ABSTRACT
Varicella (chickenpox) is a common, highly contagious disease caused by primary infection with varicella zoster virus (VZV), which can result in bacterial superinfection, central nervous system complications, and hospitalization. Stage 2 of this Phase 3 open-label study (ClinicalTrials.gov identifier: NCT03843632) enrolled 100 healthy infants, children, and adolescents (12 months–6 years, n = 37; 7–12 years, n = 33; 13–17 years, n = 30) without a clinical history of varicella. Participants aged 12 months–12 years were administered 1 dose of VARIVAX™ 0.5 mL (Varicella Virus Vaccine Live [Oka/Merck]) and adolescents aged 13–17 years were administered 2 doses 6 weeks apart. For participants seronegative at baseline (VZV antibody titer <1.25 glycoprotein enzyme-linked immunosorbent assay [gpELISA] units/mL), immunogenicity was assessed by seroconversion (VZV antibody titer ≥5 gpELISA units/mL) and VZV antibody geometric mean titers 6 weeks after the final dose. For participants who were VZV seropositive at baseline (VZV antibody titer ≥1.25 gpELISA units/mL), immunogenicity was assessed by antibody titer geometric mean fold rise and percentage of participants with ≥4-fold rise in antibody titer 6 weeks after the final dose. A Vaccine Report Card was used to report solicited and unsolicited adverse events through 42 days post-vaccination. After series completion among seronegative participants across age groups (n = 74), 98.6% demonstrated seroconversion 6 weeks post-vaccination; among seropositive participants (n = 26), 65.4% had ≥4-fold rise in antibody titer 6 weeks post-vaccination. No new safety signals were observed. Administering VARIVAX to infants, children, and adolescents resulted in an acceptable immune response with a safety profile consistent with the licensed product.
Introduction
Varicella (chickenpox) is a common and highly contagious childhood infectious disease caused by primary infection with varicella zoster virus (VZV), a double-stranded DNA α-herpes virus that can be spread via airborne trans-mission or contact with skin lesions.Citation1,Citation2 Primary varicella infection typically presents as a generalized vesicular rash that appears in crops concentrated on the head and trunk and then spreads to the extremities, which is preceded by a prodrome of malaise, loss of appetite, fever, and headache.Citation1,Citation2 For most children, the prodromal symptoms and lesion pain are mild.Citation2
In unvaccinated populations in temperate countries, >90% of people may be infected with varicella before adolescence.Citation3 In 2019, the annual incidence of varicella in Russia was 559.1 per 100,000 inhabitants, resulting in 820,000 registered cases and 5 deaths, 4 of which were in children.Citation4 Children comprise the majority of cases (94.3% in 2018), with the largest proportion (73%) in children 1 to 6 years of age; more than half (56.8%) were in children 3 to 6 years of age.Citation4 Similar annual incidence rates of more than 450 per 100,000 inhabitants have also been reported in the neighboring countries of Estonia, Lithuania, and Poland.Citation5
Complications of varicella infection, including bacterial superinfection and central nervous system complications, affect approximately 20–30% of infected children.Citation6,Citation7 VZV infection in children is also associated with arthritis, glomerulonephritis, myocarditis, and purpura fulminans, although these occurrences are rare.Citation8–10 VZV-related vasculopathy and inflammation is associated with an increased risk of ischemic stroke in children,Citation11 although the risk of stroke is not increased following vaccination against varicella.Citation12
Varicella infection in children has been associated with a significant clinical and economic burden in Central and Eastern European countries.Citation13,Citation14 Notably, 12.0% to 14.6% of children (age 1 to 12 years) infected with varicella who present to a healthcare center and are treated as out-patients in Hungary and Poland have been reported to have at least 1 complication, including keratoconjunctivitis, skin and soft tissue infection, bronchitis, pharyngitis, rhinitis, otitis media, severe pain, and facial paresis.Citation13,Citation14 In this setting, direct costs incurred by each outpatient child infected with varicella equated to approximately €50 (equivalent to US$53 cost in 2015), but indirect costs due to lost work for the child’s caregivers are estimated to cost an additional €108 to €190 (equivalent to US$101 to US$178 in 2015).Citation13,Citation14
Up to 6% of patients infected with VZV may have complications requiring hospitalization.Citation1 Most children admitted to hospital with varicella generally have 1 complication, while 21% to 38% have 2 or more complications including skin and soft tissue infections and pneumonia, all commonly experienced by outpatients as well, and then dehydration and cerebellitis.Citation13,Citation14 Consequently, the direct costs of treating children hospitalized for varicella are substantially higher than for outpatients, ranging from €554 to €959 (equivalent to US$591 to US$1023 in 2015), although indirect costs for caregivers only increase by approximately €50 (equivalent to US$53 in 2015).Citation13,Citation14 Overall, this translates into an estimated annual economic cost for treating varicella of 11 billion rubles (approximately US$143 million) in Russia,Citation15 although this estimate is likely to underestimate the true cost due to under-reporting of VZV infection.Citation16
