ABSTRACT
Analysis of the batch issued data of Chinese vaccines from 2011 to 2020 showed that the average annual dose of vaccines in China was 769.66 million doses, and the overall production was stable. Fourteen new vaccines were added, six of which were developed in China. The batch issued dose of national immunization program (NIP) vaccine was stable with slightly decreased, while the non-NIP vaccine showed an increasing trend. The development trend of combined vaccine increased significantly. Regulatory science in China’s development, promoted the China’s vaccine regulation the perfection of legal system, and the steady improvement of vaccine quality and standards, and the gradual maturity of the construction of lot release system. With the goal of being safe, effective, reasonable, and accessible, China’s vaccines encourage the research and development of new technologies and emergency vaccines to constantly improve the level of public health.
Introduction
Regulatory science is a discipline that develops new tools, standards, and approaches for assessing the safety, efficacy, quality, and performance of regulated products.Citation1 In 2005, National Medical Products Administration (NMPA) first put forward the concept of “scientific supervision,” unifying the thought of Chinese drug supervision and providing guidance and platform guarantee for the development of regulatory science in China.Citation2 In 2013, China society for drug regulation was established, dedicated to theoretical research and exchange of drug regulatory policies. In 2019, NMPA launched the scientific action plan for China’s drug supervision in the field of drugs, which is committed to launching key research projects focusing on urgent regulatory issues, including frontier and cross-disciplinary research on biological products. The development of regulatory science in China has given a strong boost to the development of the Chinese vaccine industry. Vaccines refer to preventive biological products used for human immunization with a view to preventing and controlling the occurrence and spread of disease.Citation3 Affected by science and technology, economy, environment, in the early days of the founding of the People’s Republic of China, only a few vaccines, such as vaccinia and cholera vaccines were used to prevent severe infectious diseases. Since 1949, the Chinese government has attached great importance to the development of the vaccine industry. Especially after the reform and opening up, it has vigorously promoted NIP, effectively controlling hepatitis B, measles, pertussis, encephalitis, and other diseases. By the end of 2020, China has made great progress in the variety, scale, and technology of vaccines, with continuous improvement in quality and more varieties and ways of research and development. More than 50 kinds of vaccines were approved for sale and use each year. Currently, Chinese vaccine production ranks first in the world, and it’s also one of the few countries in the world that have solved the entire vaccine immunization program by relying on its own capacity.Citation4
Regulatory science promotes the improvement of China’s vaccine regulatory system
Regulations have been constantly improved
The continuous updating and timely revision of laws and regulations is a favorable guarantee for the vaccine regulatory system. After years of development, China’s vaccine regulatory system has become increasingly mature, laws and regulations in the field of vaccines have been gradually improved. Presently, the NMPA is responsible for regulating vaccines in China. According to the latest statistics, from 2011 to 2020, vaccine regulation involves the establishment or change of 22 major regulations and guiding principles (), of which 11 are related to regulatory changes, mainly involving the management of vaccine production and operation, clinical trial quality requirements, management in the field of vaccine circulation and transportation, and monitoring of vaccine adverse reactions, etc. There are 11 guidelines for vaccines, 5 of which are for COVID-19 (Corona Virus Disease 2019) vaccine research, which provides strong technical support for promoting the phase I, II, and III clinical approval and final product marketing of China’s COVID-19 vaccine, such as inactivated COVID-19 vaccine,Citation5–7 recombinant adenovirus COVID-19 vaccine,Citation8 recombinant subunit COVID-19 vaccine,Citation9 and mRNA COVID-19 vaccine.Citation10
Table 1. Legal and regulatory documents mainly related to vaccines (2011–2020)
In 2019, China first promulgated the Vaccine Administration Law of the People’s Republic of China to support vaccine research, standardized the whole process management of vaccine R&D, production and circulation, and emphasized the principal responsibility system of vaccine enterprises and the compulsory liability insurance system of newly added vaccine manufacturers.Citation11 This is the first law specifically established for vaccines in the world. According to the Vaccine Administration Law and the newly revised Pharmaceutical Administration Law of the People’s Republic of China (2019 Revision),Citation12 Provisions of Drug Registration,Citation13 the Measures for the Supervision over and Administration of Pharmaceutical Production,Citation14 Provisions for the Lot Release of Biological ProductsCitation15 have also been revised and officially implemented in July 2020. These three regulations focus on the vaccine quality derived from the design concept, clarify the regulatory responsibility and emphasize the practicability. For example, Provisions for the Lot Release of Biological ProductsCitation15 stipulates that on the premise of ensuring the safety and effectiveness of drug quality, the applicant can apply for simultaneous lot release of biological products for emergency needs of national disease prevention and control after being approved by the NMPA. This regulation has greatly improved the R&D speed of China’s COVID-19 vaccines, and ultimately enabled China to remain at the forefront of COVID-19 vaccine research and development.
