ABSTRACT
Before the availability of COVID-19 vaccines, surveys showed that vaccine hesitancy may influence the acceptance of COVID-19 vaccination. In this cross-sectional study, we aimed to investigate COVID-19 vaccination coverage among healthcare workers (HCWs) in obstetrics and gynecology, during the first three-month period of the vaccination campaign after COVID-19 vaccines were approved. A total of 662 eligible HCWs, consisting of 250 HCWs (group one) who participated in a Jiangsu provincial symposium and 412 HCWs (group two) in the department of obstetrics and gynecology, Nanjing Drum Tower Hospital, were invited to answer a 23-question questionnaire. In total, 618 (93.4%) HCWs completed the questionnaire. The vaccine acceptance in group one was higher than that in group two (87.2% [197/226] vs 74.2% [291/392], χ2 = 14.436, P < .001). Overall, 488 (79.0%) HCWs received COVID-19 vaccination and 130 (21.0%) declined vaccination. One-third of the 488 vaccinees were not vaccinated until consulted with others or requested by employers. Adjusted logistic regression analysis showed that the decline of vaccination was associated with worry about the safety of the vaccine (OR 1.920, CI 95% 1.196–3.082; P = .007). The main reason for the decline of COVID-19 vaccination included the concern about vaccine safety, pregnancy preparation, pregnancy, or lactation. These results indicate that more safety data about COVID-19 vaccines, particularly in pregnant or lactating women, are required to promote the acceptance of COVID-19 vaccination. In addition, vaccination requests or mandates by employers may increase the acceptance of COVID-19 vaccines.
Introduction
Coronavirus disease 2019 (COVID-19), first occurrence in December 2019, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a serious public health problem, because it has been spread over the world with high mortality,Citation1 and it is still transmitting over the world.Citation2 As of September 19, 2021, a total of 227.94 million COVID-19 patients were confirmed worldwide with 4.68 million deaths.Citation2 Universal vaccination in all susceptible persons with effective COVID-19 vaccines is the most important way to control the spreading of this highly contagious disease. Several types of COVID-19 vaccine, composed of mRNA coding for spike protein of SARS-CoV-2, recombinant non-replicating adenovirus containing S gene of SARS-CoV-2, or inactivated SARS-CoV-2, have been demonstrated to be effective in preventing SARS-CoV-2 infection.Citation3–7
Traditionally, universal vaccination is usually implemented in infants and children, but not in adolescents and adults, because infants and children are susceptible populations, and the vast majority of adolescents and adults are immune due to the vaccination in infancy or childhood or natural infection during the earlier life. However, all populations are susceptible to SARS-CoV-2 because it is a novel virus. Thus, universal vaccination in all populations is critical to control the spreading of SARS-CoV-2. However, vaccine skepticism or hesitancy, a phenomenon of delay or declining to take a vaccine(s) when vaccination services are available and accessible, may hinder the control of the pandemic of infectious diseases.Citation8–10 Recent reports showed that the COVID-19 vaccination willingness among healthcare workers (HCWs) was substantially different in different countries or regions, ranging from 23.4% in TaiwanCitation11 and 34.9% in the Republic of Cyprus,Citation12 around 50–64% in Saudi Arabia,Citation13,Citation14 79.1% in China,Citation15 to as high as 90.1% in South Africa.Citation16 Even in the same country or same region, different surveys also revealed remarkably varied COVID-19 vaccination intention rates, from 46.9% to 63.7% in the USA,Citation17,Citation18 from 51.1% to 64.4% in Greece,Citation12,Citation19 and from 40.0% to 63% in Hong Kong.Citation20,Citation21 Thus, surveys on COVID-19 vaccination intention in HCWs can just provide the theoretical estimate. And the coverage of COVID-19 vaccination in HCWs is required because HCWs are the priority population for the vaccination due to the high risk of infection, and vaccination of HCWs can serve as a model for the general population.
