ABSTRACT
In the United Kingdom (UK), both the MF59-adjuvanted quadrivalent influenza vaccine (aQIV) and the high-dose QIV (QIV-HD) are preferred for persons aged 65 years and older but only aQIV is reimbursed by the National Health Service (NHS). The objective was to determine the potential cost-effectiveness of vaccinating adults aged 65 years and above with aQIV compared with QIV-HD in the UK. A dynamic transmission model, calibrated to match infection data from the UK, was used to estimate the impact of vaccination in 10 influenza seasons. Vaccine effectiveness was based on a meta-analysis that concluded the vaccines were not significantly different. Vaccine coverage, physician visits, hospitalizations, deaths, utility losses and NHS costs were estimated using published UK sources. The list price of aQIV was £11.88 while a range of prices were tested for QIV-HD. The price of the trivalent high-dose vaccine (TIV-HD) is £20.00 but a list price for QIV-HD is not yet available. The projected differences between the vaccines in terms of clinical cases and influenza treatment costs are minimal. Our analysis demonstrates that in order to be cost-effective, the price of QIV-HD must be similar to that of aQIV and may range from £7.57 to £12.94 depending on the relative effectiveness of the vaccines. The results of the analysis were most sensitive to variation in vaccine effectiveness and the rate of hospitalization due to influenza. Given the evidence, aQIV is cost-saving unless QIV-HD is priced lower than the existing list price of TIV-HD.
Introduction
In the United Kingdom (UK), seasonal influenza vaccines have been provided to those at high risk of complications as well as those aged 65 years and above since 2000.Citation1 In 2013, a phased introduction to ages 2 to 16 years also began.Citation2 Individuals aged 65 years and above are at increased risk of complications and death from influenza compared with any other age group.Citation3,Citation4 In addition, these older adults have a lower immune response to vaccines than younger peopleCitation5 and standard egg-based quadrivalent influenza vaccines have had poor effectiveness in this age group in the UK.Citation6,Citation7 The Joint Committee on Vaccination and Immunisation (JCVI) recommended the use of alternative influenza vaccines that have improved effectiveness in this age group as the preferred vaccines for the 2019/20 and 2020/21 seasons.Citation8,Citation9 While this includes both the MF59 adjuvanted trivalent influenza vaccine (aTIV) and a high-dose trivalent influenza vaccine (TIV-HD), only aTIV was reimbursed by the National Health Service (NHS) given the list price for TIV-HD.Citation10
For the 2021/22 season, the JCVI has recommended the use of the new quadrivalent versions of these vaccines,Citation11 although only the aQIV will currently be reimbursed by the NHS.Citation12 Both the MF59 adjuvanted quadrivalent influenza vaccine (aQIV) and the high-dose quadrivalent influenza vaccine (QIV-HD) are considered to be the preferred vaccines for this older age group. The JCVI considers that the evidence comparing the two vaccines is limited, inconsistent and at-risk of bias.Citation11 They require data from multiple seasons before they can determine if one of these vaccines has superior effectiveness and make a preferential recommendation for one over the other.
In this analysis, we explore and compare the clinical and economic impact of the use of each of the available enhanced vaccines. The specific objective is to determine the potential cost-effectiveness of vaccination of adults aged 65 years and above with aQIV compared with QIV-HD in the UK, using a range of pricing and relative effectiveness assumptions for QIV-HD.
Methods
In this analysis, we used a compartmental transmission model with a Susceptible-Exposed-Infectious-Recovered (SEIR) type structure to predict the number of influenza infections with and without vaccination. While the specific target population was individuals aged 65 years and older in the UK, the entire population was included as is typical for dynamic models of communicable diseases. This allowed the estimation of both the direct benefits achieved through vaccination of the specific target population and the indirect effects achieved through a reduction in transmission of the virus through the entire population. The approach to creating the transmission model was adopted from Baguelin and colleagues who have created models of independent influenza seasons in order to conduct cost-effectiveness analyses of vaccine policy changes for the UK.Citation13–17 Details on the transmission model structure and the calibration of the model inputs have been described earlier.Citation18 During calibration, we created a scenario where type A dominates and a second scenario where both types A and B are co-circulating.
