ABSTRACT
Background
In China, premature children in good health may be advised to receive routine immunization programs. However, delayed vaccination is common. This study aimed to characterize vaccination experiences of premature children and determine the impact of vaccination consultation clinic (VCC) assessment.
Methods
We performed a retrospective cohort study, including premature children visiting VCC at Children’s Hospital of Fudan University in 2017–2019. Data of these children, including demographics, recommendations of vaccination after VCC assessment, vaccination records, adverse events following immunization (AEFI), and incidence of vaccine-preventable diseases in 2017–2019, were collected retrospectively.
Results
A total of 1124 premature children were included, with vaccination uptake of 46.3% for expanded program of immunization (EPI) vaccines and 15.1% for non-EPI vaccines before VCC assessment. Furthermore, 77.5% of premature children who had not received any EPI vaccine were vaccinated after the assessment; however, most were delayed, regardless of vaccine types and recommendations. In contrast, 67.3% was vaccinated with non-EPI vaccines after the assessment. Majority (n = 35) of recorded AEFI was mild to moderate, in addition to one allergic rash. One each case of pertussis and varicella were recorded in those who had not received the corresponding vaccines.
Conclusion
Vaccination may be safe and epidemiological effective in premature children. However, it remains mostly delayed in premature children with recommendations of normal vaccination. It warrants improving implementation of VCC recommendations. In addition, there is a need of health promotion on more non-EPI vaccines for premature children.
Introduction
Approximately 15 million premature babies are born every year, accounting more than one-tenth of all newborns, according to the World Health Organization (WHO).Citation1 In China, the number of premature babies ranked second across the world, exceeding 1.17 million.Citation1 Generally, premature children have suppressive innate and adaptive immunity functions.Citation2 Immune response to T cell-dependent antigens is significantly lower in premature children, compared to full-term ones.Citation3 Similarly, levels of antibodies (IgM, IgG, and IgA) are lower in premature children, which are correlated with gestational age, birth weight, and other birth-related factors.Citation4 In addition, previous studies have shown that morbidity and mortality of vaccine-preventable diseases in premature children were higher than those in full-term ones.Citation5
It has been recommended that premature children should be fully vaccinated in accordance with existing routine immunization programs; and more important, they should receive on-time vaccination to reduce the duration of exposure.Citation6 However, premature children, especially those with minimal gestational age or very low birth weight, have relatively low immune system development and are often accompanied with various diseases at birth. It may result in delayed vaccination or missed opportunity for vaccination.Citation7 Currently, there are some expert consensuses, guiding the health care practitioners (HCPs) how to vaccinate premature children in vaccination clinics.Citation8–10 However, there remains lack of evidence demonstrating whether it is safe and effective in premature children after being vaccinated according to routine immunization programs.Citation11 It warrants developing scientific and feasible immunization program and common guidelines for premature children. In Shanghai, Vaccination Consultation Clinic (VCC) at the Children’s Hospital of Fudan University can provide professional consultation for children with special health conditions such as premature children, and then make recommendations of vaccination.Citation12 Thus, this study is aimed to characterize the vaccination experiences of premature children that had visited VCC, and then determine the occurrence of vaccine-preventable diseases and adverse events following immunization (AEFI).
Materials and methods
Study design
We conducted a retrospective cohort of premature children visiting VCC at the Children’s Hospital of Fudan University from 2017 January through 2019 December. Inclusion criteria were as follows: 1) maternal gestational age ≤37+6 weeks; 2) local children in Shanghai, or non-local children who have been vaccinated in Shanghai.