VZV vaccination substantially reduces the incidence of VZV infection and the associated complications including hospitalizations.Citation2,Citation17 A single vaccination reduces the risk of moderate or severe varicella by >80%,Citation3,Citation17–19 while a second dose reduces the risk by at least 98%.Citation3,Citation18,Citation19 It has also been demonstrated that the rate of herpes zoster is lower after VZV vaccination compared with natural infection.Citation20 In regions of Russia where VZV vaccination is part of the regional vaccination schedule, the prevalence of varicella has been reduced to levels that are 75% lower than the national average.Citation15
Accordingly, the World Health Organization recommends 2 doses of VZV vaccine when the aim is to reduce the number of cases and outbreaks and decrease varicella-related mortality and severe morbidity.Citation3 Furthermore, every US$1 spent on implementing a universal 2-dose VZV vaccination program in 2008 was estimated to save US$2.70.Citation21
Monovalent varicella vaccines are licensed and available throughout the world for the prevention of infection in healthy children, adolescents, and adults.Citation22 Varicella vaccine (VARIVAX™: Varicella Virus Vaccine Live [Oka/Merck], Merck & Co., Inc., Kenilworth, NJ, USA) was first approved in the US in 1995; however, at the time of this study, it had not been licensed in Russia. One VZV vaccine (VARILRIX®: Oka-RIT strain GlaxoSmithKline) was approved for use in Russia at the time of this study but had not been widely adopted, despite vaccination being recommended for children and adults from at-risk groups who have not previously been vaccinated and have not had varicella.Citation4 In 2019, only 110,000 people in Russia had been vaccinated against varicella (62,000 children and 48,000 adults).Citation4 VARIVAX™ has since received marketing approval in the Russian Federation.Citation23
Following successful completion of Stage 1 of this 2-stage study in adults, Stage 2 commenced to evaluate VARIVAX use in infant and pediatric participants (age 12 months–12 years) and adolescents (age 13–17 years) at 5 centers in Russia. The study was conducted to support the licensure for VARIVAX™ in the Russian Federation.
Materials and methods
Study design
Participants in this Phase 3, open-label, multicenter, single-arm trial were administered live varicella virus vaccine (Varicella Virus Vaccine Live [Oka/Merck]): 1 dose was admin-istered to participants aged 12 months–12 years; 2 doses were administered to participants aged 13–17 years, 6 weeks apart. The trial was prospectively registered in the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT identifier: 2019-003903-36) and ClinicalTrials.gov (identifier: NCT03843632) registries prior to enrolling the first participant. Information provided to participants was reviewed and approved by relevant regulatory bodies and independent ethics committees at each center prior to initiating the study (protocol V210-058). All participants provided written informed consent prior to enrollment or applicable assent where participants were under the age of consent. The study was conducted in conformance with the ethical principles originating from the Declaration of Helsinki (October 2013), Good Clinical Practice, and relevant local statutes and regulations.
Participants
Eligible participants were healthy males and females who were 12 months–17 years of age with a negative clinical history of varicella and herpes zoster infection. Individuals were excluded from participating in the study if they had a history of allergy or anaphylactic reaction to any component of the study vaccine, had received any varicella or herpes zoster vaccine at any time before the study, or been exposed to varicella or herpes zoster in the 4 weeks before the study. Eligible participants must not have had a febrile illness associated with temperature of ≥38.9°C within 72 hours of study vaccination, been vaccinated with a non-live or live vaccine ≤30 days prior or be expecting to be vaccinated during the 42-day safety follow-up period following study vaccination. Individuals who lived with a person who had, or they themselves had, immune deficiency, neoplastic disease, or depressed immunity, including receiving immunosuppressive therapy, were also excluded from the study. Female participants of reproductive potential must have had a negative pregnancy test on the day of vaccination and agreed to remain abstinent or use 2 acceptable methods of birth control for the duration of the study period.
