ABSTRACT
The 4CMenB, a protein-based vaccine, was licensed in Europe in 2013 against invasive meningococcal disease caused by serogroup B and is currently implemented in several countries although according to different national strategies. Isolate coverage estimation is required as vaccine-targeted antigens may vary among isolates over time. Several phenotypic and genotypic methods have been developed to predict strain coverage by scoring the expression and cross-reactivity of vaccine antigens using the Meningococcal Antigen Typing system (MATS), by the genetic correlation of alleles encoding these antigens and MATS expression data (gMATS) and by the Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR). We applied these approaches on meningococcal B isolates in France and compared two epidemiological years, 2013–2014 and 2018–2019. A strong correlation was observed between MATS data that were generated for the year 2013–2014 and the gMATS data extracted from whole genome sequencing. gMATS and MenDeVAR were next used to compare the two years. Using gMATS, the overall coverage was 77.2% (lower limit (LL)-upper limit (UL) 66.7–87.7) and 70.7% (LL-UL 61.5–80.0) for the two years, respectively. The reduction in coverage between the two years is mainly driven by the reduction of alleles exactly matching the vaccine antigens. A high number of unpredictable isolates was observed using the MenDeVAR and was due to lack of MATS information for new or rare alleles in particular for the year 2018–2019. Our data underline the need of continuous surveillance of strain coverage and the importance of generating phenotypic MATS data to update the genetic approaches of prediction.
Introduction
Invasive meningococcal disease (IMD) is caused by Neisseria meningitidis, a Gram-negative encapsulated bacterium that is most frequently carried in the nasopharynx. It can invade bloodstream to result in life-threatening invasive infections dominated by meningitis and septicemia. The composition of the capsular polysaccharide of N. meningitidis defines its serogroup and among the 12 known serogroups,Citation1 six of them, serogroups A, B, C, W, X and Y, are responsible for virtually all IMD worldwide.Citation2 Moreover, isolates can be classified into sequence types (ST) that are further classified into genotypes, also called clonal complexes (CC), on the basis of the polymorphisms of their DNA that is revealed by multilocus sequence typing (MLST) and more recently on the basis of whole genomesequencing (WGS).Citation3 Meningococcal epidemiology is continuously changing in terms of age and distribution of serogroups and genotypes.Citation2 Capsular polysaccharide-based vaccines (plain or conjugate polysaccharides) are available against serogroups A, C, W and Y. Protein-based vaccines are available against isolates of serogroup B. Strategies to use these vaccines require continuous surveillance to inform the best fitting vaccination schemes. This is particularly challenging as vaccine antigens may vary among serogroup B isolates in their sequences as well as in their levels of expression at the bacterial surface and consequently strain coverage needs to be monitored and updated regularly.Citation4
The 4CMenB (Bexsero, GSK) vaccine was the first licensed vaccine in Europe against serogroup B isolates in 2013 on the basis of immunogenicity, safety and breadth of coverage that was evaluated by the Meningococcal Antigen Typing System (MATS).Citation5–7 MATS is a phenotypic method that combines the sequence of porA gene segment encoding the variable region 2 (VR2) with three specialized sandwich ELISAs, one for each of the three recombinant proteins present in the 4CMenB vaccine (fHbp, NHBA, NadA) to measure the level of each antigens’ expression in the lysates of bacterial isolates. The coverage prediction is based on the presence of the