?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
Females generally have higher antibody responses to viral vaccines. Our objectives were to compare gender differences in the efficacy of COVID-19 vaccination.
Methods
Data sources: Studies from PubMed, Embase, Cochrane Central Register, Web of Science, ClinicalTrials.gov, and the World Health Organization’s International Clinical Trials Registry Platform.
Results
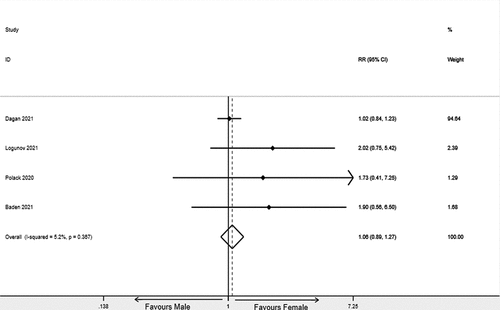
We included four eligible trials; all were categorized as having a low risk of bias. COVID-19 vaccine was significantly effective in both males and females. Slightly more SARS-CoV-2 infections were recorded in females than in males, but the difference was not significant (RR 1.064 [0.888–1.274]; p = .502, I2 = 5.7%; p = .367, 643,127 participants).
Conclusion
Despite significant biological and behavioral differences between males and females, we found no significant gender differences in the efficacy of the COVID-19 vaccines, especially in younger populations. Further pragmatic trials are needed to confirm the gender differences in protective response of different types of vaccines to different age groups.
KEYWORDS:
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of Coronavirus disease 2019 (COVID-19) originally found in December 2019. Spreadable through transmitted by contaminated surfaces, direct contact with infected individuals or via respiratory droplets, as of 14 May 2021, SARS-CoV-2 had infected over 160 million people resulting in over 3,335,000 deaths.Citation1 As of 11 May 2021, a total of 1,264,164,553 vaccine doses have been administered.Citation1 Epidemiological studies have shown a male sex-based bias in disease severity, COVID-19 produces higher mortality and more severe symptoms among males than among females.Citation2
Some mechanisms have been proposed. Recent basic studies have found that the receptors for COVID-19 (TMPRSS2 and ACE2) were co-expressed with AR in specific alveolar and bronchial epithelial cell types, and that androgens regulated the expression of TMPRSS2, and ACE2 was identified as an AR-regulated target.Citation3 Significantly, the relationship between sex differences and the susceptibility and severity of COVID-19 may exist for a variety of reasons, including sex-differences in immune response. Takahashi et al.Citation4 revealed key differences in baseline immunity between male and female patients during the disease course of SARS-CoV-2 infection. Female patients had a more robust T cell response (specifically, activated CD8 T cells) at baseline than male patients, and the poor T cell responses in male patients were associated with future disease progression, so male patients might need vaccines to enhance T cell immune responses against SARS-CoV-2.Citation4 Previous evidence on vaccines, including the yellow fever, hepatitis, measles, mumps and bacillus Calmette-Guerin vaccines, seems to illustrate higher immunoreactivity after vaccination in female compared to male.Citation5 In addition, estimates of the death reported by gender vary widely across contexts and population groups. Testing and reporting bias in women, or differences in exposure due to differences in behavior and risk, may play a role in the outcome of vaccine trials.Citation6 Equity in clinical trials begins with the consideration of both gender and sex dimensions in the study of new drugs.Citation7
To date, no specific antiviral treatment for SARS-CoV2 exists, but COVID-19 vaccines had been administered since December last year. Nearly 400 million doses of COVID-19 vaccines have been administered globally, and 16 SARS-CoV-2 vaccine candidates were in phase 3 clinical trials as of the end of January 2021.Citation8 On March 5, 2021, Vijayasingham et al.Citation9 reported that sex determinants of immunity induced by COVID-19 vaccine may exist, and future reporting on sex-disaggregated data and discussing how gender influences trial results would benefit regulatory and public decision-making and the design of mass vaccination programmes. Faced with gender differences in COVID-19 epidemiology, biology, immunology, and sociology, are sex-differentiated dosing regimens needed in vaccine studies? Therefore, we conducted a meta-analysis to explore gender differences in COVID-19 vaccine efficacy, with the hope of providing a timely reference for vaccine development and data recording.Citation5
2. Materials and methods
2.1. Search strategy
We carried on this study and reported the results in accordance with Preferred reporting items for systematic reviews and meta-analyses checklist (PRISMA).Citation10 As of April 2, 2021, 267 articles were obtained by searching databases including, PubMed, Embase, Cochrane Central Register, and Web of Science through MeSH terms such as “COVID-19 vaccine,” “SARS-CoV2 vaccine,” “sex,” “gender,” and “efficacy” without language restrictions (Supplementary Table S1). The reference lists of included trials were hand-searched for relevant citations. We searched the World Health Organization’s International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov to identify planned, ongoing, or recently completed but unpublished trials of COVID-19 vaccine.