Immunogenicity
Serum samples were collected prior to vaccination at Visit 1 and at Day 43 post-vaccination 1 (participants aged 12 months–12 years) or 2 (participants aged 13–17 years). Antibodies to VZV were detected using glycoprotein enzyme-linked immunosorbent assay (gpELISA).Citation24
For participants who were seronegative at baseline (VZV antibody titer <1.25 gpELISA units/mL), the 3 co-primary endpoints were seroconversion rate defined as a VZV antibody titer ≥1.25 gpELISA units/mL; antibody response, defined as a VZV antibody titer ≥5 gpELISA units/mL; and geometric mean titer (GMT) of VZV antibody as measured by gpELISA at 6 weeks for children and 6 weeks after Dose 2 for adolescents.
For participants who were VZV seropositive at baseline, the co-primary endpoints were the GMT, geometric mean fold rise (GMFR) in antibody titer and percentage of participants with a ≥4-fold rise in antibody titer 6 weeks post-vaccination for children and 6 weeks after Dose 2 for adolescents.
Safety
Secondary endpoints included solicited injection-site adverse events (AEs; redness, swelling, and pain/tenderness) from Day 1 through Day 5 post-dose, unsolicited injection-site AEs from Day 1–42 post-dose, maximum reported temperature ≥39.0°C oral equivalent from Day 1 through 28 post-vaccination, varicella- and herpes zoster-like rashes occurring from Day 1 through Day 42 post-vaccination, and systemic and serious AEs occurring from Day 1 until the end of study. Laboratory assessments including complete blood count, chemistry, and urinalysis were also monitored during the study for participants over the age of 7 years.
Statistical analysis
Approximately 100 children and adolescent participants were to be enrolled in Stage 2 of this study, with a minimum of 30 participants enrolled per age group (infants and children aged 12 months–6 years; children aged 7–12 years; and adolescents aged 13–17 years). Primary immunogenicity analyses were based on the per-protocol population and results are presented as descriptive statistics with 2-sided 95% confidence intervals (CIs) for antibody response rate and VZV seroconversion. CIs were computed using the exact method for a single binomial proportion. GMTs were calculated at each time point by averaging the log of titers across all participants values, and then back-transforming to the original scale with the 95% CIs for GMTs being calculated based on a t-distribution.
Summary statistics were provided for all prespecified safety events including the number and proportion of participants with AEs. AEs were reported using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0.
Results
Participants
In total, 100 participants (children aged 12 months–6 years, n = 37; children aged 7–12 years, n = 33; adolescents aged 13–17 years n = 30) were enrolled at 5 centers across Russia. Pre-vaccination investigations indicated that 8% of infants and children under the age of 7 years were seropositive for varicella compared with 33% of children aged 7–12 years and 40% of adolescents aged 13–17 years (). All participants received the protocol-specified vaccination regimen.
Table 1. Baseline characteristics
Immunogenicity
Among participants who were seronegative at baseline, the antibody response rate ranged from 95% to 100% across all age groups. Specifically for adolescents, the antibody response rate at 6 weeks post-dose 2 was 100% (n = 18; 95% CI, 81.5%–100.0%), whereas the corresponding rates were 95.5% (n = 22; 95% CI, 77.2%–99.9%) for children 7–12 years at 6 weeks post-Dose 1 and 100% (n = 34; 95% CI, 89.7%–100.0%) for children 12 months–6 years at 6 weeks post-Dose 1.
All adolescents who were seronegative at baseline (n = 18) demonstrated seroconversion 6 weeks after Dose 2, with a GMT of 78.6 gpELISA units/mL (95% CI, 46.5–133.0) after 2 doses versus 0.4 (95% CI, 0.3–0.5) gpELISA units/mL at baseline (). This represented a GMFR of 210.8 (95% CI, 117.2–379.0) in VZV antibody titer.
Figure 1. Changes in geometric mean titer from baseline following administration of varicella vaccine in (A) adolescents 13–17 years post-Dose 2, (B) children aged 7–12 years post-Dose 1 and (C) children aged 12 months–6 years post-Dose 1.CI, confidence interval; gpELISA, glycoprotein enzyme-linked immunosorbent assay.