In this study, we determined the vaccination experiences of EPI and non-EPI vaccines. EPI vaccines were as follows: Bacillus Calmette-Guerin vaccine (BCG), diphtheria tetanus pertussis vaccine (DTaP), hepatitis A vaccine (HepA), hepatitis B vaccine (HepB), Japanese encephalitis vaccine (JEV), measles-mumps-rubella vaccine (MMR), measles-rubella vaccine (MR), meningococcal serogroup A polysaccharide vaccine (MenA), and polio vaccine (PV). Of them, BCG, MMR, and MR are live attenuated vaccines, and the rest are inactivated vaccines. In addition, we included non-EPI vaccines, such as Haemophilus Influenzae type b vaccine (Hib), DTaP-Hib vaccine, DTaP-IPV/Hib vaccine, EV-71 vaccine, 13-valent pneumococcal conjugate vaccine (PCV13), rotavirus vaccine (RotV), and varicella vaccine (VarV).
Data collection
Demographic data, clinical information, and assessment by VCC of premature children were retrospectively collected from the hospital. Records of vaccination (vaccine types, doses, and date of vaccination) and AEFI were directly retrieved from Shanghai Immunization Information system. Incidence of vaccine-preventable diseases, including diphtheria, hepatitis, Japanese encephalitis, measles, mumps, pertussis, poliomyelitis, rubella, tuberculosis, and varicella was collected from the National Notifiable Disease Reported System.
Delayed duration of time in vaccination
The EPI vaccines have qualified age of vaccination. In this study, we defined qualified age of vaccination as follows: 1) for vaccines that should be vaccinated less than 12 months of age, qualified age was defined as ≤ recommended month of age plus 1 month; and 2) for vaccines that should be vaccinated above 12 months of age, qualified age was defined as ≤ recommended month of age plus 3 months. In addition, actual vaccination was defined as the first dose of vaccines.
Families with premature children may not visit VCC at birth, resulting in some of children had been partially vaccinated before the assessment by VCC. Considering delayed assessment, we defined delayed duration of time in vaccination = age of actual vaccination – qualified age of vaccination, for children vaccinated before the assessment.
If premature children received vaccines after visiting VCC, we had to exclude the influence of “waiting for assessment” on the delayed duration of time in vaccination. For children whose age of assessment was older than qualified age of vaccination, we defined delayed duration of time in vaccination = age of actual vaccination – age of assessment. For those whose age of assessment were younger than qualified age of vaccination, delayed duration of time in vaccination was = age of actual vaccination – qualified age of vaccination.
Statistical analysis
Quantitative variables with normal distributions were described as mean ± standard deviation (SD), while delayed duration of time in vaccination was presented as median (P50) and quartile range (P25, P75). Categorical variables were listed as percentages. Chi-square test, Fisher’s exact test, and t-test were used to compare the demographics between included and excluded children, and vaccination status between the recommendations by VCC across three groups (vaccinated before the assessment, vaccinated after the assessment, and not ever vaccinated in this study), where applicable. Mann–Whitney test was employed to compare the delayed duration of time in the vaccination of EPI vaccines before and after the assessment, stratified by recommendations by VCC (normal vaccination or delayed vaccination).
This study used IBM SPSS Statistics 23.0 (Armonk, NY, USA) to perform statistical analysis. A P value <.05 was considered statistically significant.
Ethics approval
This study involved the use of existing and routinely collected data at Children’s Hospital of Fudan University and Minhang District Center for Disease Control and Prevention. All data included in this study were kept confidential without personal identifiers. No additional data were collected for this study. There was no need of obtaining informed consent. This study was approved by the Institutional Review Board (IRB) of the Fudan University School of Public Health (IRB 00002408 and FWA 00002399) under IRB #2021-06-0907.
Results
Baseline characteristics in the cohort
A total of 1550 premature children were retrospectively collected, of which 426 non-local children were excluded due to having no vaccination records in Shanghai. Consequently, 1124 premature children were included in the cohort, which were more likely to have gestational age of 32–37 weeks (P = .017), be under 18 months of age at consultation (P = .027), and receive a positive recommendation by VCC (P = .026), compared to those excluded (). In addition, the vaccination uptake rate of any vaccine was 46.3% in premature children before the assessment.