Vaccine effectiveness
In those aged 65 years and older, we compared the use of aQIV and QIV-HD. The influenza vaccinations administered to the other age groups were held constant for all model runs: the cost and effectiveness of these vaccines are described in Supplemental Appendix #1. The relative vaccine effectiveness of aTIV compared with TIV-HD has been estimated in several observational cohort studies.Citation19–25 Coleman et al. conducted a systematic literature review and meta-analysis of studies with aTIV, including those comparing aTIV with TIV-HD.Citation26 Using data from four studies,Citation19,Citation20,Citation24,Citation25 they estimated that the relative vaccine effectiveness (rVE) of aTIV to TIV-HD for reducing any medical encounter due to influenza and/or pneumonia was 3.2%. The 95% confidence interval for the rVE ranged from −2.5% to 8.9%, indicating that there was no statistically significant difference in the effectiveness of the two vaccines. The relative vaccine effectiveness is defined as one minus the rate ratio of the two vaccines. The rate ratio is defined as the ratio of the incidence rates of the two vaccines.Citation19 In randomized controlled trials, the immune response in those given aQIV and QIV-HD was shown to be non-inferior to those receiving aTIV and TIV-HD respectively for homologous influenza strains and superior for the additional B strain.Citation27–29 For this analysis, we therefore assumed that the rVE of aQIV compared with QIV-HD is the same as the rVE of aTIV compared with TIV-HD. For the base case analysis, we created three effectiveness scenarios using the Coleman meta-analysis: the values for the rVE of aQIV compared with QIV-HD were set to −2.5%, 3.2% and 8.9% – corresponding to the point estimate from the meta-analysis and the ends of the confidence interval.
Time horizon
We conducted analyses across 10 different seasons, although results are presented as the average annual (i.e. per season) values. We used 2019 data for the size of the population in Year 1 and applied the average growth rate from the Office for National Statistics for each year thereafter.Citation30 The type of influenza infection across the 10 years varied according to data from the Public Health England (PHE) surveillance reports from the 2010/11 to 2019/20 seasons.Citation31–40 Consistent with these data, we assumed that an A&B season occurred for Years 1, 3, and 8 while A only was experienced in the other seasons. We also varied the proportion of A infections due to H1N1 and H3N2 infections to match the PHE data (See Supplemental Appendix #1). We acknowledge that 10 years of data is a small sample on which to base future projections, but unfortunately longer-term data are not available.
Vaccine coverage
We stratified all compartments in the transmission model into those at low and high risk (called at-risk) of complications from influenza. The proportion at-risk of complications in each age group came from presented in previously published UK analyses ().Citation14–16 Since 2004/05, vaccine coverage for those aged 65 years and above has varied between 70.5% to 75.3%.Citation1 For the base case, we sourced vaccine coverage for each age group from the previous UK analysis ().Citation17 We assumed that vaccine coverage was independent of the specific vaccination used and therefore used the same coverage level for aQIV and QIV-HD.
Table 1. Key inputs for the base case analysis
Treatment of clinical influenza
Each case of symptomatic (or clinical) influenza predicted by the transmission model enters a decision tree, which calculates the proportion of cases that receive outpatient or inpatient treatment or both. Only individuals treated in hospital faced a probability of dying from influenza. Similar to previous analyses, we assumed that 10% of clinical infections receive outpatient care for their infections.Citation13,Citation41 We used calibration to derive the probability of hospitalization () given infection so that the average rate of hospital admissions matched the overall rate of influenza admissions by age and risk group in a published analysis of the Hospital Episode Statistics database.Citation3 Case fatality rates () were based on the 30-day mortality rates in this study.Citation3
Cost and utility inputs
We used the perspective of the NHS and Personal Social Services for costs, as recommended by the National Institute for Health and Care Excellence (NICE).Citation42 The list price for aQIV is £11.88.Citation43 As a list price for QIV-HD is not yet available, the unit price of QIV-HD was varied over a range of values up to a maximum of £20.00 which is the list price for TIV-HD.Citation43 While the NHS is charged a 20% Value Added Tax (VAT) on vaccines, we did not include it in the analysis as it is considered a social transfer. Furthermore, we assumed that the cost of administration was the same regardless of the type of vaccine used.