Quality standards have been continuously improved
Regulatory scientific research has contributed to a steady improvement in China’s vaccine quality standards. The earliest standards related to vaccines were the terms on “Chinese Biological Products Regulations,” with the first regulation on biological products in China was the “Biological Product Regulation” (draft, 1952), followed by the “Biological Products Manufacturing and Verification Regulation” (1959 and 1979 version), the “Chinese Biological Product Regulation” (1990, 1993, 1995, and 2000 version), and the more perfect version was the 2000 version.Citation16 In 2005, it was incorporated into the Pharmacopoeia of the people’s Republic of China (Ch.P.), which was revised every five years, including 2010, 2015, and the latest 2020 version. The 2020 version of Ch.P.Citation17 further improved the general requirements such as the whole process control of vaccines, strengthened the technical requirements for the safety of virus contamination, and accelerated the collection of mature vaccines approved and marketed in China in recent years. A total of 54 vaccine varieties are included, and the quality control level is further consistent with the requirements of international standards. Ch.P. is the main technical basis for vaccine regulation, and China has gradually improved the national drug standard management system with the Ch.P. as the core.
Regulatory science has promoted the steady development of reference materials for vaccine quality control in China. The National Institute for Food and Drug Control (NIFDC) has gradually established a bacterial/virus seed bank in line with international standards, and established a research and supply platform, quality evaluation standards and technology platform for biological products, so as to smooth the process of R&D, storage, sales and use of reference materials.Citation18 There are nearly 40 kinds of reference materials related to vaccine quality control in the catalog of national reference material for biological products (3801) in the volume 3 of Ch.P. 2020 version, such as the national reference materials for evaluating the potency of H7N9 vaccine,Citation19 and the reference materials used for determining the control of vero cell host DNA residues in human vaccines,Citation20 etc. The above standard products were all prepared for the first time at home and abroad.
In 2013, NIFDC was approved by WHOCC (WHO Collaborating Center) and became a member of the Cooperation Center for the standardization and evaluation of biological products of the World Health Organization (WHO), marking a new breakthroughs in China’s “international voice” in the field of biological products standards,Citation4 such as China’s leading EV71 neutralizing antibody international standard, of which was approved by WHO in 2015 through the collaborative research of laboratories in many countries. This reference material plays a crucial role in the quality evaluation of EV71 vaccine, safety, and effectiveness of the vaccine.Citation21 This has also created a prerequisite for China’s vaccine to go global. China has passed the WHO certification for Japanese encephalitis vaccine, Hepatitis A vaccine, influenza vaccine, bivalent oral polio vaccine, which have been included in the United Nations vaccine procurement list presently. COVID-19 vaccine in China has passed the emergency use approval of the WHO and has been used in many countries including EU countries.
Vaccine lot release system is increasingly perfect
Vaccine lot release is listed by the WHO as one of the key functions of governments in regulating vaccines. In order to ensure the safety and effectiveness of vaccines on the market, China currently implements lot release management for the marketing of all preventive vaccines,Citation11 that is, the system of mandatory inspection and audit when each batch of products leave the factory for marketing or import. If the products fail to pass the inspection or are not approved in the audit, they shall not be marketed or imported.