matching PorA VR2 sequence P1.4 or expression levels of at least one of the three other antigens higher than a threshold called positive bactericidal threshold (PBT).Citation8 This method permitted the first prediction of coverage of serogroup B isolates in five European countries (England and Wales, France, Germany, Italy, and Norway) by MATS testing of 1052 isolates collected from July 2007 to June 2008. The overall estimated coverage was 78% (95% CI 63–90) and varied between 73% and 87% among these 5 countries, reaching 85% (95% CI 69–93) for the French panel.Citation7 To overcome the requirement of cultured isolates and resource limitations, MATS method was improved by the development of the genetic MATS (gMATS). This approach is based on the correlation between the DNA sequence of the alleles encoding the vaccine antigens and the MATS prediction data.Citation9 However, gMATS has limitations as it does not predict coverage by alleles for which no phenotypic data are available yet. Moreover, as for MATS, gMATS does not measure any synergistic effect among the different vaccine’s components and therefore may underestimate coverage.Citation4 More recently, a similar genetic method, Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR), which uses the correlation of the available MATS data and the sequence of the corresponding encoding alleles, was also reported.Citation10 However, gMATS and MenDeVAR used different criteria in defining covered alleles of fHbp and NHBA.Citation9,Citation10
The MATS data were considered by the National Immunization Technical Advisory Groups (NITAGs) to tailor vaccination strategies. In France, the 4CMenB was recommended in 2013 for at-risk subjects or in case of serogroup B outbreak due to a vaccine-covered isolate.Citation11,Citation12 This recommendation remains in force and no reevaluation of the coverage of serogroup B isolates was performed. Indeed, a recent recommendation was issued in France in June 2021 to include infant MenB immunizations in the National Immunization Program (following a 2 + 1 schedule with 4CMenB).Citation13
In this work, we aimed to perform more recent MATS, gMATS and MenDeVAR-based predictions of coverage by the 4CMenB vaccine and to compare the coverage evolution in France in more recent periods.
Materials and methods
Meningococcal isolates
The surveillance system of IMD in France was previously described and relies on the mandatory reporting that is centralized by the French National Public Health Agency (Santé Publique France) in addition to sending samples and isolates to the National Reference Center for meningococci and Haemophilus influenzae (NRCMHi).Citation14 All data and materials were collected as part of the mission of surveillance conducted by the NRCMHi and the procedure of collecting samples and information was submitted and approved by the CNIL N°1475242/2011 (Commission Nationale de l’Informatique et des Libertés). The exhaustiveness of sending the isolates was previously reported to be higher than 85%.Citation14 All cultured serogroup B isolates were from IMD cases and received at the NRCMHi from epidemiological years 2013–2014 and 2018–2019 (between 01 July and 30 June). Data on age were also included as age groups (<1 year, 1–4 years, 5–9 years, 10–14 years, 15–19 years, 20–24 years and ≥25 years).
Whole genome sequencing
Cultured isolates were confirmed for species and serogroups were determined in addition to full typing by whole genome sequencing as previously described.Citation15 Genomic DNA was extracted with the MagNA Pure 96 system (Roche Molecular System, Pleasanton, USA). Libraries preparation was performed with the Nextera® XT DNA library Preparation Kit (Illumina, San Diego, USA). WGS was performed with Illumina technology (NextSeq 500, Illumina) with paired-end strands of 150 bp and with a sequencing depth of 50X.