Table 1. Baseline characteristics between included and excluded premature children
Vaccination of EPI vaccines
For EPI vaccines, the uptake rate and on-time vaccination were 46.3% and 40.4%, respectively, before the assessment. In contrast, 77.5% of premature children who had not received any EPI vaccine were vaccinated after the assessment. We compared the vaccination experiences among three groups, including children vaccinated before the assessment by VCC, those vaccinated after the assessment, and those not ever vaccinated in this study.
For live attenuated vaccines, recommendations of normal vaccination and delayed vaccination differed significantly across the three groups in the vaccination of BCG (P < .001), MR (P < .001), and MMR (P < .001) (). In children with a recommendation of normal vaccination, delayed duration of time in those vaccinated after the assessment was similar with those vaccinated before the assessment, in the vaccination of BCG (P = .190), MR (P = .468), and MMR (P = .574). In contrast, in children with a recommendation of delayed vaccination, delayed duration of time in those vaccinated after the assessment was significantly longer in BCG (P < .001) and MR (P = .002), whereas it was shorter in MMR (P = .048), compared to those vaccinated before the assessment.
Table 2. Vaccination experiences of live attenuated vaccines included in the expanded program of immunization in premature children
For inactivated vaccines, recommendation was principally normal vaccination (98.6%). Recommendations of normal vaccination and delayed vaccination did not differ significantly across the three groups in the vaccination of DTaP (P = .097), MenA (P = .086), or JEV (P = .373) (). In children with a recommendation of normal vaccination, delayed duration of time in those vaccinated after the assessment was significantly shorter in the vaccination of HepB (P < .001), longer in PV (P < .001) and DTaP (P < .001), while similar in MenA (P = .647), JEV (P = .617), and HepA (P = .552), compared to those vaccinated before the assessment. Additionally, some vaccines may be delayed when premature children have central nervous system diseases, fever or allergy. In children with a recommendation of delayed vaccination, delayed duration of time in those vaccinated after the assessment was significantly longer than those with a recommendation of normal vaccination, in the vaccination of DTaP (P = .001), MenA (P = .011), and JEV (P = .003).
Table 3. Vaccination experiences of inactivated vaccines included in the expanded program of immunization in premature children
Vaccination of non-EPI vaccines
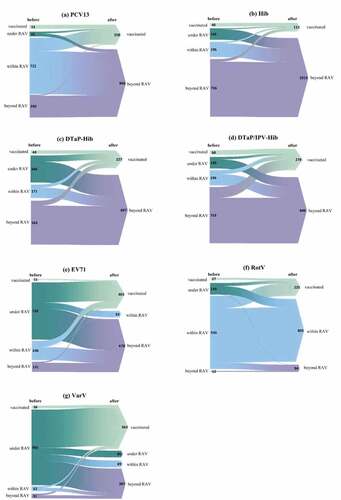
Vaccination experiences of non-EPI vaccines varied widely (), as these vaccines generally have lowest age of vaccination and no strict restriction, compared to EPI vaccines. The uptake rate was 15.1% before the assessment, while 67.3% in children who had not received any non-EPI vaccine after the assessment. Proportion of children receiving DTaP-IPV/Hib (5.3%) ranked the highest, followed by PCV13 (4.5%) before the assessment; in contrast, VarV (46.9%) ranked the highest, followed by EV-71 vaccine (31.0%) after the assessment. In addition, in children who had expired the regular age of vaccination when visiting VCC, catch-up vaccination of DTaP-IPV/Hib (18.8%) ranked the highest after the assessment.
Figure 1. Vaccination experiences of non-EPI vaccines before and after the assessment by the Vaccination Consultation Clinic (VCC) at the Children’s Hospital of Fudan University. In each panel, the height of colorful bars (left side) and triangles (right side) indicated the number of premature children in each group. Before the assessment, premature children were classified into four groups, demonstrating vaccination status on the left side of each bar, including “vaccinated,” “under RAV (recommended age of vaccination),” “within RAV,” and “beyond RAV.” After the assessment, on the right side of each triangle, premature children were classified as “vaccinated” and “beyond RAV” in the vaccination of PCV13 (panel a), DTaP-IPV/Hib (b), DTaP-Hib (c), and Hib (d); an additional status “within RAV” in EV-71 (e) and RotV (f); and another status “under RAV” in VarV (g). The flows from left bars to right triangles indicted the number of premature children who changed or remained their vaccination status before and after the assessment by VCC.