The basis of lot release involves a number of drug laws and regulations. The legal system mainly includes Vaccine Administration Law, Drug Administration Law and corresponding implementation regulations; The regulations main includes the Provisions of Drug Registration, the Provisions for the Lot Release of Biological Products and the Measures for the Supervision over and Administration of Pharmaceutical Production, etc. The Provisions for the Lot Release of Biological Products is one of the important regulations for the implementation of the lot release system of vaccines in China, which have been updated three times in 2004, 2017, and 2020.Citation15 The 2020 version strengthens the management of the whole life cycle of biological products and standardizes, and standardizes and refines the operation requirements for lot release; clarifies the responsibility of the main body of lot release, the division of responsibilities among various departments of batch issuing, especially the responsibilities of drug testing institutions.Citation15 And clarifies the investigation procedures of major quality risk products, implementing the main responsibilities of marketing authorization holders; Strengthens the risk management of lot release, and the process of supervision and inspection, and carry out extended inspection if necessary; The country will severely crack down on violations of regulations and fully implement the strictest accountability.
As the last gateway in the national vaccine regulatory system, vaccine lot release involves multi-departments cooperation in the drug regulatory system. On the administrative level, there are main the NMPA, provincial drug administration, the market supervision and management departments of the administrative areas. In terms of technical supervision, the relevant national technical supervision institutions of vaccine quality mainly include the NIFDC, Center for Drug Evaluation (CDE, NMPA), Chinese Pharmacopoeia Commission, Center for Food and Drug Inspection of NMPA, National Immunization Program of Chinese Center for Disease Control and Prevention, Center for Drug reevaluation (National Center for ADR monitoring, China).
China has always attached great importance to the construction of lot release system and inspection and detection capacity of vaccines and other biological products. At present, China is gradually improving the national vaccine lot release system with the national level (NIFDC) as the core and the participation of provincial vaccine lot release institutions.Citation22 The regulatory responsibilities at all levels are clear. National regulators should have the ability to formulate standards for vaccines and prepare reference materials, and be able to carry out all-round testing of vaccine products in accordance with legal standards. Provincial regulators need to be capable of conducting full testing of vaccines produced by enterprises within its administrative region according to legal standards.
Regulation science promotes the steady development of China’s vaccine industry
Main varieties of vaccines issued in batches in China
According to the public data of lot release of NIFDC from 2011 to 2020, an average of more than 50 kinds of vaccines are issued in the declare batch every year (), of which basically covers the types of preventive vaccines listed in other countries and can prevent and control more than 30 infectious diseases. Large dose of vaccines mainly NIP vaccines, such as BCG vaccine, measles vaccine, live attenuated poliomyelitis vaccine, recombinant hepatitis B vaccine, adsorbed acellular DPT combined vaccine, group A meningococcal vaccine, and live attenuated Japanese encephalitis vaccine.
Table 2. Classification list of vaccine batches issued varieties in China
The issuance and marketing of vaccines in China
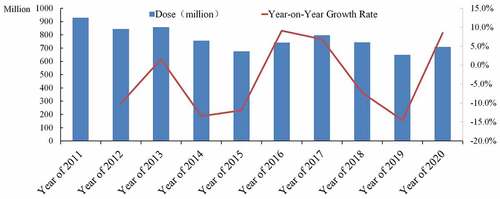
Since the trial lot release of live attenuated measles vaccine, adsorbed DPT vaccine, live attenuated poliomyelitis vaccine, hepatitis B vaccine, BCG vaccine for intradermal injection and human serum albumin in 2001,Citation4 China fully implemented the lot release system for vaccine products in 2006. NIFDC, the designated agency for the issuance of vaccine batches in China, regularly publishes the data on the issuance of vaccine batch on its official website. By querying and collecting the number of doses issued each year for all batches of vaccine varieties, it is found although the overall lot release total amount doses of vaccines in China decreased slightly since 2011, the overall annual lot release total amount was stable, with an average annual issuance amount of 770 million doses in a year-on-year growth rate from −13.5% to +9.1% ().