All de novo assemblies were performed with SPAdes (CAB, St Petersburg State University, Russia). Multilocus sequence typing (MLST) and PorA variable regions data corresponding to the studied collection were extracted from the PubMLST Neisseria database using the export dataset analysis tool.Citation16
MATS, gMATS and MenDeVAR predictions
The MATS ELISAs were performed only on the isolates of the year 2013–2014, at the NRCMHi in the Institut Pasteur to determine the specific level of expression, the relative potency (RP) for each antigen in each isolate, as previously described.Citation8,Citation17 Each isolate was tested in duplicates for each of the three antigens fHbp, NHBA and NadA using a 6 dilutions’ regression curve. The RPs were calculated using the variance-weighted regression method implemented in the StatLIA software (Brendan Technologies) that computes the RP, the lower and the upper 95% confidence limits by comparing the unknowns’ dilution curves vs the standard curves of specific reference strains selected for each antigen (H44/76 for fHbp, NGH38 for NHBA and 5/99 for NadA).Citation17 Isolates were defined as covered by the vaccine when they had matching PorA P1.4 and/or if at least one vaccine antigen had an RP value above the PBT [fHbp: 0.012 (95% CI 0.008–0.018), NHBA: 0.294 (95% CI 0.169–0.511) and NadA: 0.009 (95% CI 0.004–0.019)]. gMATS analysis was performed on both years 2013–2014 and 2018–2019 on the basis of the previously published data for fHbp and for NHBA and the presence of P1.4 VR2 of PorA.Citation9 For the prediction of coverage, antigens were first scored as covered or non-covered if they possess identical alleles of fHbp or NHBA peptides previously reported to be significantly associated with coverage or non-coverage, respectively.Citation9 If a different allele was present, for which currently there are insufficient data to be able to predict coverage, the corresponding peptide was indicated as unpredictable. After this step, each isolate was scored as (i) covered if it harbored at least one covered antigen, (ii) non-covered if all antigens were non-covered and (iii) unpredictable if it contains no covered antigen but at least one unpredictable antigen. Finally, half of these unpredictable isolates were considered as covered. The lower limit (LL) of the estimated range of gMATS covered isolates corresponds to the proportion ‘covered’ and the upper limit (UL) corresponds to the sum of the proportions of both ‘covered’ and ‘unpredictable’ isolates.Citation9
MenDeVAR analysis was performed on both years 2013–2014 and 2018–2019 using the BIGSdb tools available on the PUBMLST site (pubmlst.org/bigsdb?db = pubmlst_neisseria_isolates). MenDeVAR analysis classifies MenB isolates into four categories according to their antigen matching with the 4CMenB vaccine: (i) isolates with exact–match for at least one antigen, (ii) isolates that show at least one cross-reactive antigen, (iii) isolates that are not cross-reactive to any of vaccine antigens and (iv) isolates with insufficient data.
Statistical analysis
Data were analyzed using GraphPad Prism 5 and chi-square test or Student’s t-test. P value <.05 was considered to be statistically significant, and Bonferroni correction was applied for multiple comparisons. We used the kappa coefficient (K) to estimate the level of agreement between MATS and gMATS.Citation18
Results
Sex and age distribution in 2013/2014 versus 2018–2019
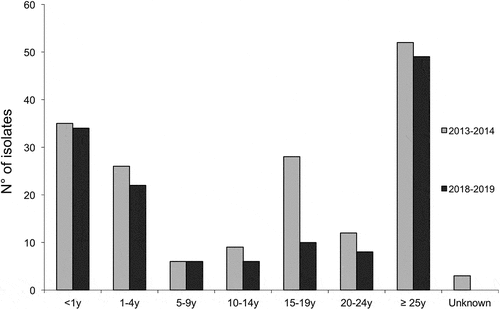
For the epidemiological year July 2013-June 2014, the NRCMHi confirmed 428 cases of IMD, including 338 (79.0%) identified by culture and 90 (21.0%) by PCR only. Among them, 235 cases were caused by MenB (55.0%), of which 64 cases were confirmed solely by PCR. For the epidemiological year July 2018-June 2019 the NRCMHi confirmed 366 cases of IMD, including 302 (82.5%) identified by culture and 64 (17.5%) by PCR. Among them, 188 cases were caused by MenB (51.4%) of which 45 cases were confirmed solely by PCR. Of the 143 cultivable isolates, 135 were successfully recovered by sub-culturing for further analysis. A total of the 171 culture-confirmed serogroup B (MenB) isolates (2013–2014) and the 135 MenB isolates (2018–2019) were all selected for further analysis. Sex ratio (M:F) was not significantly different (P = .5) for the two periods and were 1.2 and 1, respectively. Age distribution of cases is depicted in and did not significantly differ between the two periods.
