric sequelae developed in 30–50% of the survivors. There is no available antiviral therapy for JE. JE vaccines play a major role in preventing this devastating disease. The incidence of JE declined over years and the age distribution shifted toward adults in countries where JE immunization program exists. Mouse brain–JE vaccine is currently replaced by inactivated Vero cell-derived vaccine and live-attenuated vaccine using SA14-14-2 strain, and live chimeric JE vaccines. These three types of JE vaccines are associated with favorable efficacy and safety profiles. Common adverse reactions include injection site reactions and fever, and severe adverse reactions are rare.
Introduction
Japanese encephalitis (JE) was first described as an unusual outbreaks of summer encephalitis in Japan during the 19th century.Citation1,Citation2 JE virus (JEV) was later isolated from a human brain in the 1930s.Citation2 Subsequently, it was recognized as an endemic disease in the Asia-Pacific region, including Japan, China, Korea, Taiwan, India, Pakistan, the Philippines, and Australia.Citation3 JE has become a notifiable disease in Taiwan since 1955. JEV is a mosquito-borne Flavivirus belonged to the family Flaviviridae. JEV can be further divided into five genotypes according to the E gene and genomic sequences.Citation4 Wading and water birds are natural hosts and carriers of JEV. Several livestock species may serve as amplifying hosts.Citation1,Citation3 Domestic and wild pigs play the most crucial role in supplying virus in blood for infection of feeding mosquitoes.Citation1 Humans are dead-end hosts and most infected people are asymptomatic. Therefore, it is difficult to realize the accurate incidence of JE and the disease burden may be underestimated. It is estimated that less than 1% of JEV-infected humans develop disease.Citation3 Clinical manifestations range from mild disorientation or subtle personality change to confusion, delirium, and even coma. Typical thalamic lesions on computed tomography and/or magnetic resonance imaging had a sensitivity of 23% and a specificity of 100% for the diagnosis of JE.Citation5 Mortality rate varying between 3% and 30%. Long-term neuropsychiatric sequelae developed in 30% to 50% of the survivors, such as persistent motor deficit, learning difficulties, behavior problems, and personality change.Citation4,Citation6 Several reports show that JE in young children and the elderly were more likely to be associated with severe sequelae and a higher mortality rate than young adults.Citation6,Citation7
Epidemiology
According to a systematic review including 24 JE endemic countries published in 2011,Citation8 approximately 67,099 JE cases occur annually with an overall incidence of 1.8 per 1,00,000. About 50% of these cases occurred in China and near 75% were children aged 0 to 14 years old. All age groups have the risk to infect JE while most infections occurred in children in the past. However, the age distribution shifted toward adults currently in countries where JE immunization program exists.Citation7,Citation9–12 Due to the introduction of JE vaccine, urbanization and improved sanitary and public health measures, the incidence of JE decreased year by year in many endemic countries. The estimated incidence of JE in Korea was 0.1–1.8 per 1,00,000 population during 1980–1984 and decreased to 0.013–0.055 per 1,00,000 population during 2007–2010.Citation9 In Japan, the incidence dropped from over 1,000 cases every year in the 1960s to less than 10 cases annually in the 2000s. The decline of JE was also observed in China and Taiwan during the similar period.Citation4,Citation10,Citation11 However, JE has not been eliminated globally. Sporadic outbreaks are still important issues in some endemic areas, such as the outbreak in northern India and Nepal in 2005.Citation2
The first JEV isolated in 1935 belonged to genotype III that was prevalent in a broad region encompassing Japan, China, Taiwan, Vietnam, India, and Sri Lanka.Citation13 However, type III declined both in number and genetic diversity and type I has become the dominant genotype over the years.Citation4,Citation14 Genotype I had replaced genotype III in Thailand and Vietnam after the 1980s and the 1990s, respectively.Citation15,Citation16 A study from Japan also showed that all isolated JEVs have changed to genotype I since 1994.Citation13 National surveillance JEV data in China showed that genotype I accounted for 71% from 2001 to 2005.Citation14 Most JEV isolated in Australia belonged to genotype II similar with Malaysian and Indonesian isolates. However, genotype I JEV was also isolated for the first time in Australia in 2000.Citation17 The switch phenomenon of genotypes is not well explained. Possible reasons might be that genotype I JEV has increased infectivity for birds and wind-blown mosquitoes or viremic migratory birds spread JEV to new territories by chance.Citation2,Citation16