Since December 2020, several types of COVID-19 vaccines have been approved to be used in humans. As of December 30, 2020, China approved the first inactivated COVID-19 vaccine for emergency use in adult populations (18–60 years age) at risk for infection. HCWs have been prioritized by China Health Authority for vaccination against COVID-19 in the initial phase. It was planned to complete the COVID-19 vaccination in all HCWs in China from January to the end of March 2021. However, the actual acceptance of COVID-19 vaccination appeared to be not as high as expected and every effort was made to promote the acceptance of COVID-19 vaccination. HCWs in obstetrics and gynecology are at high risk for occupational SARS-CoV-2 exposure and transmission, because pregnant women with regular uterine contractions should be emergently hospitalized and there is no time to exclude the infection of SARS-CoV-2 by PCR.Citation22,Citation23 Therefore, COVID-19 vaccination in HCWs in obstetrics and gynecology should be more important. The present study aimed to survey the acceptance of COVID-19 vaccination in HCWs in obstetrics and gynecology in Jiangsu province, the eastern part of China.
Subjects and methods
Study design
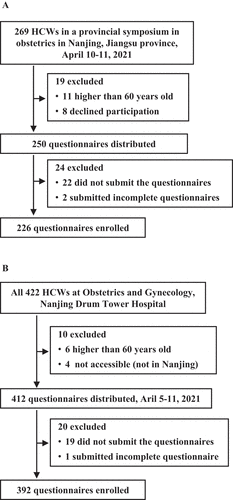
This was a cross-sectional survey about the coverage of COVID-19 vaccination among HCWs in obstetrics and gynecology in Jiangsu province, the eastern part of China. The study subjects included two groups of convenience samples (). Group one consisted of the participants in a Jiangsu provincial symposium in obstetrics and gynecology held in Nanjing city, the capital of Jiangsu province, April 10–11, 2021 (), and they were from the whole Jiangsu province. Group two included HCWs in the department of obstetrics and gynecology, Nanjing Drum Tower Hospital (). The survey was conducted by filling a Questionnaire form. For the survey among the participants in the symposium, the questionnaire form was distributed to each of the participants when they entered the conference hall, except those who were from Nanjing Drum Tower Hospital since they were allocated in group two, and was collected after the completion of the first session. For the survey among HCWs at Nanjing Drum Tower Hospital, the questionnaire forms were distributed to each of the subjects at their convenience in the department and collected four hours later. The survey at Nanjing Drum Tower Hospital was completed within seven days, April 5–11, 2021, to avoid missing due to the day-night-shift duty.
The inclusion criteria for HCWs were (1) at the age of 18–60 years because HCWs older than 60 years were not included in the initial phase of the vaccination campaign; and (2) willing to participate in the study. The subjects were informed at the beginning of the questionnaire form. This study was approved by the Ethics Committee of the Nanjing Drum Tower Hospital (2021-138-01).
Survey contents
We prepared 23 questions, including 11 questions in 3 sections (the demographic information, hospital information, and knowledge of and attitudes to COVID-19 vaccine) for all participants, one question of whether vaccinated with COVID-19 vaccine, and 10 questions for those who were vaccinated, and one question for those who were not vaccinated. The questionnaire was preliminarily prepared by reference to published articles on vaccination intentionCitation10–20 and internal discussion in the study team, and the final questionnaire form was determined by two rounds of modification based on preliminary answers by 12 physicians and 12 nurses from the department of obstetrics and gynecology, Nanjing Drum Tower Hospital, respectively. The detailed questions in Chinese and English are presented in Questionnaire-Chinese and English in Supplement file 1.
Statistical analyses
Continuous variables were described as median and range (P25–P75). Categorical variables were expressed as numbers and percentages. Rank-sum test was used to analyze the median age of different groups and to test the difference between two groups on an ordinal categorical variable. The rates or proportions of different groups were compared by the chi-squared test or the chi-squared test with Yates’ correction for continuity. Variables that were significant at P < .1 in univariate analyses were then included in a multivariable logistic regression. P < .05 was considered statistically significant. All analyses were performed using statistical analysis system software, Version 9.4 (SAS Institute Inc. Cary, USA).
Results
Participant characteristics
A total of 618 HCWs in obstetrics and gynecology submitted complete questionnaires. They were composed of two groups (). In group one, 250 eligible HCWs consented to participate in the survey; however, 22 (8.8%) did not submit the questionnaires and 2 (0.8%) submitted incomplete questionnaires. Thus, 226 (90.4%) questionnaires were included in the analysis (). In group two, of all 422 HCWs in the Department of Obstetrics and Gynecology, Nanjing Drum Tower Hospital, 412 were eligible for the study (). Of them, 19 did not submit the questionnaires and one submitted an incomplete questionnaire. Hence, 392 (95.1%) questionnaires were included in the final analysis ().