Clonal complexes distribution in 2013/2014 versus 2018–2019
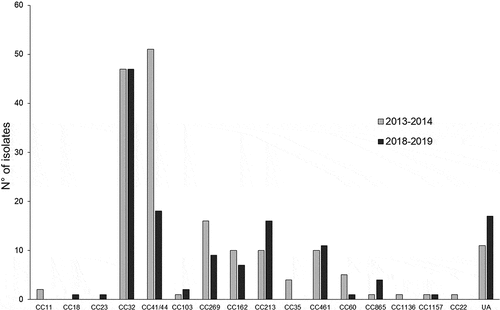
The clonal complexes were inferred from WGS data and were compared for the two periods. MenB isolates were genetically diverse and belonged to 74 STs for the year 2013–2014 that were clustered into 14 different clonal complexes with 11 isolates that did not belong to any of the currently assigned CC. The most frequent clonal complex was CC41/44 that harbored 51 isolates (29.8%) followed by CC32 that was represented by 47 isolates (27.5%) and contained the most frequent ST (ST-32) represented by 20 isolates (11.7%). The four hyperinvasive CC (CC11, CC32, CC41/44 and CC269) were represented by 116 isolates (67.8%).
MenB isolates were also diverse in the year 2018–2019 and belonged to 40 STs that were clustered into 12 different clonal complexes with 17 isolates that did not belong to any of the currently assigned CC. The most frequent CC for the year 2018–2019 was CC32 and was represented by 47 isolates (34.8%) but the most frequent ST in CC32 was ST-7460 that harbored 29 isolates (21.5%). This ST was significantly less prevalent in the year 2013–2014 with only 12 isolates (7.0%; P < .0001). The hyperinvasive CCs (CC32, CC41/44 and CC269) were represented by a lower percentage than that observed in 2013–2014 (74 isolates, 54.8%) but this difference did not reach significant level (P = .08) (). However, the isolates belonging to the CC41/44 decreased significantly in the most recent period (n = 51; 29.8%) for the period 2013–2014 versus 18 isolates (13.3%) for the period 2018–2019 (P = .0028) (). Finally, no MenB/CC11 isolates were detected in the year 2018–2019, while such isolates were present in the year 2013–2014.
Characteristics of PorA peptides among MenB isolates
For the year 2013–2014, 18 different variable regions 1 (VR1) and 36 variable regions 2 (VR2) of PorA were detected among the MenB isolates tested. These numbers were 17 and 28, respectively, for the year 2018–2019. The combination P1.7–2,4 (identical to the PorA in the 4CMenB vaccine) was the most frequent for the year 2013–2014 (n = 33, 19.3%) and mostly associated to the CC41/44 (72.7%) versus 9.6% (n = 13) for the year 2018–2019 and less frequently associated to the CC41/44 (53.8%). An additional isolate with P1.4 (P1.18–1,4) was detected in the year 2018–2019. The most frequent combination for the year 2018–2019 was P1.22,14 (n = 25, 18.5%), which was associated to several CCs versus 8.2% (n = 14) for the year 2013–2014 (Supplementary Tables 1 and 2).