In Taiwan, annual JE incidence rate of 1.65 to 2.04 cases per 1,00,000 people between 1966 and 1970 decreased gradually after 1971 because universal JE vaccination program for children older than 15 months was started in 1968. The average annual incidence rate of JE was approximately 0.118 cases per 1,00,000 people between 2002 and 2012.Citation12 The predominant genotype was genotype III from 2005 to 2007, and genotype I increased in incidence from 2008 to 2012.Citation18 Further seroprevalence study showed that the cohort born between 1963 and 1975, who generally received two or three doses of the mouse brain–derived JE vaccine and were administered the last booster dose more than 20 years ago, exhibited the lowest positive rate of neutralizing antibody against JE. Seroprevalence of JE was relatively higher in male and in people living in rural areas.Citation12 Although the incidence of JE declines over years, this devastating disease cannot be fully resolved. Similar to other countries with universal JE vaccination program, JE has become uncommon in children. The main age group of the confirmed JE cases in Taiwan has shifted from young children to adults over 30 years of age.Citation12
Safety of Japanese encephalitis vaccines
According to the recommendation from the World Health Organization (WHO), JE vaccination should be integrated into national immunization schedules in all areas where JE is recognized as a public health priority.Citation19 There are four types of JE vaccines, including (1) inactivated mouse brain–derived JE vaccine, (2) primary hamster kidney (PHK) cell–derived, live-attenuated vaccine, (3) inactivated Vero cell-derived, alum-adjuvanted JE vaccine, and (4) live-attenuated chimeric JE vaccine (). Although all current JE vaccines are derived from genotype III strains, they are found to elicit protective levels of neutralizing antibodies against heterologous strains of other genotypes.Citation18,Citation20,Citation21 Mouse brain–derived vaccine (MBDV) is currently replaced by inactivated Vero cell–derived vaccine and live-attenuated vaccine using SA14-14-2 strain and live chimeric JE vaccines. Overall, all the three currently available JE vaccines in the market have good efficacy and safety profiles according to published systematic review.Citation22
Figure 1. Timeline of the development of four main Japanese encephalitis vaccines.
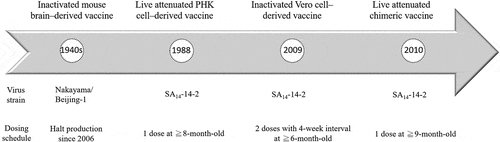
Inactivated mouse brain–derived JE vaccine
Inactivated MBDV is the first licensed JE vaccine under the trade name of JE–Vax and has been used internationally for a long time. It is produced by inoculating suckling mice intracerebrally with the virus and purifying inactivated virus suspension by ultracentrifugation.Citation18,Citation23 Inactivated mouse brain–derived JE vaccine uses two JEV stains, that is Nakayama and Beijing-1 strain. JE vaccines containing both the strains were equally capable of eliciting high levels of serum antibody and protective efficacy against a wide range of JEV strains.Citation23–26 These two inactivated mouse brain–derived JE vaccines had been used widely in endemic countries including Japan, South Korea, Taiwan, India, Vietnam, and Thailand.Citation23,Citation27 Inactivated MBDV containing Nakayama strain has been used in Taiwan for routine vaccination since 1968.Citation12 Inactivated MBDV had several disadvantages including higher reactogenicity than newer JE vaccines, a higher number of doses required and variability of manufacturing.Citation18,Citation19,Citation23 Local reactions including tenderness, swelling, and/or redness occur in around 20% of vaccinees. Systemic side effects such as headache, myalgia, fever, and gastrointestinal discomforts were reported in approximately 10% of vaccine recipients.Citation27 Post-marketing surveillance revealed a report rate of 2.8 per 1,00,000 vaccinations for total adverse events from 1996 through 1998 in Japan and 15 per 1,00,000 vaccinations during 1993 and 1998 in the United States.Citation28 Adverse events following inactivated MBDV in the United States during 1999 and 2009 was 24 per 100,000 doses distributed which consisting of 35% hypersensitivity reactions and 1% neurologic event.Citation29
Because MBDV may contain minute amounts of basic myelin protein from mouse brain, autoimmune neurological disorder including acute disseminated encephalomyelitis (ADEM) has been a theoretical safety concern of MBDV since the development of this vaccine.Citation27,Citation28,Citation30 Several children diagnosed of ADEM after receiving inactivated MBDV were reported in the 1990s in Japan.Citation31,Citation32 Notifications of neurological disorders, including ADEM, multiple sclerosis, myelitis and cognitive dysfunction, were reported in adults within one month after MBDV vaccination during 1980s and 1990s in Denmark.Citation30,Citation33 Nevertheless, there has been no case–control study to prove or disprove such an association. WHO suggested that inactivated MBDV should be replaced by the newer generation JE vaccines based on a higher incidence of other adverse reactions associated with MBDV.Citation19 The production of MBDV was then halted in 2006 and all the remaining supplies are limited.Citation34