Overall, 488 (79.0%) HCWs received COVID-19 vaccination and 130 (21.0%) did not. All 488 vaccinees received inactivated SARS-CoV-2 vaccine. Of them, 403 (82.3%) completed the second vaccine dose, and the remaining 85 (17.7%) had not yet received the second dose because of the shorter interval (less than two weeks), temporary lack of vaccine, catching a cold, acute toothache, or other reasons. The vaccine acceptance in group one was higher than that in group two (87.2% [197/226] vs 74.2% [291/392], χ2 = 14.436, P < .001) (). The detailed information about the demographic characteristics, educational levels, and professions in HCWs who took the COVID-19 vaccination and HCWs who did not take the vaccination is presented in . Whether or not getting vaccinated was not associated with sex, educational levels, or roles in hospitals, but was associated with ages, professional titles, and affiliations of universities, and was borderline associated with the hospital levels ().
Table 1. Comparison of demographic characteristics between participants vaccinated or unvaccinated with the COVID-19 vaccine
Knowledge about and attitude to COVID-19 vaccine
The first emergently approved COVID-19 vaccine in China is composed of inactivated SARS-CoV-2. Shortly before and during the vaccination campaign, Chinese experts in infectious diseases, respiratory diseases, public health, vaccinology, and other healthcare disciplines explained the safety and efficacy of the COVID-19 vaccine using various news media to the public. presents the knowledge of and the attitudes to COVID-19 vaccine in HCWs participated in this survey. Overall, the knowledge source about the COVID-19 vaccine was similar between HCWs who received the vaccination and those who declined the vaccination, except more unvaccinated HCWs obtained relevant knowledge from colleagues. However, more HCWs who declined the COVID-19 vaccination were worried about the vaccine safety and skeptical about the efficacy of COVID-19 vaccine ().
Table 2. Healthcare workers’ knowledge of and attitudes to COVID-19 vaccine
Factors associated with the decline of COVID-19 vaccination
We performed unadjusted and adjusted logistic regression analyses on the factors associated with the decline of the COVID-19 vaccination in the study subjects. shows that, after adjusted for age, profession, education, interaction between education and the subject source, professional title, hospital level and university hospital, HCWs at Nanjing Drum Tower Hospital were more likely to decline the vaccination, and the decline of vaccination was associated with worry about the safety of the vaccine, but not associated with worry about the efficacy of the vaccine.
Table 3. Factors associated with the decline of COVID-19 vaccination: logistic regression
Detailed reasons for receipt and decline of COVID-19 vaccination
Detailed reasons for the acceptance of COVID-19 vaccination in 488 HCWs are presented in . Around two-thirds of them accepted the vaccination based on their own decisions, and one-third of others had hesitancy before they were vaccinated. presents the detailed reasons for the 130 HCWs who did not receive the vaccination, which included worry about the safety of the vaccine although in good health conditions (13.1%) or due to the presence of chronic diseases (10.7%), decline by vaccination staff (14.6%), pregnancy preparation (8.4%), pregnancy (10.8%), lactation (10.8%), and HPV vaccination (7.7%), all of which accounted for 76.1% of unvaccinated subjects. In addition, 11.5% (15/130) of the unvaccinated HCWs used other excuses to decline the vaccination.
Table 4. Reasons for getting vaccinated with COVID-19 vaccine in 488 healthcare workers
Table 5. Reasons for decline of COVID-19 vaccination in 130 unvaccinated healthcare workers
Self-reported adverse events in 488 vaccinated HCWs
Of the 488 vaccinated HCWs, 268 (54.9%) had no adverse event at all and 220 (45.1%) others had one or more adverse events during the first two weeks after the first dose vaccine. The majority of the adverse events were local pain and/or redness on the injection site, and no one required hospitalization due to the adverse events. Of the 403 HCWs who received the second vaccine dose, 241 (59.8%) did not have any adverse event and 162 (40.2%) others had one or more adverse events. The adverse events were mostly mild, and none of the HCWs was hospitalized due to the adverse events.