Incidence of AEFIs
A total of 18,085 doses of above vaccines were observed in this study, of which only 36 AEFIs were reported, including 35 mild-to-moderate events (fever of 37.3–38°C, n = 29; partial redness and swelling, n = 3; endemic induration, n = 1; vomiting and diarrhea, n = 1; abnormal crying, n = 1) and 1 case of allergic rash. The incidence of AEFI was calculated to be 199.06/100,000 doses, which was much lower than that reported in all infants and children in Shanghai (312.30/100,000 doses) in 2019. In this study, PCV13 caused the highest incidence of AEFI (n = 6; 764.3/100,000 doses), followed by MenA (n = 4; 298.1/100,000 doses).
In addition, there was no significant difference between premature children with and without AEFI in gender (χ2 = 0.975, P = .323), birth weight (t = 0.527, P = .602), gestational age (t = 0.557, P = .577), or immunodeficiency (χ2 = 0.284, P = .594). In the cases with AEFI, majority (n = 32) occurred in the vaccination after the assessment, of which 16 (50.0%) were recommended delayed vaccination. Twenty-two cases occurred after receiving the first dose of vaccines.
Incidence of notifiable vaccine-preventable diseases
We identified only pertussis and varicella in both included and excluded children in this study, whereas no tuberculosis, poliomyelitis, Japanese encephalitis, diphtheria, rubella, mumps or measles. Incidence density of pertussis and varicella in included premature children was lower than those in the excluded ones, with no statistical significances (P > .05) (). Moreover, in included premature children, incidence density of pertussis and varicella in the unvaccinated ones was higher than those in the vaccinated ones, with no statistical significances (P > .05) ().
Table 4. Incidence of notifiable vaccine-preventable diseases in included and excluded premature children
Table 5. Incidence of notifiable vaccine-preventable diseases in included children
Discussion
In this study, vaccination uptake rate of any vaccine was high in premature children, especially after the assessment. For EPI vaccines, after receiving a recommendation of normal vaccination, vaccination and catch-up vaccination of HepB were accelerated, while other inactivated and live attenuated vaccines were not accelerated or continued to be delayed, before and after the assessment. In contrast, after receiving a recommendation of delayed vaccination, almost all the vaccines (except MMR) were significantly delayed. The first dose of HepB in the immunization program should be administered at birth, so catch-up vaccination of HepB may be urgent. However, there was vaccine hesitancy toward most vaccines in the recommendation of normal vaccination, whereas good compliance in the recommendation of delayed vaccination, suggesting that families with premature children were less likely to receive vaccines, regardless of vaccine types or recommendations. Most premature children in our study had comorbidities, such as congenital heart disease, growth retardation, intracranial hemorrhage, neonatal asphyxia, and genetic metabolic diseases, which may reduce the confidence of both HCPs and children’s parents in vaccinating premature children.Citation13 Previous studies have documented that HCPs at vaccination clinics were hesitant and even rejected to vaccinate premature children, due to concerns on increasing risk of vaccines.Citation14 Children’s parents had limited understanding of children’s health conditions, which might contribute to parental vaccine hesitancy toward childhood vaccination.Citation15 Thus, VCC assessment might have a limited impact on the on-time vaccination of premature children, which may be further facilitated by improvement in vaccination intent of both vaccination service providers and demanding side.