PHK cell–derived, live-attenuated vaccine
A PHK cell–derived, live-attenuated vaccine based on the SA14-14-2 strain of the JEV has been used in China since 1988.Citation18,Citation19,Citation23,Citation27 The SA14-14-2 virus was demonstrated to be highly attenuated for various JE susceptible animals and be excellent in safety and efficacy.Citation23,Citation27,Citation35 This vaccine has been widely used in some Asian countries including South Korea, Nepal, India, Cambodia, Sri Lanka, and Thailand over the past decade.Citation2,Citation23,Citation27 After the introduction of PHK cell-derived, live-attenuated SA14-14-2 JE vaccine into national immunization program, the JE incidence has declined considerably in China, India, and Nepal.Citation36–39 A case–control study in Nepal provided evidence that the efficacy of a single dose of live-attenuated SA14-14-2 was more than 90% and might confront clinical JE disease for at least 5 years.Citation36,Citation40,Citation41 Another study on immunogenicity and safety of live attenuated SA14-14-2 JE vaccine in India showed that a single dose induced protective immunity in both JE seronegative and naturally seropositive adults.Citation42 Almost 95.5% of the participants remained seroprotected at the end of 12-month follow-up.Citation42 A 30-day follow-up study of live-attenuated SA14-14-2 JE vaccine in 26,239 children showed that the incidence of encephalitis, meningitis, all-cause hospitalization or other serious adverse events was no higher than the control group.Citation43 The risk ratios of all-cause hospitalization were 0.70 (95% confidence interval 0.43–1.15), and fever was 0.79 (95% confidence interval 0.56–1.11). Live-attenuated SA14-14-2 JE was evaluated in another one-year follow-up study in 305 children. Solicited local reactions were graded as less than severe and were reported during the first three days post-vaccination mostly.Citation44 Systemic reactions were mild, including anorexia, fever, and insomnia. A total of 26 serious adverse events were recorded, and all these events were judged to be unrelated to vaccination.Citation44 According to the post-marketing surveillance in China, the reported rate of adverse events and serious adverse events following immunization in 6024 cases was 96.55 and 1.12 per million doses, respectively.Citation45 Common adverse events included local reactions such as redness (0.2–24.1%), swelling (0.4–7.9%), and pain (0.9–37.5%) at injection site. Fever (4.9–25%) was the most common systemic reaction associated with live-attenuated SA14-14-2 JE vaccine.Citation36 Several serious adverse events including death and meningoencephalitis have been reported. There was no sufficient evidence for causality based on available data.Citation45–47
Inactivated, Vero cell–derived, JE vaccine
Inactivated, Vero cell–derived, alum-adjuvanted JE vaccine is produced by purification of SA14-14-2 strain virus grown in Vero cells.Citation19 This vaccine had non-inferior immunogenicity to a MBDV and was licensed under one of the three trade names, that are IXIARO, JESPECT, and JEEV, in the USA, Europe, Canada, Australia, Hong Kong, Switzerland, and India since 2009.Citation18,Citation19,Citation23,Citation27,Citation34,Citation48 The initial licensure was limited to adults and was later approved for pediatric use in early 2013.Citation23,Citation34,Citation49 Vero cell–derived vaccine has high seroprotection rates ranging from 93 to 99% one month following completion of the two-dose primary series.Citation18,Citation19 Seroprotection rate was 95.7% one month following the booster dose among children aged 1–2 years living in endemic area.Citation18,Citation19
In contrast to MBDV, Vero cell–derived JE vaccine reduces the potential risk of contamination from host cell proteins and nucleic acids.Citation27,Citation50 Both pre-licensure clinical trials and post-marketing surveillance data demonstrated good safety profiles of the vaccine.Citation51–53 Yun et al. conducted a post-marketing surveillance to evaluate the safety of a Vero cell–derived JE vaccine booster dose following previous MBDV vaccination in children.Citation51 Solicited adverse events were mild with 44.7% of injection site tenderness, 26.2% of injection side erythema, and 1.1% of fever. In one study with enrollment of both adults and children in the USA, the reporting rates of adverse events and serious adverse events following immunization were 14.8 and 1.1 per 100,000 doses distributed, respectively.Citation52 A few local and systemic reactions were observed after Vero cell–derived JE vaccination in 152 Thai children. Serious adverse events were reported in 21 children and were considered to be unrelated to vaccination.Citation53 A systematic review of reports from Asia-Pacific area showed that both PHK cell–derived, live-attenuated SA14-14-2 JE vaccine and inactivated, Vero cell–derived, alum-adjuvanted JE vaccine had acceptable safety without significant difference. The pooled adverse reaction rates were 18.09% and 12.49%, respectively.Citation54