Characteristics of fHbp peptides among MenB isolates
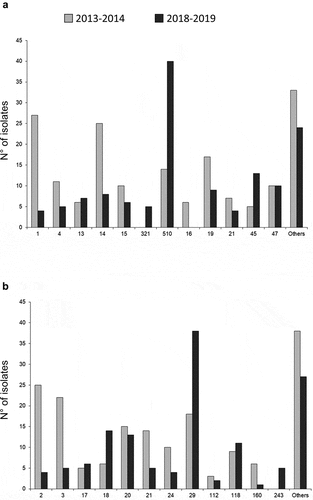
fHbp peptides in isolates of the year 2013–2014 were diverse, and 36 different peptides were detected among the tested MenB isolates with 21 peptides appearing only once and 3 new peptides. Most fHbp peptides belonged to variant 1 (n = 101; 59%) which is the variant in the 4CMenB vaccine. Variants 2 and 3 peptides accounted for 47 isolates (27.5%) and 23 (13.5%), respectively. fHbp sub-variant 1.1 (included in the 4CMenB vaccine) was the most frequent (n = 27; 15.8%) of all MenB isolates of 2013–2014. For the year 2018–2019, fHbp peptides were also diverse, and 30 different peptides were detected among the tested MenB isolates, with 17 peptides appearing only once and 3 new peptides. fHbp peptides belonged mainly to variant 1 (n = 86; 63.7%). Variants 2 and 3 peptides accounted for 19 isolates (14.1%) and 30 isolates (22.2%), respectively. Peptide 510 (of the variant 1) was the most frequent (n = 40; 29.6% of all MenB isolates of 2018–2019 versus n = 14; 8.2% for the year 2013–2014, P < .0001). Variant 1.1 (included in the 4CMenB vaccine) was present only in 4 isolates (3.0% versus 15.8% in 2013–2014, P = .0005) (). Indeed, the overall distribution of fHbp peptides differed significantly (P < .0001) between the two periods (). All the isolates harboring the fHbp peptides 1 or 510 belonged to the CC32.
Table 1. Peptide identification number according to PubMLST
Characteristics of NHBA peptides among MenB isolates
Forty-one NHBA peptides were detected (including six new peptides) for the year 2013–2014. Among these peptides, 23 were found only once. The NHBA peptide 2 (n = 25, 14.6%; peptide included in the vaccine) was the most frequent peptide (). These isolates belonged mainly to the CC41/44 (n = 24; 96%) and 11 isolates also harbored the PorA P1.4 peptide. The second most prevalent NHBA peptide was peptide 3 (n = 22; 12.9%) that were all associated with CC32 isolates other than ST-7460 isolates (Supplementary Table 1).
Thirty-six NHBA peptides were also detected (including eight new peptides) for the year 2018–2019, including 21 found only once. The most frequent NHBA peptide was 29 (n = 38, 28.1% versus n = 18; 10.5% for the year 2013–2014; P = .001). NHBA Peptide 2 (included in the 4CMenB vaccine) was present only in four isolates (3.0% versus 14.6% in 2013–2014, P = .0003) (). Indeed, the overall distribution of peptides differed significantly (P < .0001) between the two periods (). All the isolates harboring the NHBA peptide 29 belonged to the CC32 and also shared the fHbp peptide 510 (Supplementary Table 2).
Characteristics of NadA peptides among MenB isolates
The nadA gene was detected in 49 isolates (28.7%) for the year 2013–2014 and in 57 (42.2%) for the year 2018–2019. These isolates with nadA most frequently belonged to the CC32 and harbored NadA peptide 1 (variant 1), which is cross-reactive with the peptide 3 present in the 4CMenB vaccine.
MATS-based predicted 4CMenB vaccine coverage
MATS was performed on 171 culture confirmed isolates for the year 2013–2014 only and showed that 70.8% (95% CI: 60.2–80.1%) of all isolates are predicted to be covered by the 4CMenB vaccine by at least one antigen. This prediction was lower than that of the year 2007–2008, estimated to be 85% (95% CI: 69–93%).Citation7 Overall, 50 isolates (29.2%) were not covered by any antigen, 58 (33.9%) of the MenB isolates were covered by one vaccine antigen, 51 (29.8%) by two and 12 (7.0%) by three antigens. fHbp contributed to most of the predicted coverage: alone in 42 isolates (24.6%) or in several combinations: fHbp+NHBA (26 isolates, 15.2%), fHbp+PorA (14 isolates, 8.2%), fHbp+NadA (6 isolates, 3.5%) and fHbp+NHBA+PorA (12 isolates, 7.0%). Thirteen isolates (7.6%) were covered by NHBA alone, 2 isolates (1.2%) by PorA alone, one isolate (0.6%) by NadA alone and 5 isolates (2.9%) by NHBA+PorA. MATS-based coverage due to fHbp or NHBA was mainly due to the presence of fHbp variant 1 peptide 1 or NHBA peptide 2 (which are the antigen variants included in the vaccine). However, only one of the two isolates with nadA gene variant 3 had RP values for this antigen above the PBT.