For non-EPI vaccines, vaccination coverage in general children remains usually low, due to self-paid vaccination, less advocacy, and lower awareness of children’s parents, compared to EPI vaccines.Citation16 In our study, DTaP-IPV/Hib ranked the highest in both the coverage before the assessment and catch-up vaccination after the assessment, in children who had exceeded the vaccination date on the schedule when visiting VCC. It may be interpreted that the first dose of DTaP-IPV/Hib is early recommended at 2 months of age; additionally, DTaP-IPV/Hib is popular probably because it can prevent five infectious diseases, reduce the frequency of visiting vaccination clinics, and contain DTaP components (EPI vaccine), as described elsewhere.Citation2 Moreover, the coverage rate of VarV (54.2%) and EV71 (32.3%) were the highest in this study, possibly because varicella and hand-foot-and-mouth disease (HFMD) were non-EPI vaccine-preventable infectious diseases with the highest incidence in Shanghai, which have enhanced publicity by vaccination clinics.Citation17 VarV has been included in local EPI in Shanghai in 2018, which also led to an increase in the coverage.Citation18 It warrants further health promotion on more non-EPI vaccines for premature children.
Previous studies have confirmed that immune and epidemiological effects by vaccines are similar between premature children and full-term children, such as inactivated or live attenuated PV (UK, 2005), inactivated flu vaccine (USA, 2011), PCV (Germany, 2010), and HepB (Israel, 1998; China, 2012).Citation19–22 In our study, we identified one each case of varicella and pertussis in children unvaccinated with VarV or DTaP, so the vaccination protection rate was 100%. No tuberculosis, poliomyelitis, Japanese encephalitis, diphtheria, rubella, mumps or measles were reported in our study. The annual incidence (/100,000) was 25.69 (tuberculosis), 0.21 (rubella), 10.91(mumps) and 0.18 (measles), while no poliomyelitis, Japanese encephalitis, or diphtheria notified in Shanghai in 2017–2019.Citation23,Citation24 Some vaccine-preventable diseases are not notifiable in China such as meningitis, pneumonia, and rotavirus-diarrhea, so we could not determine the effects of these vaccines. However, majority of vaccination may be considered effective in protecting premature children against infectious diseases, indicating routine immunization is necessary.
In addition, vaccination in premature children has been proven safe in this study. The incidence of AEFI in premature children was approximately 64% of that in Shanghai Municipality in 2019, and similar or lower compared to those documented in multiple areas of China.Citation25,Citation26 Clinical manifestation and severity of AEFI in premature children in our study were common, mild and moderate, similar to those in general children.Citation27 Furthermore, it was not significantly associated with gender, birth weight, gestational age or immunodeficiency. On the other hand, incidence of AEFI is related to local reporting mechanism and health care service.Citation28 In Shanghai, a large metropolitan area in China, on-time report, diagnosis, and treatment of AEFI could be assured, which would facilitate promoting more routine immunization in premature children.
There are several limitations in this study. First, premature children who were born at the Children’s Hospital of Fudan University but had no vaccination records in Shanghai were excluded in this study. It might induce a selection bias, as the excluded children were possibly received vaccination elsewhere. Furthermore, in 2017–2019, there was only this VCC available in Shanghai, which might limit the representativeness of the findings in this study. Second, we studied only the first dose of vaccines, while did not evaluate subsequent doses, which may be normal or continue to be delayed. Third, we determined the vaccination experiences of premature children based on VCC assessment and vaccination records, whereas did not follow-up the health status of premature children that may have a direct impact on the parents’ and HCPs’ attitude toward the vaccination. In addition, we did not include premature children who did not receive VCC assessment, which may be a control to our findings. They might receive normal or delayed vaccination; however, it is difficult to include them as they might not actively report the premature status when vaccinated. It warrants further study for clarification.
Conclusion
Vaccination uptake in premature children was high, which has been confirmed safe and effective in this study. Majority of vaccination after VCC assessment in premature children were delayed, regardless of vaccine types and recommendations. Therefore, implementation of VCC recommendations may be strengthened to avert unnecessary delayed vaccination. In addition, EPI vaccines were more widely accepted compared to non-EPI vaccines, suggesting a need of health promotion on more non-EPI vaccines for premature children.