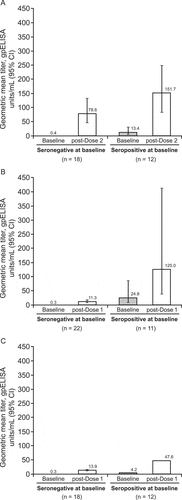
Among seronegative children and infants, 95.5% (21/22) and 100% (34/34) aged 7–12 years and 12 months–6 years, respectively, demonstrated seroconversion 6 weeks after a single dose with a GMT of 11.3 (95% CI, 7.3–17.5) gpELISA units/mL for children aged 7–12 years and 13.9 (95% CI, 11.2–17.2) gpELISA units/mL for children and infants aged 12 months–6 years after 1 dose versus 0.3 (95% CI, 0.3–0.4) gpELISA units/mL at baseline for both groups (). This represented GMFRs of 32.8 (95% CI, 21.4–50.4) and 40.8 (95% CI, 31.7–52.5) in VZV antibody titer, respectively.
Among adolescents who were seropositive at baseline, 66.7% (8/12) had a ≥ 4-fold rise in antibody titer 6 weeks after Dose 2 with a GMT of 151.7 (95% CI, 92.5–248.6) gpELISA units/mL versus 13.4 (95% CI, 5.8–31.0) gpELISA units/mL at baseline (), representing a GMFR of 11.3 (95% CI, 4.6–27.5). A ≥ 4-fold rise in antibody titer 6 weeks after a single dose was observed in 54.5% (6/11) and 100% (3/3) of children and infants aged 7–12 years and 12 months–6 years, respectively, who were seropositive at baseline. GMT for seropositive children aged 7–12 years was 125.0 (95% CI, 37.9–411.7) gpELISA units/mL after 1 dose versus 24.8 (95% CI, 7.2–85.5) gpELISA units/mL at baseline (), representing a GMFR of 5.0 (95% CI, 2.6–9.7). GMT was 47.6 gpELISA units/mL 6 weeks after a single dose in the 3 seropositive infants and children aged 12 months–6 years versus 4.2 gpELISA units/mL at baseline, representing a GMFR of 11.2 ().
Safety
Over the course of the study, AEs were reported by 52% of participants (52/100; all-participants-as-treated population). Vaccine-related injection-site reactions () were reported by 30% (9/30) of adolescents after Dose 1 and 23% (7/30) after Dose 2, 33% (11/33) of children aged 7–12 years, and 19% (7/37) of infants and children, aged 12 months–6 years, after a single dose. All injection-site AEs occurred 1 to 5 days after dosing and were mild in intensity, except for 2 instances of moderate injection-site pain. All instances of injection-site erythema and injection-site swelling were ≤2.4 cm in size.
Table 2. Injection-site adverse events
The most frequently reported systemic AEs were respiratory tract infections, increased body temperature (not meeting the protocol-defined criteria for fever), and headache (). Vaccine-related systemic AEs were reported by 1 adolescent, 3 children aged 7–12 years, and 4 children aged 12 months–6 years.
Table 3. Systemic adverse events
Protocol-defined fever (maximum oral or oral equivalent temperature ≥39.0°C) was observed in 1 child aged 7–12 years and 3 infants/children aged 12 months–6 years. A varicella-like rash was reported by 2 children aged 7–12 years and 1 child aged 12 months–6 years. All varicella-like rashes were mild in severity and no herpes zoster-like rashes were observed.
One serious AE of severe headache was reported 1 month after Dose 1 for an adolescent that resulted in hospitalization, which was considered to be unrelated to study vaccination. This severe headache was concurrent with mild pharyngitis, which was also considered to be unrelated to study vaccination.
Laboratory abnormalities were generally not considered clinically meaningful, and none were reported as vaccine-related AEs. No deaths were reported during the study, and no participants discontinued the study vaccine due to AEs.
Discussion
Varicella vaccine (VARIVAX™) induced an acceptable immune response and was generally well tolerated in adolescents receiving 2 doses and infants and children aged 12 months–12 years administered a single dose. The response rates, GMTs, and seroconversion rates in this pediatric and adolescent population were consistent with those reported previously for children administered this vaccine.Citation25,Citation26 Likewise, there is ample real-world evidence on the effectiveness and safety of varicella vaccine since its licensure in 1995,Citation18,Citation27 and there were no unexpected safety or tolerability concerns identified in this study compared with the vaccine’s >25 year real-world experience. The efficacy and safety data observed in Stage 2 of this study were also consistent with those observed in Stage 1 in adults (reported separately).