Discussion
Recent reports showed that the COVID-19 vaccination willingness among HCWs was substantially varied, ranging from 23.4% to 90.1% in different countries or regions.Citation8–16 Even in the same country or same region, different surveys also revealed remarkably varied COVID-19 vaccination intention rates.Citation17–21 Thus, surveys on COVID-19 vaccination intention in HCWs can just provide the theoretical estimate. The actual acceptance of COVID-19 vaccination in HCWs is required, because HCWs are the priority population for the vaccination due to the high risk of infection and vaccination of HCWs can serve as a model for the general population. In the present investigation, we revealed that 79.0% (488/618) of HCWs in obstetrics and gynecology already received COVID-19 vaccination in the first three-month period (January to March 2021) of the vaccination campaign against COVID-19 in China. The actual COVID-19 vaccination coverage appears to be moderately high. The results indicate that request or mandate by employers may increase the coverage of COVID-19 vaccination, and the main reasons for the decline of COVID-19 vaccination appear to be the concern about the safety of the vaccine, pregnancy preparation, pregnancy, and lactation.
Surveys among HCWs in mainland China showed that the rate of COVID-19 vaccination intention ranged from 27.7% to 77.3%.Citation24,Citation25 In the present survey, the coverage of COVID-19 vaccination was 79.0% in HCWs in obstetrics and gynecology, higher than the reported vaccination intention rates. This may be associated with vaccination requests or mandates by the employers and vaccination educations, because one-third of the vaccinees were reluctant before getting vaccinated (). Notably, 17.4% of the vaccinated HCWs did not receive the vaccination until the requests or mandates by employers (). Therefore, the vaccination requests or mandates by the employers can increase the acceptance of COVID-19 vaccination in HCWs. It should be emphasized that vaccination request or mandate by employers is not a threat, and this is ethical because the acceptance of COVID-19 vaccination is beneficial to both the vaccinees and others. Some other vaccines, including measles vaccination, are required for children to attend a school or were historically mandated by the governments.Citation26–28 Actually, various means have been applied to promote the COVID-19 vaccination, such as providing lottery tickets, gift cards, college tuition compensations, and more for persons who received COVID-19 vaccination in other parts of the world.Citation29
In this study, the coverage of COVID-19 vaccination in HCWs of group one who were from the whole Jiangsu province was 87.2% (197/226), very similar to the real-world acceptance of 86.2% (906/1051) in HCWs in perinatal medicine in mainland China during the first three-month period of the vaccination campaign.Citation30 However, the actual acceptance (74.2%) of COVID-19 vaccination in HCWs in Nanjing Drum Tower Hospital (group two), one of the hospitals at the top level in China, was relatively lower. This acceptance rate is similar to a coverage of 75.4% among 11951 HCWs in intensive care units from 252 prefecture-level regions of mainland China surveyed between March 24 and April 10, 2021.Citation31 Our finding is also in agreement with the observation that HCWs in perinatal medicine from hospitals at high levels are less likely to accept COVID-19 vaccination.Citation30 In the USA, the actual COVID-19 vaccination coverage in the first month was just 37.5% in the staff members in the long-term care facilities, much lower than 77.8% in the residences in the same facilities.Citation32 In Spain, 85.9% (608/708) nephrologists received the COVID-19 vaccination.Citation33 These data, together with the results in our investigation, indicate that actual acceptance of COVID-19 vaccination in HCWs is not high as expected. Because the real-world acceptance of COVID-19 vaccination in HCWs has been rarely reported, it is unknown whether such a phenomenon is unique or common, which merits further observation.
The main reasons for the decline of COVID-19 vaccination in the 130 unvaccinated HCWs included the concern about the vaccine safety (), decline by vaccination staff, pregnancy preparation, pregnancy or lactation, and vaccination against human papillomavirus (HPV) and other excuses (). The COVID-19 vaccine used in China in the initial phase is composed of inactivated SARS-CoV-2,Citation34,Citation35 which has been demonstrated to be highly safe.Citation6,Citation34,Citation35 Studies showed that controlled chronic diseases, such as diabetes mellitus, hypertension, chronic kidney disease, and others, are not the contradiction of vaccination.Citation36,Citation37 Even in organ transplant recipients, COVID-19 vaccination showed to be generally safe.Citation38–41 In theory, vaccination with vaccines that contain no viable or non-replicable components has no additional adverse effects in women who are preparing for pregnancy, or in pregnant or lactating women,Citation42 which has been demonstrated in recent studies.Citation43,Citation44 However, because the relevant study in these special populations has been rarely reported, it is understandable that around two-thirds of pregnant women did not want to be vaccinated with COVID-19 vaccines.Citation45 Therefore, more safety data about the COVID-19 vaccination in women during pregnancy or lactation are urgently required to increase the vaccination coverage. In addition, whether COVID-19 vaccination may influence the efficacy of the HPV vaccine, or vice versa, requires investigation.