Analysis of national immunization program (NIP) and non-NIP vaccines
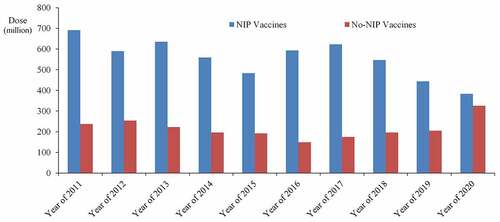
According to the Regulation on the Management of Vaccine Circulation and Use, vaccines can be divided into NIP vaccines and non-NIP vaccines. The NIP vaccines are provided free charge by the government and are distributed to citizens in accordance with regulations. Chinese NIP vaccines currently have 14 to prevent 15 infectious diseases, mainly including hepatitis B vaccine, DTP combined vaccine, BCG vaccine, polio vaccine, measles vaccine, diphtheria tetanus combined vaccine, acellular DTP combined vaccine, measles mumps, and rubella (MMR) Combined attenuated vaccine, attenuated Japanese encephalitis vaccine, hepatitis B vaccine, meningococcal vaccine (meningococcal polysaccharide vaccine). Local governments can also vaccinate other vaccines based on their geographical characteristics. In addition, there are three immunization program vaccines for adults or priority populations, including hemorrhagic fever with renal syndrome (HFRS) vaccine, anthrax vaccine, and leptospira vaccine.Citation23 The overall situation of NIP vaccine from 2011 to 2020 is shown in . As can be seen from the figure, vaccines in NIP showed a downward trend, which is partly related to the annual population growth in China.
Non-NIP vaccines are vaccines that are administered voluntarily by citizens at their own expense, mainly including Haemophilus influenzae type b (Hib) conjugate vaccine, meningococcal ACWY, meningococcal group A&C conjugate vaccine, rabies vaccine, rotavirus vaccine, influenza vaccine, human papillomavirus (HPV) vaccine, live attenuated varicella vaccine, 13-valent pneumococcal polysaccharide conjugate vaccine (PPCV), 23-valent pneumococcal polysaccharide vaccine, etc. With the development of China’s economy and the needs of disease prevention, some non-NIP vaccines may also be included in the immunization program such as Shanghai, China, provided free varicella vaccine to school-age children.Citation24 It can also be seen that non-NIP vaccines have an increasing trend from .
Scientific research on drug regulation promotes the development of China’s innovative vaccine industry
Regulatory science facilitate new vaccines to be approved for marketing
Vaccine R&D is a long-term cooperation process of multi-department and multi-disciplinary joint research. The development of regulatory science straightened out the responsibilities of each department, shortened the vaccine R&D time, and accelerated the ability of vaccine emergency R&D and industrialization. Taking EV71 vaccine as an example, during 2008 to 2016, more than 16 million infants and young children in China suffered from hand-foot-mouth disease (HMFD) and 3,548 died, causing great pain and medical burden to many families.Citation4 Vaccines are the most effective means to control the pandemic, and regulatory scientific research has played a very important role in vaccine R&D. The regulatory authorities and scientific research enterprises jointly solved the problem that vaccines could not be quantified by taking EV71 national reference materials as the mail line, whose achievement has attracted the attention of WHO. After collaborative research by laboratories in many countries, WHO approved the international standard of EV71 neutralizing antibody in 2015.Citation25 The above research results were applied to the quality evaluation of EV71 vaccine, ensuring the safety and effectiveness of the vaccine. In 2016, the EV71 inactivated vaccine was introduced, which effectively reduced the incidence of HMFD, and greatly reduced the severe and fatal cases of the disease. From 2016 to 2020, the EV71 vaccine batches issued were 8.75 million doses, 14.92, 30.52, 18.85 and 24.78 million doses, which are of great importance in protecting children’s life and health.