2.2. Inclusion criteria
All clinical trials or cohort studies that adopted COVID-19 vaccines (intervention group) and non-COVID-19 component vaccine or the placebo (control group) to vaccinate adolescents or adults were identified. Studies that did not meet the inclusion criteria and study outcomes, as well as those that provided only study protocols without data, were excluded.
2.3. Outcome measurement
The relevant outcome of efficacy was participants without serologic or virologic evidence of SARS-CoV-2 infection did not develop COVID-19 after COVID-19 vaccination during the study period. COVID-19 infection was defined as at least one nasopharyngeal swab, nasal swab, saliva sample, or respiratory sample that was positive for SARS-CoV-2 by reverse-transcriptase–polymerase-chain-reaction (RT-PCR) test.
2.4. Study selection
Two reviewers independently screened all citations and abstracts for identified trials, excluding trials that clearly did not meet the inclusion criteria. Reviewers searched the full text of potential qualification trials for further evaluation. According to the Cochrane Guidelines, disagreements between the two reviewers were resolved through discussion.
2.5. Data extraction
Two investigators (ZZ and LX) extract the following data from eligible studies: 1. general information, including authors, year of publication; 2. research type; 3. participants’ characteristics and sample size; 4. intervention, including vaccination name, regimen and follow-up time; 5. vaccine efficacy. Data extraction was done independently by two reviewers. Any differences were resolved through discussion or consultation with a third independent reviewer.
2.6. Risk of bias assessment
We used the Cochrane Collaboration’s Risk of Bias Tool to evaluate internal validity.Citation11 The tool consists of seven domains, and classifies the total deviation into risk of bias. Each item was valued as “low risk,” “high risk” or “unclear risk.” The Newcastle Ottawa scale (NOS) was used to assess the risk of bias in nonrandomized studies.Citation12 They are divided into three main aspects: selection, comparability, and exposure. The highest score for each study was 9, studies with a score above 7 were considered to be of high quality, excluding articles of poor quality (0–3).
2.7. Statistical analysis
The statistical analysis was performed by STATA 15.1 software to assess gender differences in the efficacy of COVID-19 vaccines. We calculated the pooled RRs of vaccine efficacy and the rate ratio (RR) and 95% confidence intervals (CI) for COVID-19 infection in females and males. Heterogeneity was assessed using the Cochrane Q Chi-Squared and I2 tests. When the heterogeneity test showed P < .1 or I2 > 50%, the data was considered to have high heterogeneityCitation13. When the I2 > 50%, the random-effect model was chosen to calculate the pooled effect, otherwise the fixed-effect model was used instead. We conducted sensitivity analysis using two techniques, respectively ignoring studies one by one, repeating meta-analysis by using a random-effects model. Because fewer than 10 studies were included, publication bias was not assessed. For other analysis, if not specifically mentioned, a P value of 0.05 was considered statistically significant. The estimation of raw data was calculated using the following formula:
where a is the number of vaccinated participants with COVID-19, b is the number of vaccinated participants without COVID-19, c is the number of unvaccinated participants with COVID-19, and d is the number of unvaccinated participants without COVID-19.