Author contribution
YL conceived of this study. JJ and JL collected data. CZ, JJ, and YL conducted the analysis and drafted initial manuscript. YL and PZ critically revised the manuscript and interpreted data. All authors reviewed the final manuscript.
Acknowledgments
We thank a lot to the health care practitioners and staffs in Vaccination Consultation Clinic of Children’s Hospital of Fudan University and Minhang District Center for Disease Control and Prevention, for their contribution to our study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Born too soon: the global action report on preterm birth. 2012 Nov 30 [accessed 2021 July 29]. https://www.who.int/publications/i/item/9789241503433 .
- Zhang X. Vaccination strategies of premature infants and low birth weight infants. Chin J Appl Clin Pediatr. 2017;32(14):1045–51. doi:10.3760/cma.j.2095-428X.2017.14.002.
- Walker JC, Smolders M, Gemen E, Antonius T, Leuvenink J, Vries ED. Development of lymphocyte subpopulations in preterm infants. Scand J Immunol. 2011;73(1):53–58. doi:10.1111/j.1365-3083.2010.02473.x.
- Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110(8):2948–54. doi:10.1182/blood-2007-01-069245.
- Gaudelus J, Pinquier D, Romain O, Thiebault G, Le Sage FV, Dommergues MA, Hau I, Bakhache P, Virey B, Dufour V, et al. Is the new vaccination schedule recommended in France adapted to premature babies? Archives De Pediatrie. 2014;21(9):1062–70. doi:10.1016/j.arcped.2014.06.020.
- Langkamp DL, Davis JP. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. Pediatr. 1996;128(5):654–59. doi:10.1016/s0022-3476(96)80131-4.
- Tozzi AE, Piga S, Corchia C, Di Lallo D, Carnielli V, Chiandotto V, Fertz MC, Miniaci S, Rusconi F, Cuttini M. Timeliness of routine immunization in a population-based Italian cohort of very preterm infants: results of the ACTION follow-up project. Vaccine. 2014;32(7):793–99. doi:10.1016/j.vaccine.2013.12.044.
- Hangzhou Center for Disease Control and Prevention, Suzhou Center for Disease Control and Prevention, Shanghai Center for Disease Control and Prevention. Expert consensus on immunization in children with special state: vaccination of preterm infants. Chin J Prac Pediatr. 2018;33:737–38. doi:10.19538/j.ek2018100601.
- Wang F, Xiao L, Huang R, Tang Y, Peng T, Peng X, Guan W, Li C, Zhang S, Li Y, et al. Vaccination suggestions for children with underlying diseases based on multidisciplinary expert consultation. Chin J Vaccines Immunization. 2020;26(1):116–27.
- Pediatrics Branch of Guangdong Medical Doctor A. Expert consensus on immunization in children with special state(Guangdong). Chin J Appl Clin Pediatr. 2020;35(6):401–10. doi:10.3760/cma.j.cn101070-20200309-00351.
- Hu Y, Li Q. Safety of vaccination for premature or low birth weight infants. Chin J Vaccines Immunization. 2016;22:339–44.
- Guo X. The first Chinese vaccination consultation clinic was established in Shanghai. China Hospital CEO. 2017;4:26. doi:10.3969/j.1674-3989.2017.04.004.
- Fortmann I, Dammann M-T, Humberg A, Siller B, Stichtenoth G, Engels G, Marissen J, Faust K, Hanke K, Goedicke-Fritz S, et al. Five year follow up of extremely low gestational age infants after timely or delayed administration of routine vaccinations. Vaccines. 2021;9(5):493. doi:10.3390/vaccines9050493.
- Sisson H, Gardiner E, Watson R. Vaccination timeliness in preterm infants: an integrative review of the literature. J Clin Nurs. 2017;26(23–24):4094–104. doi:10.1111/jocn.13916.