There are several limitations in the present study. First, the participants were mostly from hospitals at high levels in cities, because HCWs in communities or towns are less likely to attend academic symposia. Thus, the coverage of COVID-19 vaccination in the present survey might not be generalized to HCWs in obstetrics and gynecology in other hospitals at low levels. Second, this survey was based on the convenience sampling of the attendees at a provincial symposium and HCWs from a large tertiary hospital. The results might not be extended in all HCWs in obstetrics and gynecology in Jiangsu province. However, the 87.2% (197/226) acceptance rate in HCWs from whole Jiangsu province and the 74.2% (291/392) from Nanjing Drum Tower Hospital observed in the present study are comparable to the 86.2% (906/1051) acceptance rate in the whole mainland China surveyed from April 9 to April 21, 2021Citation30 and the 75.4% of 11951 HCWs in intensive care units from 252 prefecture-level regions of mainland China surveyed between March 24 and April 10, 2021,Citation31 respectively. Thus, we considered that the results in the present study may to some extent represent the situations in Jiangsu province and in hospitals at the top level in China, respectively. Third, this survey only evaluated the coverage during the first three months of the vaccination campaign. The vaccination coverage in HCWs might have increased, as China has started the universal vaccination campaign against COVID-19 in all adult populations, including subpopulations at-risk for infection, since April 1, 2021. Some unvaccinated HCWs during the first period of the vaccination campaign may take COVID-19 vaccination later.
In conclusion, the present survey shows that the coverage of COVID-19 vaccination was moderately high among HCWs in obstetrics and gynecology during the initial phase of the vaccination campaign. Vaccination requests or mandates by employers may increase the vaccination acceptance. Concern about the safety of COVID-19 vaccine seems to be the main reason for the decline of COVID-19 vaccination. The results indicate that more safety data about COVID-19 vaccine are urgently required to increase the COVID-19 vaccination coverage.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1997297.
Additional information
Funding
References
- Peng Y, Xu B, Sun B, Han G, Zhou YH. Importance of timely management of patients in reducing fatality rate of coronavirus disease 2019. J Infect Public Health. 2020;13(6):890–92. doi:10.1016/j.jiph.2020.04.015 .
- World Health Organization. COVID-19 weekly epidemiological update edition 58. Published 2021 Sep 21 [accessed 2021 Sep 22]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—21-september-2021 .
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320–29. doi:10.1056/NEJMoa2107058.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi:10.1001/jama.2021.8565.
- Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove C, Galloway J, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021;385:1172–83. doi:10.1056/NEJMoa2107659.
- Jaca A, Iwu-Jaja CJ, Balakrishna Y, Pienaar E, Wiysonge CS. A global bibliometric analysis of research productivity on vaccine hesitancy from 1974 to 2019. Hum Vaccines Immunother. 2021;17(9):3016–22. doi:10.1080/21645515.2021.1903294.
- Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39(15):2024–34. doi:10.1016/j.vaccine.2021.02.005.
- Smith-Norowitz TA, Silverberg JI, Norowitz EM, Kohlhoff S, Hammerschlag MR. Factors impacting vaccine hesitancy toward coronavirus disease-19 (COVID-19) vaccination in Brooklyn, New York. Hum Vaccines Immunother. 2021:1–2. doi:10.1080/21645515.2021.1948786.
- Kukreti S, Lu MY, Lin YH, Strong C, Lin CY, Ko NY, Chen PL, Ko WC. Willingness of Taiwan’s healthcare workers and outpatients to vaccinate against COVID-19 during a period without community outbreaks. Vaccines (Basel). 2021;9(3):246. doi:10.3390/vaccines9030246.
- Raftopoulos V, Iordanou S, Katsapi A, Dedoukou X, Maltezou HC. A comparative online survey on the intention to get COVID-19 vaccine between Greek and Cypriot healthcare personnel: is the country a predictor? Hum Vaccines Immunother. 2021;17:2397–404. doi:10.1080/21645515.2021.1896907.