3. Results
3.1. Search results
Our search retrieved 267 records, of which 40 were duplicates and excluded. Of the 124 articles excluded due to obvious irrelevance, 103 full texts were retrieved (). A total of 99 records did not meet the inclusion criteria, 97 of which had not published the experimental results, and 2 did not display the data in an appropriate format. Four articles provided gender-specific data on vaccine efficacy that met the inclusion criteria.Citation14–17
3.2. Description of the included trials
The sample sizes of the included trials were ranged from 19,866 to 1,193,236, with a total of 1,279,015 participants in the 4 studies. Our study included 643,127 vaccinated patients without COVID-19, 49.6% of whom were female. The participants were aged from 45 to 52 years old. Four trials were considered to have low risk of bias (Supplementary Table S2). Four studies were conducted in Israel, Russia, Argentina, Brazil, South Africa, and the United States, and efficacy was assessed between 7 days before the second dose and 14 days after the second dose. One of the studies was vaccinated with an adenovirus vectored vaccine,Citation15 while the other three were vaccinated with an mRNA vaccine. Only one study was observational;Citation14 the other three were randomized controlled trials. The characteristics of the included studies are shown in .
Table 1. Characteristics of included studies
3.3. Efficacy
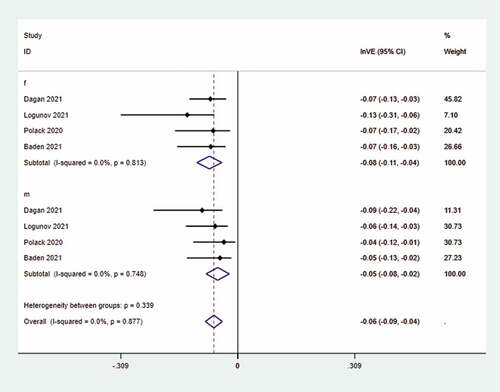
The pooled effect of COVID-19 vaccine in females and males was 92.3% (I2 = 0.0%; p = .813) and 95.1% (I2 = 0.0%; p = .877), respectively (See ). Among those who received the COVID-19 vaccine, the intervention group was female and the control group was male. Statistical analysis of the vaccinated population showed that slightly more SARS-CoV-2 infections were recorded in females than in males, but the difference was not significant (RR 1.064 [0.888–1.274]; p = .502, I2 = 5.7%; p = .367) (See ). Moreover, the heterogeneity of the above researches was negligible. The results of sensitivity analysis showed that this study had a large impact on the pooled effect,Citation14 and the results were robust using the random-effect model (Supplementary Figure 1S).
4. Discussion
Our study showed that COVID-19 vaccines were significantly effective in both males and females. In any clinical trial of COVID-19 vaccines, VE>90% can be interpreted as having a protective effect against SARS-CoV-2 infection. Besides, a statistical analysis of the raw data of the vaccinated population showed that there was no correlation between sex-differences and the COVID-19 vaccine efficacy. The stability of the combined estimate was good when using the random effects model. However, the leave-one-out sensitivity analysis showed that one study resulted in a change in the pooled estimates. Although this study was not a randomized controlled trial,Citation14 it was a low-risk bias study published in the New England Journal of Medicine that matched the age, sex and other factors of vaccine recipients and controls for variables related to the probability of vaccination and COVID-19 infection. Moreover, the number of people included in the study was more than 40 times that of the other three clinical trials. Therefore, we believed that this paper could not be excluded. What’s more, Dagan showed that the estimated vaccine efficacy for documented SARS-CoV-2 infection in subpopulations defined by sex were consistent with similar efficacy across age groups, which supported our findings. Therefore, the result that COVID-19 vaccine efficacy did not differ between males and females can still be confirmed.