A total of 14 vaccines were newly marketed in China between 2011 and 2020 ().Citation26 Among them, vaccines independently developed in China include hepatitis E virus vaccine (HepE), poliomyelitis inactivated vaccine (Sabin strain),Citation27 EV71 vaccine, recombinant Ebola virus vaccine,Citation28 quadrivalent influenza vaccine,Citation29 and SARS-CoV-2 vaccines.
Table 3. Newly launched vaccine in China from 2011 to 2020
Regulatory science facilitate the development of combined vaccines
According to the NIP, generally speaking, each individual needs at least 30 doses of vaccine. Such a heavy vaccination task not only greatly increase the pain of vaccination for children and adds inconvenience to parents and medical staff, but also increases the possibility of missed, incorrect vaccination and late vaccination, which has a negative impact on the guarantee of vaccine coverage rate and immune effect.Citation30 Developing combination vaccine is one of the effective ways to solve the above problems. The combined vaccine refers to the vaccine preparation made by physical mixing of two or more antigens, including multi-diseases vaccine and multi-valent vaccine.Citation30 Multi-diseases vaccine is a vaccine made from a mixture of antigens from different pathogens, which can prevent several different diseases, such as DPT vaccine, on which can prevent three diseases, that is pertussis, diphtheria, and tetanus. Multi-valent vaccine is a vaccine mixed with different serotypes of the same pathogen and protect against only one disease, such as 23-valent pneumococcal vaccine, which contains pneumococcal capsular polysaccharide antigens of 23 serotypes, but only against pneumococcal infections.
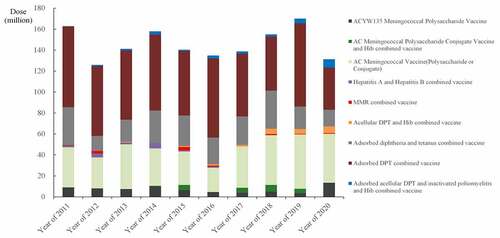
Depending on their antigenic composition, multi-disease vaccines can be divided into four categories: based on DPT vaccine; based on measles vaccine; based on hepatitis A/B vaccine; or other multi-disease vaccines (). Among them, DPT-based the multi-disease vaccines account for the vast majority of multi-disease vaccine, which is the most important basis for the development of multi-disease vaccine. The issuance of adsorbed acellular DPT combined vaccine has always been the first place, with an average issuance of 64.99 million doses from 2011 to 2020, followed by group A&C meningococcal polysaccharide vaccine and adsorbed diphtheria tetanus combined vaccine, with 10-year average issuance of 31.01 and 25.78 million doses. In recent years, the adsorbed acellular DPT vaccine, inactivated poliomyelitis vaccine, Hib combined vaccine, and ACYW135 meningococcal polysaccharide vaccine showed a significant increase trend, and the decreasing trend is hepatitis A and B combined vaccine ().
Considering the latest trend of vaccine R&D at home and abroad, multi-disease vaccine and multi-valent vaccine has become a hot pot. By the end of July 2021, there are 65 vaccine manufacturers registered with the NMPA. Many enterprises in China are vigorous developing the R&D and production of multi-disease and multi-valent vaccines, of which was encouraged by its government. Published laws and policies, such as the general office of state council on further strengthening vaccine circulation and vaccination management, all put forward suggestions on increasing financial support in the development of new vaccines such as multi-disease and multi-valent vaccines and encouraging the R&D and industrialization of combined vaccines.