Earlier studies have indicated VZV vaccination reduces the risk of VZV infection, especially with a 2-dose regimen.Citation3,Citation18,Citation19,Citation21 Furthermore, epidemiologic data have confirmed that widespread utilization of VARIVAX reduces the incidence of varicella in both vaccinated and unvaccinated individuals (herd immunity), an effect that is maintained at the population level over at least 15 years for both vaccinated and unvaccinated individuals.Citation28 However, while children in this study were only administered 1 dose, the US Centers for Disease Control and health authorities in many other countries recommend 2 doses to reduce the risk of breakthrough VZV infection and transmission to at-risk populations, including immunosuppressed individuals and adolescents and adults without evidence of immunity, who have a higher risk of severe disease.Citation18,Citation29
Many physicians may consider varicella infection to be a relatively benign condition, but complications associated with VZV infection can result in hospitalization and potentially death.Citation29 VZV vaccination reduces clinic visits, overall varicella-related morbidity and mortality, and overall medical expenditure associated with varicella infection.Citation1 Analyses performed in Central and Eastern European countries have suggested that vaccinating for VZV has the potential to reduce the clinical economic burden of treating patients with varicella,Citation13,Citation14,Citation29 which continues to be high in Russia in the absence of widespread vaccination.Citation4
Primary infection with wild-type VZV also carries a higher risk of VZV establishing a latent infection in the dorsal root ganglia, which can reactivate, causing herpes zoster, often many years after the initial infectionCitation1,Citation2 However, vaccination for VZV reduces the risk of developing herpes zoster later in life compared with wild-type VZV infection.Citation1,Citation29
This study was limited by its sample size and inadvertent inclusion of children over the age of 7 and adolescents who were seropositive at baseline despite a negative clinical history for varicella. Strengths of the study included evaluation of all age groups across Stage 1 and Stage 2 of the trial, which encompassed adolescents and adults not routinely assessed in clinical trials for pediatric vaccines. Also, the study evaluated individuals who were seropositive at baseline, showing an increase in antibody titers in all these participants that is indicative of a boosting effect with vaccination.
In conclusion, varicella vaccine (VARIVAX™: Varicella Virus Vaccine Live [Oka/Merck], Merck & Co., Inc., Kenilworth, NJ, USA) has demonstrated immunogenicity and safety in children and adolescents that is consistent with previously published studies, supporting its licensure in the Russian Federation.
Acknowledgments
The authors thank the participants and clinical research staff who participated in this trial. Medical writing and editorial assistance, under the direction of the authors, were provided by Toinette Labuschagné of Apothecom in accordance with Good Publication Practice (GPP3) guidelines.
Disclosure statement
EP, XC, and HLP are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). OT and AF are employees of MSD, Russia. FW and VJ are employees of MSD, Switzerland.
Additional information
Funding
References
- Papaloukas O, Giannouli G, Papaevangelou V. Successes and challenges in varicella vaccine. Ther Adv Vaccines. 2014;2:39–55. doi:10.1177/2051013613515621.
- Freer G, Pistello M. Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41:95–105.
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014;89:265–88.
- Federal Service for Supervision of Consumer Rights Protection and Human Welfare. On the state of sanitary and epidemiological well-being of the population in the Russian Federation in 2019: State report. Moscow (Russia); 2020.
- Mészner Z, Wysocki J, Richter D, Zavadska D, Ivaskeviciene I, Usonis V, Pokorn M, Mangarov A, Jancoriene L, Man SC, et al. Burden of varicella in Central and Eastern Europe: findings from a systematic literature review. Expert Rev Vaccines. 2019;18(3):281–93. doi:10.1080/14760584.2019.1573145.
- Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. J Infect Dis. 2000;182:383–90. doi:10.1086/315714.
- Jaeggi A, Zurbruegg RP, Aebi C. Complications of varicella in a defined central European population. Arch Dis Child. 1998;79:472–77. doi:10.1136/adc.79.6.472.
- Bevilacqua S, Poircuitte JM, Boyer L, May T, Lascombes P, Venard V. Varicella arthritis in childhood: a case report and review of the literature. Pediatr Infect Dis J. 2011;30:980–82. doi:10.1097/INF.0b013e318223c0d8.
- Majumdar A, Atam V, Mishra M. Rare case of post-varicella membranoproliferative glomerulonephritis presenting with massive proteinuria. BMJ Case Rep. 2020;13(3):e233084. doi:10.1136/bcr-2019-233084.
- Azak E, Cetin II. Acute myocarditis following varicella zoster infection in an immunocompetent adolescent: an uncommon complication. Arch Argent Pediatr. 2020;118:e284–e7.