Live-attenuated chimeric JE vaccine
Live-attenuated chimeric JE vaccine was created by replacing the premembrane (prM) and envelope (E) coding sequences of the yellow fever live-attenuated 17D vaccine virus with the analogous sequences coding for the antigenic determinants from the SA14-14-2 live-attenuated JE vaccine virus. The vaccine virus is produced in Vero cells.Citation18,Citation19 The yellow fever 17D vaccine virus was chosen due to commendable safety and efficacy for several decades.Citation27,Citation55,Citation56 Live-attenuated chimeric JE vaccine is also unlikely to be transmitted by mosquitoes from vaccinated persons to other hosts.Citation23,Citation27,Citation57 This vaccine is designated JE–CV, ChimeriVax–JE, IMOJEV, or THAIJEV and is licensed in Australia and Thailand since 2010.Citation23,Citation56 High seroprotection rates were reported from children from endemic countries and from adults from non-endemic countries one month after administration of a single dose of liver chimeric JE vaccine.Citation18,Citation19,Citation56
In a randomized, controlled, double-blind Phase III study performed in Australia and the USA comparing the immunogenicity and safety of JE–CV and MBDV showed that JE–CV had a significant lower adverse reaction rate (67.6%) than that of MBDV (82.2%) (p < .001). The reactogenicity profile of JE–CV was comparable with that of placebo.Citation58 According to another two comparative studies, JE–CV also have lower estimates of reactogenicity and other safety endpoints including serious adverse events, injection site reactions, and systemic reactions than live-attenuated SA14-14-2 JE vaccine.Citation59,Citation60 Moreover, clinical studies and post-marketing surveillance all showed favorable safety profile of JE–CV ().Citation61–68 The most common adverse effects were injection site reaction with incidence of 6.3% to 37.8% and fever (3.6%–39.0%). Solicited adverse reactions following vaccination were most frequently reported in children younger than 24 months of age.Citation63,Citation68 Few serious adverse reactions had been reported, but further investigations showed unrelatedness to JE–CV vaccination.
Table 1. Summary of investigations providing safety data of the live-attenuated chimeric Japanese encephalitis vaccine
MBDV in national immunization program in Taiwan has been replaced by JE–CV since May 2017. A post-licensure safety surveillance of JE-CV was conducted in Taiwan with a total of 1,078,248 JE-CV doses distributed from May 2017 to 2018.Citation62 A total of 51 adverse events were reported from 30 vaccinees, corresponding to a reporting rate of 2.8 per 100,000 doses distributed. Adverse events occurred a median of one day after vaccination. Fever (39%), systemic rashes (20%), local redness (8%), fatigue/irritability (8%) and decreased appetite (8%) accounted for the majority. No neurological and severe hypersensitivity adverse reaction was recorded. The reporting rate of serious adverse events was 0.37 per ,100,000 doses distributed, which included viral respiratory tract infection, cellulitis, focal segmental glomerulosclerosis, and febrile seizures. These serious adverse events were not considered to be attributed to the use of JE–CV.
Conclusion
JE is believed to be an underestimated disease because most infected people are asymptomatic. The mortality rate is up to 30% and 30%–50% of survivors have neuropsychiatric sequelae. Vaccination plays the most important role in reducing the disease burden. Nowadays, inactivated MBDV was replaced by three newer JE vaccines, which had good efficacy and safety. Common adverse reactions include injection site reactions and fever, and severe adverse reactions were rare. Clinical studies and post-marketing surveillance data also proved favorable safety profile of SA14-14-2 JE vaccine and JE–CV. Continuous surveillance of the safety of JE vaccines is still imperative in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all the committee members of the Advisory Committee on Immunization Practices in Taiwan for their great efforts in constitution and promotion of Japanese encephalitis vaccination program.
References
- Mansfield KL, Hernández-Triana LM, Banyard AC, Fooks AR, Johnson N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol. 2017;201:85–92. doi:10.1016/j.vetmic.2017.01.014.
- Solomon T. Control of Japanese encephalitis–within our grasp? N Engl J Med. 2006;355:869–71. doi:10.1056/NEJMp058263.
- Filgueira FL. Review of emerging Japanese encephalitis virus: new aspects and concepts about entry into the brain and inter-cellular spreading. Pathogens. 2019;8(3):111. doi:10.3390/pathogens8030111.