The costs of outpatient and inpatient treatment were obtained from Pitman and colleagues,Citation44 and inflated to 2020 values.Citation45 These outpatient costs, presented in , included both consultations and broad-spectrum antibiotics associated with complications such as acute otitis media, pneumonia, and sinusitis. We also added the cost of recommended anti-viral treatment for high-risk individualsCitation46 to a portion of casesCitation47 using the British National Formulary costs.Citation48 The age-specific hospitalization costs in were derived by Pitman et al. from NHS reference costs for admissions for lobar, atypical or viral pneumonia without complications.
We assigned a disutility of 0.0075Citation15,Citation49 to uncomplicated cases of influenza and 0.0180Citation15,Citation50 to hospitalized cases of influenza. We calculated quality-adjusted life-year (QALY) decrements for nonfatal influenza cases by multiplying the disutility by the time spent in the corresponding health state. We calculated the discounted number of QALYs lost due to death from influenza using expected survivalCitation51 and expected age-specific utility values.Citation52
We applied a discount rate of 3.5% for both costs and outcomes in line with NICE recommendations.Citation42
Sensitivity analyses
We performed a pricing analysis to determine an economically justifiable price for QIV-HD for the different effectiveness scenarios assuming various thresholds for an acceptable cost per QALY. We ran several additional scenarios to test the sensitivity to variables other than vaccine effectiveness. First, in order to simulate the impact of more or less severe influenza seasons, we doubled and then halved the hospitalization rates. Second, we varied the QALY decrements using their 95% confidence intervals: 0.0002 to 0.0271 for uncomplicated disease and 0.0145 to 0.0217 for hospitalizations. Third, we increased and decreased the baseline utility values by 10% in order to vary the QALY loss associated with early death due to influenza. Finally, based on a recent methods review in which NICE acknowledged that ‘the evidence suggests there is a case to change the reference discount rate to 1.5% per year for both costs and effects,’ we performed a scenario analysis using this rate for both costs and outcomes.Citation53
Results
In the base case analysis, there is a small difference favoring aQIV when the rVE of aQIV versus QIV-HD is 3.2% and 8.9% or favoring QIV-HD when rVE is −2.5%. The difference in the number of predicted clinical influenza cases in the UK population is less than 2% for all scenarios () while the difference in hospitalizations is 3% or less for all scenarios. The change in the average annual discounted NHS treatment costs for influenza range from a decrease of 1% to an increase of less than 4% (). As most deaths due to influenza occur in those age 65 years and older, vaccinating these individuals with a more effective vaccine has a slightly greater impact on the overall number of deaths in the population. If aQIV has a rVE of 8.9% compared with QIV-HD, then the use of QIV-HD in place of aQIV would lead to approximately 6% more deaths. Changes in number of deaths due to influenza impacts the QALYs lost predicted with influenza more than the changes in the loss of QALYs due to symptoms of influenza (). However, the absolute value of the change in average annual discounted QALYs when QIV-HD is implemented in place of aQIV is expected to be less than 1% in all scenarios.