- Qattan AMN, Alshareef N, Alsharqi O, Al Rahahleh N, Chirwa GC, Al-Hanawi MK. Acceptability of a COVID-19 vaccine among healthcare workers in the Kingdom of Saudi Arabia. Front Med (Lausanne). 2021:8. doi:10.3389/fmed.2021.644300.
- Alshahrani SM, Dehom S, Almutairi D, Alnasser BS, Alsaif B, Alabdrabalnabi AA, Bin Rahmah A, Alshahrani MS, El-Metwally A, Al-Khateeb BF, et al. Acceptability of COVID-19 vaccination in Saudi Arabia: a cross-sectional study using a web-based survey. Hum Vaccines Immunother. 2021;17(10):3338–47. doi:10.1080/21645515.2021.1936869.
- Wang J, Feng Y, Hou Z, Lu Y, Chen H, Ouyang L, Wang N, Fu H, Wang S, Kan X, et al. Willingness to receive SARS-CoV-2 vaccine among healthcare workers in public institutions of Zhejiang Province, China. Hum Vaccines Immunother. 2021;17(9):2926–33. doi:10.1080/21645515.2021.1909328.
- Adeniyi OV, Stead D, Singata-Madliki M, Batting J, Wright M, Jelliman E, Abrahams S, Parrish A. Acceptance of COVID-19 vaccine among the healthcare workers in the Eastern Cape, South Africa: a cross sectional study. Vaccines (Basel). 2021;9(6):666. doi:10.3390/vaccines9060666.
- Gadoth A, Halbrook M, Martin-Blais R, Gray A, Tobin NH, Ferbas KG, Aldrovandi GM, Rimoin AW. Cross-sectional assessment of COVID-19 vaccine acceptance among health care workers in Los Angeles. Ann Intern Med. 2021;174(6):882–85. doi:10.7326/M20-7580.
- Kuter BJ, Browne S, Momplaisir FM, Feemster KA, Shen AK, Green-mckenzie J, Faig W, Offit PA. Perspectives on the receipt of a COVID-19 vaccine: a survey of employees in two large hospitals in Philadelphia. Vaccine. 2021;39(12):1693–700. doi:10.1016/j.vaccine.2021.02.029.
- Maltezou HC, Pavli A, Dedoukou X, Georgakopoulou T, Raftopoulos V, Drositis I, Bolikas E, Ledda C, Adamis G, Spyrou A, et al. Determinants of intention to get vaccinated against COVID-19 among healthcare personnel in hospitals in Greece. Infect Dis Health. 2021;26(3):189–97. doi:10.1016/j.idh.2021.03.002.
- Wang K, Wong ELY, Ho KF, Cheung AWL, Chan EYY, Yeoh EK, Wong SYS. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the coronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. 2020;38(45):7049–56. doi:10.1016/j.vaccine.2020.09.021.
- Kwok KO, Li KK, Wei WI, Tang A, Wong SYS, Lee SS. Editor’s choice: influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: a survey. Int J Nurs Stud. 2021;114:103854. doi:10.1016/j.ijnurstu.2020.103854.
- Chen D, Yang H, Cao Y, Cheng W, Duan T, Fan C, Fan S, Feng L, Gao Y, He F, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet. 2020;149(2):130–36. doi:10.1002/ijgo.13146.
- Kumar R, Yeni CM, Utami NA, Masand R, Asrani RK, Patel SK, Kumar A, Yatoo MI, Tiwari R, Natesan S, et al. SARS-CoV-2 infection during pregnancy and pregnancy-related conditions: concerns, challenges, management and mitigation strategies-a narrative review. J Infect Public Health. 2021;14(7):863–75. doi:10.1016/j.jiph.2021.04.005.
- Li M, Luo Y, Watson R, Zheng Y, Ren J, Tang J, Chen Y. Healthcare workers’ (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. 2021. doi:10.1136/postgradmedj-2021-140195.
- Yu Y, Lau JTF, She R, Chen X, Li L, Li L, Chen X. Prevalence and associated factors of intention of COVID-19 vaccination among healthcare workers in China: application of the Health Belief Model. Hum Vaccines Immunother. 2021;17(9):2894–902. doi:10.1080/21645515.2021.1909327.