In response to the COVID-19 outbreak in late 2019, the scientific research team under the joint Prevention and Control Mechanism of The State Council set up a special vaccine team to coordinate vaccine departments to promote the research, approval and production of COVID-19 vaccines under the premise of ensuring the safety and effectiveness of COVID-19 vaccines. For example, rolling submission of data in approval process, pre-registration inspection, emergency inspection, and testing have greatly shortened the time of vaccine research and development to market. Under the guidance of the special vaccine team of the joint prevention and control mechanism of The State Council, China National Biotec Group completed the pre-clinical research work such as the breeding of production strains, the establishment of production strains, the development of pilot technology, pharmacodynamic evaluation, and safety evaluation in 98 days. On April 12, 2020, it obtained the human clinical trial license of the world’s first inactivated COVID-19 vaccine. On June 23, 2020, the company initiated phase III in the United Arab Emirates for its inactivated vaccine, which was approved for emergency use on July 23, 2020, and officially received national marketing approval on December 30, 2020. It takes less than one year from R&D to formal approval for listing, while it generally takes 6–8 years for conventional vaccines to go on the market.
Concluding remarks
From 2011 to 2020, China has steadily improved the quality of vaccines, gradually established NIP vaccines, vigorously developed multi-disease and multi-valent vaccines, carried out technological upgrading and process optimization of existing vaccines, established quality key node control in vaccine production process, and improved the circulation cold chain traceability system to ensure the quality and safety of vaccines.Citation31 The development of drug regulatory science has promoted the improvement of the vaccine regulatory system and accelerated the development of innovative vaccines and emergency vaccines. However, how to achieve the safety, effectiveness, and accessibility of vaccines is still a hot topic for further discussion. The strict supervision of the vaccine industry has also become a prominent feature of the industry. Vaccine regulators also need to further explore new mechanisms, new methods, new technologies and new standards to ensure the safety, effectiveness, reasonable and accessible, and continuously improved public health.Citation32
Acronyms
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Liu C, Cheng Y, Fan X. Translational research: development of regulatory science from regulatory science to scientific regulation. Drug Eval Res. 2014;37(5):385–91. doi:10.7501/j.1674-6376.2014.05.001.
- Shao M. The establishment and practice of bestowing the thought on scientific supervision. Manage World. 2006;(11):1–5. doi:CNKI:SUN:GLSJ.0.2006-11-000.
- Gerberding JL, Haynes BF. Vaccine innovations - past and future. N Engl J Med. 2021;384(5):393–96. doi:10.1056/NEJMp2029466.
- Wang J. Overview of biopharmaceuticals regulatory science development in China. Chin New Drugs J. 2018;27(21):2465–71. doi:CNKI:SUN:ZXYZ.0.2018-21-001.
- Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, Xu W, Zhao Y, Li N, Zhang J, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–21. doi:10.1016/j.cell.2020.06.008.
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi:10.1126/science.abc1932.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–60. doi:10.1001/jama.2020.15543.
- Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, Wu SP, Wang BS, Wang Z, Wang L, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–54. doi:10.1016/S0140-6736(20)31208-3.
- Yang S, Li Y, Dai L, Wang J, He P, Li C, Fang X, Wang C, Zhao X, Huang E, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–19. doi:10.1016/S1473-3099(21)00127-4.
- Zhang NN, Li XF, Deng YQ, Zhao H, Huang YJ, Yang G, Huang WJ, Gao P, Zhou C, Zhang RR, et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182(5):1271–83. doi:10.1016/j.cell.2020.07.024.
- Standing Committee of the National People’s Congress. Vaccine administration law of the People’s Republic of China (2019 issued, order No. 30 of President of the People’s Republic of China).
- Standing Committee of the National People’s Congress. Pharmaceutical administration law of the People’s Republic of China (2019 issued, order No. 31 of President of the People’s Republic of China).
- State Administration for Market Regulation. Provisions for drug registration (2020 issued, order No. 27).
- State Administration for Market Regulation. Measures for the supervision over and administration of pharmaceutical production (Order No. 27).
- State Administration for Market Regulation. Provisions for the lot release of biological products (SFDA Order No. 33).