Table 2. Base Case Results: Cases of clinical infection, hospitalizations, deaths, health-care system costs, and quality-adjusted life years with aQIV and QIV-HD under three relative effectiveness scenarios. All numbers, except for deaths from influenza, are presented rounded to the thousands
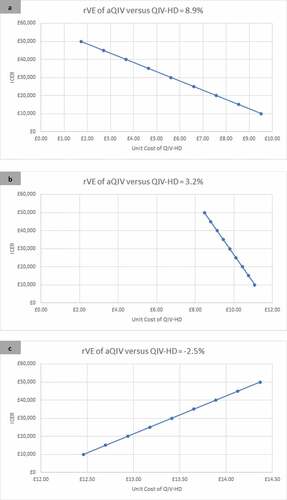
The incremental cost-effectiveness ratio (ICER) of QIV-HD compared with aQIV is shown in for the three effectiveness scenarios, for a range of unit prices for QIV-HD. In order for the ICER to fall below a cost per QALY willingness-to-pay threshold of £20,000, the unit price of QIV-HD needs to be less than £12.94 if the rVE is −2.5%, less than £10.44 if the rVE is 3.2% and less than £7.67 if the rVE is 8.9%.
Figure 1. The incremental cost-effectiveness of QIV-HD compared with aQIV over a range of unit prices for three relative effectiveness scenarios (rVE of aQIV versus QIV-HD): 8.9% (Panel A); 3.2% (Panel B); −2.5% (Panel C). A positive rVE implies that aQIV is more effective than QIV-HD while a negative rVE implies that QIV-HD is more effective than aQIV.
The impact of the scenario analyses on the potential price of QIV-HD is shown in . For all effectiveness scenarios, the economically justifiable unit cost of QIV-HD is affected most by changes in the hospitalization rates, followed by changes in the QALY decrements associated with influenza, followed by the changes in the baseline utility. The discount rate has a minimal impact. For example, for the rVE of aQIV versus QIV-HD of 3.2%, the price of QIV-HD should be below the aQIV price of £11.88 given the extra effectiveness regardless of the scenario. At a £20,000 willingness-to-pay threshold, the economically justifiable price of QIV-HD varies between £9.21 with the high hospitalization rate to £11.05 with the low hospitalization rate. In other words, the small increase in vaccine effectiveness with aQIV in this scenario is worth more during a more severe influenza season than a less season influenza season. For this same analysis with rVE set to −2.5% (QIV-HD is more effective), the economically justifiable price of QIV-HD varied from £12.49 (fewer hospitalizations) to £13.84 (more hospitalizations). When the rVE was 8.9% (aQIV more effective), the economically justifiable price of QIV-HD varied from £3.90 (more hospitalizations) to £9.40 (fewer hospitalizations).
Figure 2. Scenario analyses: The incremental cost-effectiveness of QIV-HD compared with aQIV over a range of unit prices for three relative effectiveness scenarios (rVE of aQIV versus QIV-HD): 8.9% (Panel A); 3.2% (Panel B); −2.5% (Panel C). A positive rVE implies that aQIV is more effective than QIV-HD while a negative rVe implies that QIV-HD is more effective than aQIV.

Discussion
We constructed three effectiveness scenarios using the results of a meta-analysis of the impact of aTIV and TIV-HD on the rate of medical encounters due to influenza and/or pneumonia. As the effectiveness of the vaccines was not statistically significantly different, our projections of the implications of this result show that the differences between the vaccines in terms of clinical cases and influenza treatment costs are minimal. While the NHS list price for aQIV has been set to £11.88, the list price for QIV-HD is not yet available. Our analysis demonstrates that in order to be cost-effective, the price of QIV-HD must be similar to that of aQIV and may range from £7.57 to £12.94 depending on the value assumed for rVE. The results are most sensitive to changes in the rate of hospitalization due to influenza and least sensitive to the discount rate. Typically, quadrivalent versions of vaccines cost more than the trivalent versions. Given that the latest list price of TIV-HD was £20.00, a considerable reduction in price would be required in order for QIV-HD to be considered cost-effective if effectiveness is similar to aQIV.