- Opel DJ, Omer SB. Measles, mandates, and making vaccination the default option. JAMA Pediatr. 2015;169(4):303–04. doi:10.1001/jamapediatrics.2015.0291.
- Mignot A, Le Maréchal M, Guimier L, Epaulard O. Mandatory vaccination in France: perception by outpatients and self-evaluation of its impact on their vaccine confidence. Hum Vaccines Immunother. 2021:1–6. doi:10.1080/21645515.2021.1949952.
- Batniji R. Historical evidence to inform COVID-19 vaccine mandates. Lancet. 2021;397(10276):791. doi:10.1016/S0140-6736(21)00267-1.
- Persad G, Emanuel EJ. Ethical considerations of offering benefits to COVID-19 vaccine recipients. JAMA. 2021;326(3):221–22. doi:10.1001/jama.2021.11045.
- Xu B, Gao X, Zhang X, Hu Y, Yang H, Zhou YH. Real-world acceptance of COVID-19 vaccines among healthcare workers in perinatal medicine in China. Vaccines (Basel). 2021;9(7):704. doi:10.3390/vaccines9070704.
- Huang W, Shao X, Wagner AL, Chen Y, Guan B, Boulton ML, Li B, Hu L, Lu Y. COVID-19 vaccine coverage, concerns, and preferences among Chinese ICU clinicians: a nationwide online survey. Expert Rev Vaccines. 2021:1–7. doi:10.1080/14760584.2021.1971523.
- Gharpure R, Guo A, Bishnoi CK, Patel U, Gifford D, Tippins A, Jaffe A, Shulman E, Stone N, Mungai E, et al. Early COVID-19 first-dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy partnership for long-term care program - United States, December 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5):178–82. doi:10.15585/mmwr.mm7005e2.
- Quiroga B, Sánchez-Álvarez E, Goicoechea M, de Sequera P, Spanish Society of Nephrology Council. COVID-19 vaccination among Spanish nephrologists: acceptance and side effects. J Healthcare Qual Res. 2021. doi:10.1016/j.jhqr.2021.05.002.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–60. doi:10.1001/jama.2020.15543.
- Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, Li M, Jin H, Cui G, Chen P, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–12. doi:10.1016/S1473-3099(20)30987-7.
- Leong DP, Banerjee A, Yusuf S. COVID-19 vaccination prioritization based on cardiovascular risk factors and number-needed-to-vaccinate to prevent death. Can J Cardiol. 2021;37(7):1112–16. doi:10.1016/j.cjca.2021.04.012.
- Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15(2):505–08. doi:10.1016/j.dsx.2021.02.026.
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–62. doi:10.1056/NEJMc2108861.
- Yi SG, Knight RJ, Graviss EA, Moore LW, Nguyen DT, Ghobrial RM, Gaber AO, Huang HJ. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021;105(7):e72–e73. doi:10.1097/TP.0000000000003764.
- Aslam S, Danziger-Isakov L, Mehra MR. COVID-19 vaccination immune paresis in heart and lung transplantation. J Heart Lung Transplant. 2021;40(8):763–66. doi:10.1016/j.healun.2021.04.018.
- Boyarsky BJ, Chiang TP, Ou MT, Werbel WA, Massie AB, Segev DL, Garonzik-Wang JM. Antibody response to the Janssen COVID-19 vaccine in solid organ transplant recipients. Transplantation. 2021;105(8):e82–e83. doi:10.1097/TP.0000000000003850.
- Advisory Committee on Immunization Practices (ACIP). Guidelines for vaccinating pregnant women. 2016 Aug [accessed 2021 May 21]. https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/guidelines.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fpregnancy%2Fhcp%2Fguidelines.html .
- Collier AY, McMahan K, Yu J, Tostanoski LH, Aguayo R, Ansel J, Chandrashekar A, Patel S, Apraku Bondzie E, Sellers D, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325(23):2370–80. doi:10.1001/jama.2021.7563.
- Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Sapir E, Regev Yochay G, et al. Short-term outcome of pregnant women vaccinated by BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58(3):450–56. doi:10.1002/uog.23729.
- Goncu Ayhan S, Oluklu D, Atalay A, Menekse Beser D, Tanacan A, Moraloglu Tekin O, Sahin D. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet. 2021;154(2):291–96. doi:10.1002/ijgo.13713.