- Xu M, Liang Z, Xu Y, Wang J. Chinese vaccine products go global: vaccine development and quality control. Expert Rev Vaccines. 2015;14(5):763–73. doi:10.1586/14760584.2015.1012503.
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China (2020 version).
- Liang Z, Mao Q, Wang Y, Li C, Gao K, Wang J. Regulatory science accelerates the development of biotechnology drugs and vaccines by NIFDC. Emerg Microbes Infect. 2014;3(9):e67. doi:10.1038/emi.2014.66.
- Li C, Xu K, Hashem A, Shao M, Liu S, Zou Y, Gao Q, Zhang Y, Yuan L, Xu M, et al. Collaborative studies on the development of national reference standards for potency determination of H7N9 influenza vaccine. Hum Vaccin. 2015;11(6):1351–56. doi:10.1080/21645515.2015.1032490.
- Cao S, Dong G, Tang J, Li J, Liu J, Shi L, Li C, Wang J. Development of a vero cell DNA reference standard for residual DNA measurement in China. Hum Vaccin Immunother. 2013;9(2):413–19. doi:10.4161/hv.22699.
- Reed Z, Cardosa MJ. Status of research and development of vaccines for enterovirus 71. Vaccine. 2016;34(26):2967–71. doi:10.1016/j.vaccine.2016.02.077.
- Hu Y. Improving vaccine supply and regulatory system in China. Chin J Drug Eval. 2014;31(3):175–79. doi:10.3969/j.2095-3593.2014.03.012.
- Jiang Y, Wang X, Zhu W, Qu D. The current vaccination program and related safety problem in China. J Microbes Infect. 2019;14(6):375–86. doi:10.3969/j.1673-6184.2019.06.010.
- Wang W, Wang H. Status and influencing factors of vaccination with non-expanded program on Immunization vaccines in China. Chin J Vaccines Immunization. 2020;26(1):93:97. doi:1006-916X (2020) 01-0093-05.
- Mao Q, Li N, Yu X, Yao X, Li F, Lu F, Zhuang H, Liang Z, Wang J. Antigenicity, animal protective effect and genetic characteristics of candidate vaccine strains of enterovirus 71. Arch Virol. 2012;157(1):27–41. doi:10.1007/s00705-011-1136-3.
- Yu Z, Luo H, Fan H, Su Y, Yu X. Review and future perspectives of vaccine technology and industry development in China in the 70 years after the founding of the People’s Republic of China. Chin Med. 2019;14(7):961–65. doi:10.3760/j.1673-4777.2019.07.001.
- Jiang R, Liu X, Sun X, Wang J, Huang Z, Li C, Li Z, Zhou J, Pu Y, Ying Z, et al. Immunogenicity and safety of the inactivated poliomyelitis vaccine made from Sabin strains in a phase IV clinical trial for the vaccination of a large population. Vaccine. 2021 Mar 1;39(9):1463–71. doi:10.1016/j.vaccine.2021.01.027.
- Pan W, Liu Y. Progress in the research of Ebola virus vaccines. Med Inf. 2020;33(1):36–38,42. doi:10.3969/j.1006-1959.2020.01.013.
- Zhang P, Meng L, Guo X, Cai L, Pan R, Fan B, Han B. Experimental study on immunogenicity and safety of influenza vaccine. Chin J PHM. 2020;36(6):900–02. doi:10.19568/j.cnki.23-1318.2020.06.044.
- Yang X. A review of combined immunization: current research situation and its promising future. Chin J Epidemiol. 2020;41(1):120–26. doi:10.3760/cma.j.0254-6450.2020.01.022.
- Wang G, Shao M. Marketing and prospects for human vaccines in China. Chin J Vaccines Immunization. 2021;27(1):116–20. doi:10.19914/j.CJVI.2021016.
- Shao M. Thinking on scientific research framework of drug regulation in China. China Food Drug Administration. 2019;17(12):4–9. doi:10.3969/j.1673-5390.2019.12.