As with any decision analysis, there are several limitations associated with our analysis comparing aQIV and QIV-HD. Foremost is the lack of data on the relative effectiveness of the two vaccines. While there are some data comparing the trivalent versions of the vaccines, these are from observational analyses of large databases in the United States. As the JCVI states, additional effectiveness data are required from multiple seasons before they might recommend one vaccine as preferred to the other. The meta-analysis used in this cost-effectiveness study did summarize data from multiple seasons but found no significant differences between the trivalent versions of the vaccines. The quadrivalent formulations of these vaccines are new, but as they will both be available in upcoming seasons, comparative data will be available in the future from countries that have adopted both vaccines.
In this analysis, we estimated the number of influenza infections with a transmission model that has a number of limitations associated with the model structure and inputs. Similar to previously published influenza models,Citation47,Citation54,Citation55 the model structure allowed the vaccine to reduce infection only and did not allow a differential response according to the severity of disease. Therefore, the same effectiveness was applied to reduction of transmission of disease, reduction in symptomatic disease and reduction in hospitalizations. The effectiveness data used were derived from the reduction of medical encounters including general practitioner visits, emergency room visits and hospitalizations. Furthermore, we assumed a constant strain-specific effectiveness for all 10 years but in reality, vaccine effectiveness varies each year depending on how well the strains chosen by the World Health Organization match the strains that circulate locally during the influenza season. Given this inherent variation in influenza vaccine effectiveness, we conducted our pricing analyses with several effectiveness scenarios to estimate the impact of variation on the estimated economically attractive price for QIV-HD.
One further limitation is the limited variability in influenza infection rates in our model across the 10 seasons. While the burden of influenza varies by season, the model contains only two calibrated scenarios for an unvaccinated population: a representative A only and a representative A and B scenario. Our model does capture some additional variation in annual consequences of infection because vaccine effectiveness varies from season to season as the proportion of H1N1 and H3N2 circulating is varied. Overall, the model does not predict the same variability in outcomes across seasons that may be observed in the UK. PHE, for example, reports that the deaths in England attributed to influenza may vary from 4,000 to 22,000 annually,Citation40 while the number of deaths predicted by the model varied only from approximately 2,000 to 4,000 each year. To counter this limitation, we conducted a set of analyses where we doubled and halved the hospitalization rate associated with our base case analyses. As all of the deaths associated with influenza in this model were assumed to occur in hospitalized individuals, this varied the mortality rate as well. Increasing the hospitalizations and deaths associated with influenza meant that a larger price differential could be justified between the less effective and the more effective vaccine if the willingness-to-pay for QALYs gained is held constant.
We chose the inputs for the economic portion of the model that calculated the costs and quality of life implications of this model to be consistent with past cost-effectiveness analyses published for the UK.Citation15,Citation44 We conducted several scenario analyses where these were varied. While there are some limitations with these data, they appear to have a smaller impact on the cost-effectiveness results and the economically justifiable price of QIV-HD than vaccine effectiveness or severity of influenza. This is consistent with past analyses where vaccine effectiveness and severity of influenza have been the most important drivers of cost-effectiveness.Citation56
Conclusion
The current evidence of the rVE of aQIV and QIV-HD is not sufficient to suggest that either aQIV or QIV-HD is more effective. In this analysis, we used a range of rVE values from a recent meta-analysis that did not find a statistically significant difference in the effectiveness of the two vaccines to conduct a cost-effectiveness analysis for a range of unit prices for QIV-HD. Given the effectiveness evidence, aQIV is cost-saving compared to QIV-HD priced at the existing list price of TIV-HD.
Disclosure of potential conflicts of interest
MK is a shareholder in Quadrant Health Economics Inc., who was contracted by Seqirus USA Inc. to conduct this study. MM, MCW and MD are consultants at Quadrant Health Economics Inc. JMQ is employed by Seqirus and a shareholder of CSL Limited.