The coverage rate by the 4CMenB differed between the clonal complexes and in particular it was significantly higher for isolates belonging to the hyperinvasive clonal complexes (81.0%) versus 49.1% for the other isolates (P < .0001). The coverage for the hyperinvasive isolates was 83.0%, 86.3% and 62.5% for CC32, CC41/44 and CC269, respectively.
Age data were available for 168 of the tested isolates of the year 2013–2014 (predicted vaccine coverage 70.2%). Age distribution for covered and not covered isolates differed according to the age with the lowest coverage rate for infants under 1 year of age (45.7%; 95% CI 37.1–57.1 P = .0011) ().
Table 2. Coverage rates prediction for the tested isolates according to MATS (with 95% CI) and gMATS (LL-UL)
gMATS -based predicted 4CMenB vaccine coverage
We next evaluated the coverage of the MenB isolates of the year 2013–2014 using the gMATS approachCitation9 that classifies the isolates into covered, non-covered or unpredictable. When applied to the 171 MenB isolates of the year 2013–2014, the gMATS predicted 114 covered isolates, 21 non-covered isolates and 36 unpredictable isolates. gMATS predicted an overall level of coverage of 77.2% (LL-UL 66.7–87.7%). A strong agreement was observed between MATS and gMATS using the predictable isolates with 94.1% of observed agreement with a kappa coefficient of 0.80. gMATS approach was also applied on MenB isolates for the year 2018–2019 resulting in an overall coverage of 70.7% (LL-UL 61.5–80.0%) with 83 covered-, 27 non-covered- and 25 unpredictable isolates.
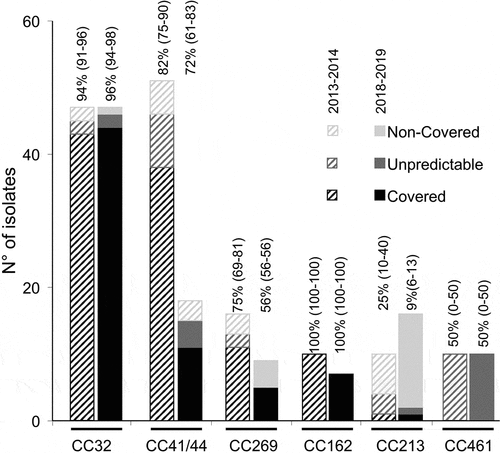
Clonal complexes-based coverage as predicted by gMATS was calculated for CC that are shared by more than 5 isolates in each year. Similar high coverage rates were observed for CC32 and CC162 for the two study periods. However, CC41/44, CC269 and CC213 showed a non-significant decreasing trend of coverage levels between the two periods ().
Figure 4. gMATS-based coverage distribution of isolates by clonal complexes represented by more than 5 isolates in each period (two bars per CC; on the left the year 2013–2014 and on the right the year 2018–2019). The isolates of each CC were categorized in covered, unpredictable or non-covered as indicated. The coverage rate with the LL and UL for each CC are indicated above each corresponding bar.
Age-related distributions of coverage on the basis of MATS (only for 2013–2014) and gMATS were calculated and compared for the two periods (by gMATS). Although a slight overall decrease was observed during the year 2018–2019 compared to the year 2013–2014 (70.7% versus 77.2%), the difference was not significant. Moreover, non-significant variations in coverage were observed for the specific age groups between the two periods (). Finally, gMATS estimated higher coverage than MATS for the <1 year old for the period 2013–2014 ().