- Zheng Y, Li M, Wang H, Liang G. Japanese encephalitis and Japanese encephalitis virus in mainland China. Rev Med Virol. 2012;22:301–22. doi:10.1002/rmv.1710.
- Dung NM, Turtle L, Chong WK, Mai NT, Thao TT, Thuy TT, Kneen R, Phu NH, Wills B, Farrar J, et al. An evaluation of the usefulness of neuroimaging for the diagnosis of Japanese encephalitis. J Neurol. 2009;256:2052–60. doi:10.1007/s00415-009-5249-5.
- Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171–94.
- Xiong W, Lu L, Xiao Y, Li J, Zhou D. Mortality and disability due to Japanese encephalitis in elderly adults: evidence from an adult tertiary care center in West China. Frontiers in Neurology. 2019;10:918. doi:10.3389/fneur.2019.00918.
- Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–74, 74a-74e. doi:10.2471/BLT.10.085233.
- Lee DW, Choe YJ, Kim JH, Song KM, Cho H, Bae GR, Lee JK. Epidemiology of Japanese encephalitis in South Korea, 2007-2010. Int J Infect Dis. 2012;16:e448–52. doi:10.1016/j.ijid.2012.02.006.
- Choe YJ, Taurel AF, Nealon J, Seo HS, Kim HS. Systematic review of seroepidemiological studies on Japanese encephalitis in the Republic of Korea. Int J Infect Dis. 2018;67:14–19. doi:10.1016/j.ijid.2017.11.023.
- Chang YK, Chang HL, Wu HS, Chen KT. Epidemiological features of Japanese encephalitis in Taiwan from 2000 to 2014. Am J Trop Med Hyg. 2017;96:382–88. doi:10.4269/ajtmh.16-0330.
- Hsu LC, Chen YJ, Hsu FK, Huang JH, Chang CM, Chou P, Lin IF, Chang FY. The incidence of Japanese encephalitis in Taiwan–a population-based study. PLoS Negl Trop Dis. 2014;8:e3030. doi:10.1371/journal.pntd.0003030.
- Ma SP, Yoshida Y, Makino Y, Tadano M, Ono T, Ogawa M. Short report: a major genotype of Japanese encephalitis virus currently circulating in Japan. Am J Trop Med Hyg. 2003;69:151–54. doi:10.4269/ajtmh.2003.69.151.
- Pan XL, Liu H, Wang HY, Fu SH, Liu HZ, Zhang HL, Li MH, Gao XY, Wang JL, Sun XH, et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol. 2011;85:9847–53. doi:10.1128/JVI.00825-11.
- Nitatpattana N, Dubot-Pérès A, Gouilh MA, Souris M, Barbazan P, Yoksan S, De Lamballerie X, Gonzalez JP. Change in Japanese encephalitis virus distribution, Thailand. Emerg Infect Dis. 2008;14:1762–65. doi:10.3201/eid1411.080542.
- Nga PT, Parquet MDC, Cuong VD, Ma SP, Hasebe F, Inoue S, Makino Y, Takagi M, Nam VS, Morita K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J Gen Virol. 2004;85:1625–31. doi:10.1099/vir.0.79797-0.
- Pyke AT, Williams DT, Nisbet DJ, van den Hurk AF, Taylor CT, Johansen CA, Macdonald J, Hall RA, Simmons RJ, Mason RJ, et al. The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am J Trop Med Hyg. 2001;65:747–53. doi:10.4269/ajtmh.2001.65.747.
- Lee PI, Huang YC, Hwang KP, Liu CC, Chiu CH, Chen PY, Lu CY, Chen CJ, Chang LY, Chiu NC, et al. Recommendations for the use of Japanese encephalitis vaccines. Pediatr Neonatol. 2020;61:3–8. doi:10.1016/j.pedneo.2019.11.009.
- World Health Organization. Japanese encephalitis vaccines: WHO position paper – February 2015. Wkly Epidemiol Rec 2015;90:69–87.
- Erra EO, Askling HH, Yoksan S, Rombo L, Riutta J, Vene S, Lindquist L, Vapalahti O, Kantele A. Cross-protective capacity of Japanese encephalitis (JE) vaccines against circulating heterologous JE virus genotypes. Clin Infect Dis. 2013;56:267–70. doi:10.1093/cid/cis883.
- Beasley DW, Li L, Suderman MT, Guirakhoo F, Trent DW, Monath TP, Shope RE, Barrett ADT. Protection against Japanese encephalitis virus strains representing four genotypes by passive transfer of sera raised against ChimeriVax-JE experimental vaccine. Vaccine. 2004;22:3722–26. doi:10.1016/j.vaccine.2004.03.027.