Interestingly, past studies on vaccines had produced results different from ours, including the measles and mumps vaccine, the bacillus Calmette-Guerin vaccine, in which females generally developed higher antibody responses to viral infection and vaccines than males.Citation5 This may be related to the fact that E2 and P4 favor a state of decreased innate immune inflammatory response while enhancing immune tolerance and antibody production. However, this was only from the perspective of molecular biology, and it is still unknown whether sex hormones affect the human body to produce antibodies against COVID-19 vaccines. Significantly, the investigation results for the real-world support our research conclusion. As of April 26, 2021, the CDC reported 9245 cases of reinfection, 63% of which were women, after more than 95 million people in the United States had been vaccinated.Citation18 Females are more vulnerable to the threat of COVID-19 because of gender differences in biology and behavior. Biological differences such as immunity, hormones, genetics,Citation19 as well as behavioral differences such as vaccination opportunities and willingness,Citation5 and professional exposing risks may lead to differences in vaccine responses between the sexes.Citation6 However, in the Clinical trials of COVID-19 vaccine, gender differences in antibody protection and seroconversion rates have not been considered. Whether gender affects the antibody production in SARS-CoV-2 infection is still unknown.
Both influenza virus and SARS-CoV-2 belong to single-stranded mRNA virus, a recent systematic review of influenza vaccines had shown that no clear conclusion could be drawn regarding the effect of sex on the efficacy of seasonal influenza vaccine.Citation20 At the same time, age and vaccine type are also the regulatory factors of gender response. An observational study found that across most outcomes and seasons,Citation21 gender has a modest effect on influenza VE. The gender effects were age-dependent, and adults age ≥50 years had a greater impact than those age <50 years. In our study, more than half of the participants were younger than 50 years old, which might be one of the reasons why gender differences had a modest effect on vaccine efficacy. Notably, it has been found that sex bias in the efficacy of influenza inactivated vaccine with high-dose vaccine and adjuvant supplementation compared to the standard-dose vaccination.Citation22This meta-analysis included a large population, but the primary interventions were adenovirus vectored vaccine or mRNA vaccine. Therefore, the results of phase III trials with more types of COVID-19 vaccines are needed in the future to further explore this issue.
4.1. Limitations
This meta-analysis was the first study on gender differences in the efficacy of COVID-19 vaccine, which included high-quality literature and a large number of participants. Since the results of phase III clinical trials of some types of vaccines have not been published, only adenovirus vectored vaccine or mRNA vaccine were included in this study. Moreover, age as a common regulatory factor has an impact on the incidence rate and severity of COVID-19 and the gender effect of vaccines. Due to limited data, age and other factors could not be eliminated. Finally, due to the inability to eliminate high-quality studies, we used reliable statistical methods to estimate the required data without original data, but this would inevitably lead to small systematic bias.
4.2. Future direction
With the development of various types of vaccines, the gender inequality caused by social and psychological factors such as exposure risk, vaccination opportunity, vaccination willingness and psychological stress should be considered in clinical trials. If possible, females’ biases toward effective and adverse effects of the COVID-19 vaccine should be considered, and gender specific doses should be tested. National policies should consider the clinical and logistical aspects of the safe development, equitable delivery, and scientific management of COVID-19 vaccines. To ensure that the antibody response is positive and effective in different genders and ages, which can also provide a better vaccination experience for the public and increase the willingness to vaccinate.
5. Conclusions
In summary, our study showed that there was no significant difference in the efficacy of COVID-19 vaccines in males and females, and that it was slightly more effective in males than in females. Even though there are significant biological and behavioral differences between males and females, these differences did not translate into clinical infection among younger patients.
Highlights
COVID-19 vaccines were significantly effective in both males and females.
Statistical analysis of the vaccinated population showed that slightly more SARS-CoV-2 infections were recorded in females than in males, which was in line with the latest research data in the real-world.
There was no significantly correlation between sex-differences and the COVID-19 vaccine efficacy.