Supplemental Material
Download MS Word (50.8 KB)Acknowledgments
This manuscript was funded by Seqirus USA Inc. We would like to acknowledge Shannon Cartier, consultant to Quadrant Health Economics Inc., for assisting in development of model inputs.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1971017.
Additional information
Funding
References
- Chapter 19 Influenza. In: Ramsay M, editor. Immunisation against infectious disease. Public Health England; August 2018[accessed 2020 December 16]. https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book#the-green-book.
- Joint Committee on Vaccination and Immunisation (JCVI) scientific secretariat. JCVI statement on the annual influenza vaccination programme – extension of the programme to children. 25 July 2012 [accessed 2018 December 10]. Available online at: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation.
- Cromer D, van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014;68(4):363–71. doi:10.1016/j.jinf.2013.11.013.
- Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health. 2016;16(1):481. doi:10.1186/s12889-016-3128-4.
- Haralambieva IH, Painter SD, Kennedy RB, Ovsyannikova IG, Lambert ND, Goergen KM, Oberg AL, Poland GA. The impact of immunosenescence on humoral immune response variation after influenza A/H1N1 vaccination in older subjects. PLoS One. 2015;10(3):e0122282. doi:10.1371/journal.pone.0122282
- Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, Reynolds A, Gunson R, Thompson C, Galiano M, et al. End-of-season influenza vaccine effectiveness in adults and children, United Kingdom, 2016/17. Euro Surveill. 2017 Nov;22(44):17-00306. doi:10.2807/1560-7917.ES.2017.22.44.17-00306. PMID:29113630; PMCID:PMC5710133.
- Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, Reynolds A, Gunson R, Thompson C, Galiano M, et al. Uptake and effectiveness of influenza vaccine in those aged 65 years and older in the United Kingdom, influenza seasons 2010/11 to 2016/17. Euro Surveill. 2018 Sep;23(39):1800092. doi:10.2807/1560-7917.ES.2018.23.39.1800092. PMID:30280688; PMCID:PMC6169201.
- Joint Committee on Vaccination and Immunisation (JCVI) scientific secretariat. JCVI advice on influenza vaccines for the 2020/21 influenza season. September 2019 [accessed 2020 October 2]. Available online at: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation.
- Joint Committee on Vaccination and Immunisation (JCVI) scientific secretariat. JCVI advice on influenza vaccines for the 2019/2020 influenza season. October 2018 [accessed 2020 October 16]. Available online at: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation.
- National Health Service. The national flu immunisation programme 2020 to 2021 - update (Letter: August 5, 2020). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907149/Letter_annualflu_2020_to_2021_update.pdf.
- Joint Committee on Vaccination and Immunisation (JCVI) scientific secretariat. JCVI advice on influenza vaccines for the 2021/22 influenza season. November 2020 [accessed 2020 October 5]. Available online at: www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation.
- National Health Service. Vaccines reimbursed as part of the NHS seasonal influenza immunisation programme 2021/22 (Letter: February 3, 2021) [accessed 2021 February 5]. Available online at: https://www.england.nhs.uk/wp-content/uploads/2021/02/C1076-nhsei-flu-reimbursement-letter-3-feb-21.pdf.
- Baguelin M, Jit M, Miller E, Edmunds WJ. Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine. 2012;30(23):3459–62. doi:10.1016/j.vaccine.2012.03.019.
- Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ, Leung GM. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527. doi:10.1371/journal.pmed.1001527.
- Baguelin M, Camacho A, Flasche S, Edmunds WJ. Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: a cost-effectiveness study. BMC Med. 2015;13(1):236. doi:10.1186/s12916-015-0452-y.
- Thorrington D, van Leeuwen E, Ramsay M, Pebody R, Baguelin M. Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in England. BMC Med. 2017;15(1):166. doi:10.1186/s12916-017-0932-3.
