ABSTRACT
Vaccination-induced demyelination is a rare, unusual side effect of certain vaccines which can cause significant damage to patient’s sensory-motor abilities within days. Although COVID-19 vaccines go through rigorous clinical trials before they are injected to the general population, certain unexpected side effects remain inevitable. We herein describe a case of a 42-year-old woman who experienced acute demyelination 10 days after the Oxford-AstraZeneca vaccination. To the best of our knowledge, this is the first case of such nature and severity described regarding COVID-19 vaccines’ side effects.
Introduction
As the COVID-19 pandemic continues to progress worldwide, scientists, epidemiologists, public health researchers, and policymakers are challenged to provide efficient treatment and preventive modalities.Citation1 From the initial stages of the pandemic, developing a safe, effective, affordable, and deployable vaccine against COVID-19 deemed necessary with public and private sectors uniting together to develop and evaluate the safety and efficacy of the candidate vaccines to end this global disaster.Citation2 So far, several vaccines against SARS-CoV-2 have been developed, some of which are the Adenovirus Type-5 (Ad5)-Vectored COVID-19 Vaccine, Sputnik V vaccine (Gam-COVID-Vac), Janssen Ad26.COV2.S COVID-19 Vaccine, Moderna (mRNA-1273) Vaccine, BNT162b1 vaccine (mRNA vaccine), and the Oxford–AstraZeneca COVID-19 ChAdOx1 nCoV-19 (AZD1222) vaccine.Citation2–9
The results on the efficacy and safety of the AZD1222 vaccine from the first interim analysis for a viral vector coronavirus vaccine, ChAdOx1 nCoV-19, evaluated in four continents, showed significant vaccine efficacy of 70.4% after two doses and protection of 64.1% after at least one standard dose, against symptomatic disease, with no safety concerns.Citation2 However, here we report a case of a 42-year-old woman with cerebellar ataxia who experienced acute demyelination 10 days after the first dose of the AZD1222 vaccine.
Patient presentation
The patient was a 42-year-old Iranian female hospital staff who was diagnosed with hereditary cerebellar ataxia 5 years ago. She has had difficulty walking from childhood which exacerbated in her mid-twenties. Our patient’s parents have consanguine marriage and her oldest sister developed disequilibrium, episodes of sudden fallings and related forms of motor dysfunction at a young age. Her sister’s MRI showed cerebellar atrophy and was diagnosed as a hereditary cerebellar ataxia; She passed away a few years after the initial onset of the disease.
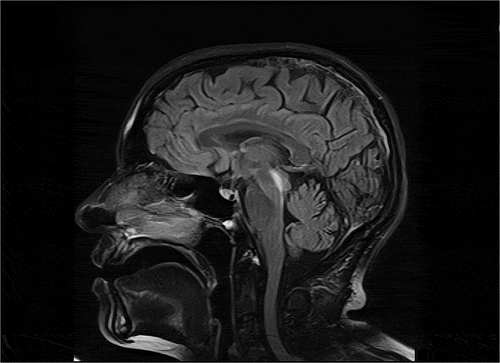
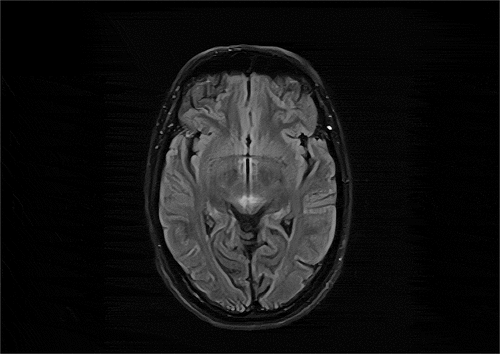
The patient’s conditions had remained stable until she received the first dose of the AZD1222 vaccine on 22 April 2021. For the first 9 days following the vaccination, she had not complained of any sort of side effects, including fatigue, a fever, headaches, body aches, chills, or nausea. On the tenth day, her vision got blurred and she developed vertical diplopia due to which she visited an ophthalmologist the next day and an MRI scan was indicated. MRI images showed results consistent with demyelination patterns (), and she was referred to our MS clinic where a complete patient history was obtained and hematologic laboratory tests were ordered, including the Neuromyelitis Optica Antibody test which turned out negative. Moreover, some lab tests were done to rule out vasculitis disorders. Eventually, a diagnosis of acute demyelination was made and after three consecutive days of pulse corticosteroids therapy, Methylprednisolone 1 g/day, her vision resolved about 60%. Her gait and motor functions have remained stable throughout the follow-up period and she has resumed daily activities.