- Tillmann BU, Tillmann HC, Nars PW, Weber P. Vaccination rate and age of premature infants weighing < 1500 g: a pilot study in north-western Switzerland. Acta Paediatr. 2001;90(12):1421–26. doi:10.1080/08035250152708842.
- Hu Y, Luo S, Tang X, Lou L, Chen Y, Guo J. Comparative assessment of immunization coverage of migrant children between national immunization program vaccines and non-national immunization program vaccines in East China. Hum Vaccines Immunother. 2015;11(3):761–68. doi:10.1080/21645515.2015.1012015.
- Tao F, Feng W, Wang Y, Han R, Gu B, Wu H. Epidemiological characteristics of infectious disease automatic early warning signals in Shanghai, 2008–2016. Pract Prev Med. 2019;26(10):1186–90. doi:10.3969/j.1006-3110.2019.10.009.
- Hu J, Sun X. Retrospect and prospect of immunization programme in Shanghai. Chin Health Resources. 2019;22(4):262–5,8. doi:10.3969/j.1007-953X.2019.04.004.
- D’Angio CT, Heyne RJ, Duara S, Holmes LC, O’Shea TM, Wang H, Wang D, Sanchez PJ, Welliver RC, Ryan RM, et al. Immunogenicity of trivalent influenza vaccine in extremely low-birth-weight, premature versus term infants. Pediatr Infect Dis J. 2011;30(7):570–74. doi:10.1097/INF.0b013e31820c1fdf.
- Rueckinger S, Van Der Linden M, von Kries R. Effect of heptavalent pneumococcal conjugate vaccination on invasive pneumococcal disease in preterm born infants. BMC Infect Dis. 2010:10. doi:10.1186/1471-2334-10-12.
- Blondheim O, Bader D, Abend M, Peniakov M, Reich D, Potesman I, Handsher R, Gidoni I, Linder N. Immunogenicity of hepatitis B vaccine in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;79(3):F206–F8. doi:10.1136/fn.79.3.F206.
- Zhang L, Zhai X, Li Y, Zhang W, Zhu F, Huang T, Yan B, Liu J, Li L, Gong X, et al. Multi-center matching study on antibody response between preterm and full-term infants after primary immunization of hepatitis B vaccine. Chin J Epidemiol. 2012;33(2):185–88. doi:10.3760/cma.j.0254-6450.2012.02.013.
- Shanghai Municipal Health Commission. Epidemic situation on notifiable infectious diseases in Shanghai, 2018. 2019 Apr 23 [accessed 2021 Sept 13]. https://wsjkw.sh.gov.cn/yqxx/20190507/0012-63903.html .
- Shanghai Municipal Health Commission. Epidemic situation on notifiable infectious diseases in Shanghai, 2019. 2020 July 3 [accessed 2021 Sept 13]. https://wsjkw.sh.gov.cn/yqxx/20200703/ce47739b555e4b70bea9f704678524ee.html .
- Cao J, Liang F, Qin Y. Analysis on causes and strategy of adverse events following immunization in children. Mod Prev Med. 2012;39:601–3+10.
- Chen J, Yang J. Surveillance and analysis of suspected AEFI in 2013 in Dingxi city. Prog Microbiol Immunol. 2014;5:43–47. doi:10.13309/j.cnki.pmi.2014.05.009.
- Hu R, Peng S, Liu Y, Tang F, Wang Z, Zhang L, Gao J, Guo H. The characteristics and trend of adverse events following immunization reported by information system in Jiangsu province, China, 2015–2018. BMC Public Health. 2021;21(1). doi:10.1186/s12889-021-11387-3.
- Guo B, Page A, Wang H, Taylor R, McIntyre P. Systematic review of reporting rates of adverse events following immunization: an international comparison of post-marketing surveillance programs with reference to China. Vaccine. 2013;31(4):603–17. doi:10.1016/j.vaccine.2012.11.051.