- Thorrington D, van Leeuwen E, Ramsay M, Pebody R, Baguelin M. Assessing optimal use of the standard dose adjuvanted trivalent seasonal influenza vaccine in the elderly. Vaccine. 2019;37(15):2051–56. doi:10.1016/j.vaccine.2019.03.002.
- Kohli MA, Maschio M, Mould-Quevedo JF, Ashraf M, Drummond MF, Weinstein MC. The cost-effectiveness of expanding vaccination with a cell-based influenza vaccine to low risk adults aged 50 to 64 years in the United Kingdom. Vaccines (Basel). 2021;9(6):598. doi:10.3390/vaccines9060598.
- Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis. 2019;220(8):1255–64. doi:10.1093/infdis/jiy716.
- Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Wernecke M, MaCurdy T, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018–2019. J Infect Dis. 2020;222(2):278–87. doi:10.1093/infdis/jiaa080.
- Izurieta HS, Lu M, Kelman J, Lu Y, Lindaas A, Loc J, Pratt D, Wei Y, Chillarige Y, Wernecke M, et al. Comparative effectiveness of influenza vaccines among US medicare beneficiaries ages 65 years and older during the 2019–2020 Season. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1727.
- van Aalst R, Gravenstein S, Mor V, Mahmud SM, Wilschut J, Postma M, Chit A. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: a retrospective cohort study. Vaccine. 2020;38(2):372–79. doi:10.1016/j.vaccine.2019.09.105.
- Divino V, Krishnarajah G, Pelton SI, Mould-Quevedo J, Anupindi VR, DeKoven M, Postma MJ. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine. 2020;38(40):6334–43. doi:10.1016/j.vaccine.2020.07.023.
- Pelton SI, Divino V, Shah D, Mould-Quevedo J, DeKoven M, Krishnarajah G, Postma MJ. Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017-2018 Influenza Season. Vaccines (Basel). 2020 Aug 7;8(3):446. doi:10.3390/vaccines8030446. PMID:32784684; PMCID: PMC7563546.
- Boikos C, Fischer L, O’Brien D, Vasey J, Sylvester G, Mansi J Relative effectiveness of aTIV versus TIVe, QIVe, and HD-TIV in preventing medical encounters during the 2017–18 and 2018–19 influenza season in the United States. National Foundation for Infectious Diseases. Virtual, 2020.
- Coleman BL, Sanderson R, Haag M, McGovern I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses. 2021;00:1–11.
- Essink B, Fierro C, Rosen J, Figueroa AL, Zhang B, Verhoeven C, Edelman J, Smolenov I. Immunogenicity and safety of MF59-adjuvanted quadrivalent influenza vaccine versus standard and alternate B strain MF59-adjuvanted trivalent influenza vaccines in older adults. Vaccine. 2020;38(2):242–50. doi:10.1016/j.vaccine.2019.10.021.
- Fluad. Summary of product characteristics. Updated 13 August 2018. Seqirus UK Limited.
- Quadrivalent influenza vaccine (split virion, inactivated) high-dose. summary of product characteristics. sanofi pasteur. [accessed 2020 November 20]. Available at: https://www.hpra.ie/homepage/medicines/medicines-information/find-a-medicine/results/item?pano=PA2131/015/001&t=Quadrivalent%20Influenza%20Vaccine%20(Split%20Virion,%20Inactivated)%20High-Dose.
- Office for National Statistics. 2016-based National Population Projections [accessed 2019 April 16]. Available Online at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections.
- Health Protection Agency. Surveillance of influenza and other respiratory viruses in the UK, 2010/11. London (UK). 2011.
- Health Protection Agency. Surveillance of influenza and other respiratory pathogens in the UK. October 2011 - April 2012. London (UK). 2012.
- Public Health England. Surveillance of influenza and other respiratory viruses, including novel respiratory viruses, in the United Kingdom: winter 2012/13. London (UK). 2013.
- Public Health England. Surveillance of influenza and other respiratory viruses in the United Kingdom: winter 2013/14. London (UK). 2014.