Discussion
Demyelination is defined as the damage of myelin, the protective coating layer of nerve cells. As such happens, neurological problems can occur. It can result from various medical conditions, including Multiple Sclerosis (MS). Demyelination is primarily an autoimmune response, stemming from a break within the peripheral tolerance mechanism.Citation10,Citation11 Immune tolerance has been shown to play a significant role in the prevention of autoimmunity, which in this patient may have been affected by the injection of the Oxford–AstraZeneca COVID-19 ChAdOx1 nCoV-19 (AZD1222) vaccine.
The publicly available safety data based on current COVID-19 vaccines’ trials revealed no cases of acute demyelination in all trials combined. However, other neurological side effects have seldom been reported.Citation2,Citation9 A review study conducted in 2021 reported cases of transverse myelitis in phase three volunteers of the AZD1222 vaccine most of which were declared irrelevant to COVID-19 vaccines.Citation12 Previously, the association between the incidence of acute demyelination and other vaccines has been discussed. In a study conducted in the United States of America in 2016, an association was found between the incidence of acute demyelination and the use of inactivated influenza vaccine, measles, mumps, rubella vaccine, and varicella vaccine.Citation13 Other studies have also pointed out some mild connections between the yellow fever vaccine and the risk of demyelination.Citation14 One theory suggests that vaccines could be associated with autoimmune phenomena occurring via mimicry of host molecules by the vaccinal antigen or the bystander activation of dormant auto-reactive T-cells.Citation12,Citation15–17
To our knowledge, this is the first case report about acute demyelination after AZD1222 vaccination The timing of the patient’s demyelination and the mode of onset suggest the association of this clinical condition with vaccination in this case. However, any conclusions should be made carefully as the patient’s cerebellar ataxia might have been a predisposing condition to the induction of CNS demyelination. Subsequently, further research is needed to establish a strong association and to elucidate the mechanism of it.
Authors’ contributions
Investigation: OM, VSh.
Supervision: OM, VSh.
Validation: OM, VSh.
Writing – original draft: SB.
Writing – review & editing: SB, OM, VSh.
Consent for publication
The patient signed written consent for publishing her information and photos.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical considerations
The patient signed written consent for publishing her information and photos.
Acknowledgments
The authors would like to express their gratitude to the patient who signed written consent for publishing her information and photos.
References
- Blandenier E, Habibi Z, Kousi T, Sestito P, Flahault A, Rozanova L. Initial COVID-19 outbreak: an epidemiological and socioeconomic case review of Iran. Int J Environ Res Public Health. 2020;17:1–13. doi:10.3390/ijerph17249593.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi:10.1056/NEJMoa2034577.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–93. doi:10.1038/s41586-020-2639-4.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–81. doi:10.1016/S0140-6736(21)00234-8.
- Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi:10.1016/S0140-6736(20)32623-4.
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–32. doi:10.1056/NEJMoa2026920.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383:1920–31. doi:10.1056/NEJMoa2022483.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. doi:10.1016/S0140-6736(20)31604-4.
- Goverman JM. Immune tolerance in multiple sclerosis. Immunol Rev. 2011;241:228–40. doi:10.1111/j.1600-065X.2011.01016.x.
- Racke MK, Ratts RB, Arredondo L, Perrin PJ, Lovett-Racke A. The role of costimulation in autoimmune demyelination. J Neuroimmunol. 2000;107:205–15. doi:10.1016/S0165-5728(00)00230-7.
- Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–24. doi:10.1016/j.autrev.2013.10.003.
- Baxter R, Lewis E, Goddard K, Fireman B, Bakshi N, DeStefano F, Gee J, Tseng HF, Naleway AL and Klein, NP. Acute demyelinating events following vaccines: a case-centered analysis. Clin Infect Dis. 2016;63:1456–62. doi:10.1093/cid/ciw607.
- McMahon AW, Eidex RB, Marfin AA, Russell M, Sejvar JJ, Markoff L, Hayes EB, Chen RT, Ball R, Braun MM, et al. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine. 2007;25:1727–34. doi:10.1016/j.vaccine.2006.11.027.
- Wood H. Demyelinating disease: new study refutes link between vaccines and demyelination. Nat Rev Neurol. 2014;10(673):673–673. doi:10.1038/nrneurol.2014.215.
- Principi N, Esposito S. Do vaccines have a role as a cause of autoimmune neurological syndromes? Front Public Health. 2020;8:1–9.
- Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956. doi:10.1136/bmj.d5956.