- Amlie-Lefond C, Gilden D. Varicella zoster virus: a common cause of stroke in children and adults. J Stroke Cerebrovasc Dis. 2016;25:1561–69. doi:10.1016/j.jstrokecerebrovasdis.2016.03.052.
- Donahue JG, Kieke BA, Yih WK, Berger NR, McCauley JS, Baggs J, Zangwill KM, Baxter R, Eriksen EM, Glanz JM, et al. Varicella vaccination and ischemic stroke in children: is there an association? Pediatrics. 2009;123(2):e228–e34. doi:10.1542/peds.2008-2384.
- Wysocki J, Malecka I, Stryczynska-Kazubska J, Rampakakis E, Kuter B, Wolfson LJ. Varicella in Poland: economic burden in children 1-12 years of age in Poland, 2010-2015. BMC Public Health. 2018;18:410. doi:10.1186/s12889-018-5298-8.
- Meszner Z, Molnar Z, Rampakakis E, Yang HK, Kuter BJ, Wolfson LJ. Economic burden of varicella in children 1-12 Years of age in Hungary, 2011-2015. BMC Infect Dis. 2017;17:495. doi:10.1186/s12879-017-2575-6.
- Baryshev MA, Chernyavskaya OP, Saltykova TS. Experience of the varicella vaccine introduction into regional vaccine schedule of the Russian Federation. Epidemiol Vaccin Protect. 2019;18:67–74. doi:10.31631/2073-3046-2019-18-6-67-74.
- Henry O, Brzostek J, Czajka H, Leviniene G, Reshetko O, Gasparini R, Pazdiora P, Plesca D, Desole MG, Kevalas R, et al. One or two doses of live varicella virus-containing vaccines: efficacy, persistence of immune responses, and safety six years after administration in healthy children during their second year of life. Vaccine. 2018;36(3):381–87. doi:10.1016/j.vaccine.2017.11.081.
- Seward JF, Marin M, Vázquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis. 2008;197(Suppl 2):S82–9. doi:10.1086/522145.
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137:e20153741. doi:10.1542/peds.2015-3741.
- Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, Kim LL, Lupinacci L, Hartzel J, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23(2):132–37. doi:10.1097/01.inf.0000109287.97518.67.
- Sadaoka T, Depledge DP, Rajbhandari L, Venkatesan A, Breuer J, Cohen JI. In vitro system using human neurons demonstrates that varicella-zoster vaccine virus is impaired for reactivation, but not latency. Proc Natl Acad Sci USA. 2016;113:E2403–12. doi:10.1073/pnas.1522575113.
- Zhou F, Ortega-Sanchez IR, Guris D, Shefer A, Lieu T, Seward JF. An economic analysis of the universal varicella vaccination program in the United States. J Infect Dis. 2008;197:S156–S64. doi:10.1086/522135.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15:645–57. doi:10.1080/21645515.2018.1546525.
- Ministry of Health of the Russian Federation. State Register of Medicines; 2021.
- Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006;78(12):1679–87. doi:10.1002/jmv.20754.
- Ngai AL, Staehle BO, Kuter BJ, Cyanovich NM, Cho I, Matthews H, Keller P, Arvin AM, Watson B, White CJ. Safety and immunogenicity of one vs. two injections of Oka/Merck varicella vaccine in healthy children. Pediatr Infect Dis J. 1996;15(1):49–54. doi:10.1097/00006454-199601000-00011.
- Silber JL, Chan IS, Wang WW, Matthews H, Kuter BJ. Immunogenicity of Oka/Merck varicella vaccine in children vaccinated at 12-14 months of age versus 15-23 months of age. Pediatr Infect Dis J. 2007;26:572–76. doi:10.1097/INF.0b013e318060d33d.
- Chaves SS, Haber P, Walton K, Wise RP, Izurieta HS, Schmid DS, Seward J. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S170–7. doi:10.1086/522161.
- Baxter R, Ray P, Tran TN, Black S, Shinefield HR, Coplan PM, Lewis E, Fireman B, Saddier P. Long-term effectiveness of varicella vaccine: a 14-Year, prospective cohort study. Pediatrics. 2013;131(5):e1389–96. doi:10.1542/peds.2012-3303.
- Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, Rentier B, Rümke H, Sadzot-Delvaux C, Senterre J, et al. Varicella vaccination in Europe - taking the practical approach. BMC Med. 2009;7:26. doi:10.1186/1741-7015-7-26.