- Li X, Ma SJ, Liu X, Jiang LN, Zhou JH, Xiong YQ, Ding H, Chen Q. Immunogenicity and safety of currently available Japanese encephalitis vaccine: a systemic review. Hum Vaccin Immunother. 2014;10:3579–93. doi:10.4161/21645515.2014.980197.
- Yun SI, Lee YM. Japanese encephalitis: the virus and vaccines. Hum Vaccin Immunother. 2014;10:263–79. doi:10.4161/hv.26902.
- Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, Kotchasenee S, Gingrich JB, Latendresse J, Fukai K, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319:608–14. doi:10.1056/NEJM198809083191004.
- Nimmannitya S, Hutamai S, Kalayanarooj S, Rojanasuphot S. A field study on Nakayama and Beijing strains of Japanese encephalitis vaccines. Southeast Asian J Trop Med Public Health. 1995;26:689–93.
- Kurane I, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains. Vaccine. 2000;18(Suppl 2):33–35. doi:10.1016/S0264-410X(00)00041-4.
- Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines. 2011;10:355–64. doi:10.1586/erv.11.7.
- Takahashi H, Pool V, Tsai TF, Chen RT. Adverse events after Japanese encephalitis vaccination: review of post-marketing surveillance data from Japan and the United States. The VAERS Working Group. Vaccine. 2000;18:2963–69.
- Lindsey NP, Staples JE, Jones JF, Sejvar JJ, Griggs A, Iskander J, Miller ER, Fischer M. Adverse event reports following Japanese encephalitis vaccination in the United States, 1999-2009. Vaccine. 2010;29:58–64. doi:10.1016/j.vaccine.2010.10.016.
- Plesner AM, Arlien-Søborg P, Herning M. Neurological complications and Japanese encephalitis vaccination. Lancet. 1996;348:202–03. doi:10.1016/S0140-6736(05)66156-9.
- Ohtaki E, Murakami Y, Komori H, Yamashita Y, Matsuishi T. Acute disseminated encephalomyelitis after Japanese B encephalitis vaccination. Pediatr Neurol. 1992;8:137–39. doi:10.1016/0887-8994(92)90036-X.
- Ohtaki E, Matsuishi T, Hirano Y, Maekawa K. Acute disseminated encephalomyelitis after treatment with Japanese B encephalitis vaccine (Nakayama-Yoken and Beijing strains). J Neurol Neurosurg Psychiatry. 1995;59:316–17. doi:10.1136/jnnp.59.3.316.
- Plesner AM, Arlien-Soborg P, Herning M. Neurological complications to vaccination against Japanese encephalitis. Eur J Neurol. 1998;5:479–85. doi:10.1046/j.1468-1331.1998.550479.x.
- Fischer M, Lindsey N, Staples JE, Hills S. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1–27.
- Yu Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine. 2010;28:3635–41. doi:10.1016/j.vaccine.2010.02.105.
- Ginsburg AS, Meghani A, Halstead SB, Yaich M. Use of the live attenuated Japanese Encephalitis vaccine SA14-14-2 in children: a review of safety and tolerability studies. Hum Vaccin Immunother. 2017;13:2222–31. doi:10.1080/21645515.2017.1356496.
- Gao X, Li X, Li M, Fu S, Wang H, Lu Z, Cao Y, He Y, Zhu W, Zhang T, et al. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951-2011. PLoS Negl Trop Dis. 2014;8:e3015. doi:10.1371/journal.pntd.0003015.
- Upreti SR, Janusz KB, Schluter WW, Bichha RP, Shakya G, Biggerstaff BJ, Shrestha MM, Sedai TR, Fischer M, Gibbons RV, et al. Estimation of the impact of a Japanese encephalitis immunization program with live, attenuated SA14-14-2 vaccine in Nepal. Am J Trop Med Hyg. 2013;88:464–68. doi:10.4269/ajtmh.12-0196.
- Upreti SR, Lindsey NP, Bohara R, Choudhary GR, Shakya S, Gautam M, Giri JN, Fischer M, Hills SL. Updated estimation of the impact of a Japanese encephalitis immunization program with live, attenuated SA14-14-2 vaccine in Nepal. PLoS Negl Trop Dis. 2017;11:e0005866. doi:10.1371/journal.pntd.0005866.
- Ohrr H, Tandan JB, Sohn YM, Shin SH, Pradhan DP, Halstead SB. Effect of single dose of SA14-14-2 vaccine 1 year after immunisation in Nepalese children with Japanese encephalitis: a case-control study. Lancet. 2005;366:1375–78. doi:10.1016/S0140-6736(05)67567-8.
- Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, Ohrr HC, Tang JL, Halstead SB. Efficacy of single-dose SA14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet. 2001;358:791–95. doi:10.1016/S0140-6736(01)05967-0.
- Khan SA, Kakati S, Dutta P, Chowdhury P, Borah J, Topno R, Jadhav SM, Mohapatra PK, Mahanta J, Gupte MD. Immunogenicity & safety of a single dose of live-attenuated Japanese encephalitis vaccine SA14-14-2 in adults. Indian J Med Res. 2016;144:886–92. doi:10.4103/ijmr.IJMR_712_15.
- Liu ZL, Hennessy S, Strom BL, Tsai TF, Wan CM, Tang SC, Xiang CF, Bilker WB, Pan XP, Yao YJ, et al. Short-term safety of live attenuated Japanese encephalitis vaccine (SA14-14-2): results of a randomized trial with 26,239 subjects. J Infect Dis. 1997;176:1366–69. doi:10.1086/517323.
- Wijesinghe PR, Abeysinghe MRN, Yoksan S, Yao Y, Zhou B, Zhang L, Fleming JA, Marfin AA, Victor JC. Immunogenicity of live attenuated Japanese encephalitis SA14-14-2 vaccine among Sri Lankan children with previous receipt of inactivated JE vaccine. Vaccine. 2016;34:5923–28. doi:10.1016/j.vaccine.2016.10.028.
- Wang Y, Dong D, Cheng G, Zuo S, Liu D, Du X. Post-marketing surveillance of live-attenuated Japanese encephalitis vaccine safety in China. Vaccine. 2014;32:5875–79. doi:10.1016/j.vaccine.2014.08.001.
- Liu Y, Lin H, Zhu Q, Wu C, Zhao Z, Zheng H. Safety of Japanese encephalitis live attenuated vaccination in post-marketing surveillance in Guangdong, China, 2005-2012. Vaccine. 2014;32:1768–73. doi:10.1016/j.vaccine.2013.11.107.
- Sanchayan K, Fernandopulle R, Amarasinghe A, Thiyahiny SN, Sri Ranganathan S. Safety of live attenuated Japanese encephalitis vaccine given at the age of 9 months in National Immunisation Programme of Sri Lanka. Ceylon Med J. 2016;61:99–105. doi:10.4038/cmj.v61i3.8344.
- Jelinek T. Ixiaro: a new vaccine against Japanese encephalitis. Expert Rev Vaccines. 2009;8:1501–11. doi:10.1586/erv.09.112.
- Butler S, Sutter D, Maranich A. Tolerability of Japanese encephalitis vaccine in pediatric patients. J Pediatric Infect Dis Soc. 2017;6:149–52.
- Srivastava AK, Putnak JR, Lee SH, Hong SP, Moon SB, Barvir DA, Zhao B, Olson RA, Kim SO, Yoo WD, et al. A purified inactivated Japanese encephalitis virus vaccine made in Vero cells. Vaccine. 2001;19:4557–65. doi:10.1016/S0264-410X(01)00208-0.
- Yun KW, Lee HJ, Park JY, Cho HK, Kim YJ, Kim KH, Kim NH, Hong YJ, Kim DH, Kim HM, et al. Long-term immunogenicity of an initial booster dose of an inactivated, Vero cell culture-derived Japanese encephalitis vaccine (JE-VC) and the safety and immunogenicity of a second JE-VC booster dose in children previously vaccinated with an inactivated, mouse brain-derived Japanese encephalitis vaccine. Vaccine. 2018;36:1398–404.
- Walker WL, Hills SL, Miller ER, Fischer M, Rabe IB. Adverse events following vaccination with an inactivated, Vero cell culture-derived Japanese encephalitis vaccine in the United States, 2012–2016. Vaccine. 2018;36:4369–74.
- Chanthavanich P, Limkittikul K, Sirivichayakul C, Chokejindachai W, Hattasingh W, Pengsaa K, Surangsrirat S, Srisuwannaporn T, Kaewma B, Yoksan S, et al. Immunogenicity and safety of inactivated chromatographically purified Vero cell-derived Japanese encephalitis vaccine in Thai children. Hum Vaccin Immunother. 2018;14:900–05. doi:10.1080/21645515.2017.1414763.
- Wang SY, Cheng XH, Li JX, Li XY, Zhu FC, Liu P. Comparing the immunogenicity and safety of 3 Japanese encephalitis vaccines in Asia-Pacific area: a systematic review and meta-analysis. Hum Vaccin Immunother. 2015;11:1418–25. doi:10.1080/21645515.2015.1011996.
- Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–49. doi:10.1016/j.vaccine.2009.09.098.
- Appaiahgari MB, Vrati S. Clinical development of IMOJEV ®–a recombinant Japanese encephalitis chimeric vaccine (JE-CV). Expert Opin Biol Ther. 2012;12:1251–63. doi:10.1517/14712598.2012.704908.
- Bhatt TR, Crabtree MB, Guirakhoo F, Monath TP, Miller BR. Growth characteristics of the chimeric Japanese encephalitis virus vaccine candidate, ChimeriVax-JE (YF/JE SA14–14–2), in Culex tritaeniorhynchus, Aedes albopictus, and Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2000;62:480–84. doi:10.4269/ajtmh.2000.62.480.
- Torresi J, McCarthy K, Feroldi E, Méric C. Immunogenicity, safety and tolerability in adults of a new single-dose, live-attenuated vaccine against Japanese encephalitis: randomised controlled phase 3 trials. Vaccine. 2010;28:7993–8000. doi:10.1016/j.vaccine.2010.09.035.
- Kim DS, Houillon G, Jang GC, Cha SH, Choi SH, Lee J, Kim HM, Kim JH, Kang JH, Kim JH, et al. A randomized study of the immunogenicity and safety of Japanese encephalitis chimeric virus vaccine (JE-CV) in comparison with SA14-14-2 vaccine in children in the Republic of Korea. Hum Vaccin Immunother. 2014;10:2656–63. doi:10.4161/hv.29743.
- Feroldi E, Pancharoen C, Kosalaraksa P, Chokephaibulkit K, Boaz M, Meric C, Hutagalung Y, Bouckenooghe A. Primary immunization of infants and toddlers in Thailand with Japanese encephalitis chimeric virus vaccine in comparison with SA14-14-2: a randomized study of immunogenicity and safety. Pediatr Infect Dis J. 2014;33:643–49. doi:10.1097/INF.0000000000000276.
- Chotpitayasunondh T, Pruekprasert P, Puthanakit T, Pancharoen C, Tangsathapornpong A, Oberdorfer P, Kosalaraksa P, Prommalikit O, Tangkittithaworn S, Kerdpanich P, et al. Post-licensure, phase IV, safety study of a live attenuated Japanese encephalitis recombinant vaccine in children in Thailand. Vaccine. 2017;35:299–304. doi:10.1016/j.vaccine.2016.11.062.
- Ma HY, Lai CC, Chiu NC, Lee PI. Adverse events following immunization with the live-attenuated recombinant Japanese encephalitis vaccine (IMOJEV®) in Taiwan, 2017-18. Vaccine. 2020;38:5219–22. doi:10.1016/j.vaccine.2020.06.008.
- Vu TD, Nguyen QD, Tran HTA, Bosch-Castells V, Zocchetti C, Houillon G. Immunogenicity and safety of a single dose of a live attenuated Japanese encephalitis chimeric virus vaccine in Vietnam: a single-arm, single-center study. Int J Infect Dis. 2018;66:137–42.
- Kim DS, Jang GC, Cha SH, Choi SH, Kim HM, Kim JH, Kang JH, Kim JH, Kim KH, Bang J, et al. Immunogenicity and safety of a booster dose of a live attenuated Japanese encephalitis chimeric vaccine given 1 year after primary immunization in healthy children in the Republic of Korea. Pediatr Infect Dis J. 2016;35:e60–4.
- Feroldi E, Capeding MR, Boaz M, Gailhardou S, Meric C, Bouckenooghe A. Memory immune response and safety of a booster dose of Japanese encephalitis chimeric virus vaccine (JE-CV) in JE-CV-primed children. Hum Vaccin Immunother. 2013;9:889–97. doi:10.4161/hv.23087.
- Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Sabchareon A, Pancharoen C, Bouckenooghe A, Gailhardou S, Boaz M, Feroldi E. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J. 2010;29:1111–17. doi:10.1097/INF.0b013e3181f68e9c.
- Feroldi E, Pancharoen C, Kosalaraksa P, Watanaveeradej V, Phirangkul K, Capeding MR, Boaz M, Gailhardou S, Bouckenooghe A. Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12-18 months: randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccin Immunother. 2012;8:929–37. doi:10.4161/hv.20071.
- Kim HS, Oh Y, Korejwo J, Castells VB, Yang K. Post-marketing surveillance of adverse events following vaccination with the live-attenuated Japanese encephalitis chimeric virus vaccine (Imojev(®)) in South Korea, 2015-2019. Infect Dis Ther. 2020;9:589–98. doi:10.1007/s40121-020-00305-6.