Author contributions
Gang Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Zheng Zhu and Lizhen Xu reviewed the literature, extracted the data and completed the analysis. Zheng Zhu provided edits of the manuscript.
Supplemental Material
Download ()Disclosure statement
We declare no competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1984135.
Additional information
Funding
References
- World Health Organisation Coronavirus disease (COVID-19) Situation dashboard; 2021 May 14 [accessed 2021 May 14]. https://covid19.who.int/ .
- Xu L, Mao Y, Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging. 2020;12(12):12410–21. doi:10.18632/aging.103383.
- Qiao Y, Wang XM, Mannan R, Pitchiaya S, Zhang Y, Wotring JW, Xiao L, Robinson DR, Wu Y-M, Tien JC-Y, Cao X, Simko SA, Apel IJ, Bawa P, Kregel S, Narayanan SP, Raskind G, Ellison SJ, Parolia A, Zelenka-Wang S, McMurry L, Su F, Wang R, Cheng Y, Delekta AD, Mei Z, Pretto CD, Wang S, Mehra R, Sexton JZ, Chinnaiyan AM. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci USA. 2020;118:e2021450118.
- Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–20. doi:10.1038/s41586-020-2700-3.
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33(1):577–99. doi:10.1146/annurev-cellbio-100616-060718.
- Bischof E, Oertelt-Prigione S, Morgan R, Klein S. Towards precision medicine: inclusion of sex and gender aspects in COVID-19 clinical studies-acting now before it is too late-a joint call for action. Int J Environ Res Public Health. 2020;17(10):3715. doi:10.3390/ijerph17103715.
- Tannenbaum C, Ellis RP, Eyssel F, Zou J, Schiebinger L. Sex and gender analysis improves science and engineering. Nature. 2019;575(7781):137–46. doi:10.1038/s41586-019-1657-6.
- Kim JH, Hotez P, Batista C, Ergonul O, Figueroa JP, Gilbert S, Gursel M, Hassanain M, Kang G, Lall B, Larson H, Naniche D, Sheahan T, Shoham S, Wilder-Smith A, Strub-Wourgaft N, Yadav P, Bottazzi ME. Operation warp speed: implications for global vaccine security. Lancet Global Health. 2021;9:e1017–e1021.
- Vijayasingham L, Bischof E, Wolfe J. Sex-disaggregated data in COVID-19 vaccine trials. The Lancet. 2021;397(10278):966–67. doi:10.1016/S0140-6736(21)00384-6.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Med PGJP. 2009;6:e1000097.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Peterson J, Welch V, Losos M, Tugwell PJOOHRI The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 2011.
- Higgins JP, Thompson S. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine. 2002;21(11):1539–1558.
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. doi:10.1056/NEJMoa2101765.
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. 2021;397(10275):671–81. doi:10.1016/S0140-6736(21)00234-8.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403‐16. doi:10.1056/NEJMoa2035389.
- COVID-19 breakthrough case investigations and reporting; 2021 May 7 [accessed 2021 April 26]. https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html .
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. doi:10.1038/nri.2016.90.
- Tadount F, Doyon-Plourde P, Rafferty E, MacDonald S, Sadarangani M, Quach C. Is there a difference in the immune response, efficacy, effectiveness and safety of seasonal influenza vaccine in males and females? - A systematic review. Vaccine. 2020;38(3):444–59. doi:10.1016/j.vaccine.2019.10.091.
- Chambers C, Skowronski DM, Rose C, Serres GD, Winter A-L, Dickinson JA, Jassem A, Gubbay JB, Fonseca K, Drews SJ, et al. Should sex be considered an effect modifier in the evaluation of influenza vaccine effectiveness? Open Forum Infect Dis. 2018;5(9):ofy211. doi:10.1093/ofid/ofy211.
- Dhakal S, Klein Sabra L, Coyne Carolyn B. Host factors impact vaccine efficacy: implications for seasonal and universal influenza vaccine programs. J Virol. 2019;93(21):e00797–19. doi:10.1128/JVI.00797-19.