- Public Health England. Surveillance of influenza and other respiratory viruses in the United Kingdom: winter 2014 to 2015. 2015.
- Public Health England. Surveillance of influenza and other respiratory viruses in the United Kingdom: winter 2015 to 2016. London (UK). 2016.
- Public Health England. Surveillance of influenza and other respiratory viruses in the UK: winter 2016 to 2017. London (UK). 2017.
- Public Health England. Surveillance of influenza and other respiratory viruses in the UK: winter 2017 to 2018. London (UK). 2018.
- Public Health England. Surveillance of influenza and other respiratory viruses in the UK: winter 2018 to 2019. London (UK). 2019.
- Public Health England. Surveillance of influenza and other respiratory viruses in the UK: winter 2019 to 2020. London (United Kingdom). 2020.
- Tilston NL, Eames KT, Paolotti D, Ealden T, Edmunds WJ. Internet-based surveillance of Influenza-like-illness in the UK during the 2009 H1N1 influenza pandemic. BMC Public Health. 2010;10(1):650. doi:10.1186/1471-2458-10-650.
- National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. Process and Methods [PMG9]. April 2013 [accessed 2019 April 16]. Available online at: https://www.nice.org.uk/process/pmg9/chapter/foreword.
- British medical association and royal pharmaceutical society of Great Britain. British National Formulary. London (UK). 2021.
- Pitman RJ, Nagy LD, Sculpher MJ. Cost-effectiveness of childhood influenza vaccination in England and Wales: results from a dynamic transmission model. Vaccine. 2013;31(6):927–42. doi:10.1016/j.vaccine.2012.12.010.
- Curtis LA, Burns A Unit costs of health and social care 2018. Project report. Canterbury: University of Kent, UK. 2018. Avaliable Online at: 10.22024/UniKent/01.02.70995.
- Public Health England guidance on the use of antiviral agents for the treatment and prophylaxis of seasonal influenza. Version 9.1. London (UK). 2019.
- Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, Nicholson K. Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation. Health Technol. Assess. 2009;13(11):iii,ix–xii, 1–246. doi:10.3310/hta13110.
- British medical association and royal pharmaceutical society of Great Britain. British National Formulary Vol. 67. London (UK). 2019.
- van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ, Cowling B. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PLoS One. 2011;6(3):e17030. doi:10.1371/journal.pone.0017030.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14. doi:10.1016/j.vaccine.2004.05.003.
- Office for National Statistics. National life tables: United Kingdom, 2015-17. [accessed 2021 May 2]. Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables.
- Meier G, Gregg M, Poulsen Nautrup B. Cost-effectiveness analysis of quadrivalent influenza vaccination in at-risk adults and the elderly: an updated analysis in the UK. J Med Econ. 2015;18(9):746–61. doi:10.3111/13696998.2015.1044456.
- National Institute for Health and Care Excellence. The NICE methods of health technology evaluation: the case for change. (November 6, 2020) [accessed 2021 March 5]. Available online at: https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/nice-guidance/chte-methods-consultation/NICE-methods-of-health-technology-evaluation-case-for-change.docx.
- De Boer PT, van Maanen BM, Damm O, Ultsch B, Dolk FCK, Crepey P, Pitman R, Wilschut JC, Postma MJ. A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev Pharmacoecon Outcomes Res. 2017;17(3):249–65. doi:10.1080/14737167.2017.1343145.
- Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton Nj, Ades AE, Sutton A, Cooper N, Elliot AJ, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technol. Assess. 2009;13(58):iii–iv. doi:10.3310/hta13580.
- Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, et al. Relative Effectiveness of Cell-Cultured and Egg-Based Influenza Vaccines Among Elderly Persons in the United States, 2017-2018. J Infect Dis. 2019 Sep 13;220(8):1255-1264. doi:10.1093/infdis/jiy716. Erratum in: J Infect Dis. 2019 Jun 5;220(1):179. PMID:30561688.