MenDeVAR -based prediction of 4CMenB vaccine coverage
MenDeVAR analysis was also used and classified the 171 MenB isolates (for the period 2013–2014) into 4 categories: exact match (n = 72), cross-reactive (n = 17), none-covered (n = 13) and insufficient data (n = 69) analysis. These data indicate that only 102 isolates (59.6%) showed predictable data using MenDeVAR. A strong correlation was also observed between MenDeVAR and both MATS and gMATS for the predictable isolates with an observed agreement of 98.0% and 100%, respectively (Kappa coefficient of 0.92 and 1 respectively). MenDeVAR analysis on the 135 MenB isolates of the year 2018–2019 classified them into exact–match (n = 21), cross-reactive (n = 17), none (n = 18) and insufficient data (n = 79). A complete agreement was found between gMATS and MenDeVAR for the predictable isolates (). However, these data indicate that MenDeVAR allowed the prediction of only 56 (41.5%) isolates for the period 2018–2019 with coverage rate of 67.9%. The predictable fraction of the isolates showed a significant reduction (P = .028) compared to the 102 isolates (59.6%) for the period 2013–2014 with coverage rate of 87.2%.
Figure 5. Prediction of 4CMenB coverage by MATS, gMATS and MenDeVAR (for the epidemiological year 2013–2014) and by gMATS and MenDeVAR (for the epidemiological year 2018–2019) of all the tested MenB isolates. The percentages of isolates within each predicted category are shown. For MenDeVAR, covered isolates accounted for both ”exact-match” + ”cross-reactive”. The number of isolates is indicated in the corresponding bars. The estimations of MATS and gMATS coverage are depicted in the .
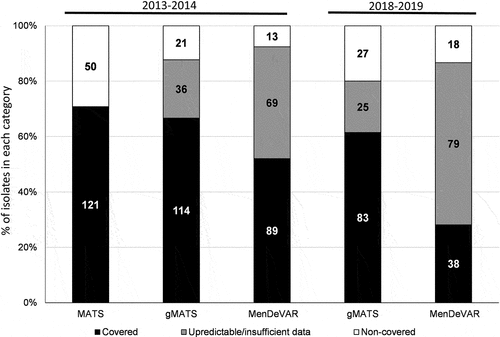
gMATS prediction was possible on a higher fraction of the panel and, unlike MenDeVAR, did not differ significantly between the two periods: 110 (81.5%) isolates for 2018–2019 and 135 (78.9%) isolates for 2013–2014 (P = .80). Moreover, coverage rates were also similar for the two periods ().
Discussion
This study provides data on the evolution of potential coverage of meningococci B isolates in France by the 4CMenB vaccine over a period of 5 years. The need for such data is warranted by the changing epidemiology of IMD. The evolution of genotypes and age distributions were analyzed between the year 2013–2014 and 2018–2019. Our data showed that significant changes were observed in the clonal complexes with a significant decrease in the CC41/44 isolates in 2018–2019 and an increase in the number of non-hyperinvasive clonal complexes. Although other clonal complexes did not change significantly, the STs belonging to these CCs changed over time. In particular, the ST-7460, a member of the CC32, increased significantly from 7.0% of MenB isolates in 2013–2014 to 21.5% in the year 2018–2019. This ST was not reported in the previously published period for the year 2007–2008.Citation7 These observations suggest a new clonal expansion within the CC32 with a possible replacement of the ST-32 isolates (P1.7,16) that were prevalent during the first decade of the twenty-first century,Citation19 by the ST-7460 isolates that showed several other combinations of PorA variable regions as well as different fhbp and nhba alleles (Supplementary Tables 1 and 2).
The decrease in MATS-based coverage prediction in 2013–2014 compared to that of the year 2007–2008 was driven by the lower proportion of isolates harboring the NHBA peptides 2 and 3 from 40.5% in the year 2007–2008Citation7 to 27.5% in the year 2013–2014. This epidemiological change was also reflected by the decrease of CC41/44 (harboring NHBA peptide 2) from 40.5% in the year 2007–2008Citation7 to 29.8% in the year 2013–2014. Such changes prior the licensure and the introduction of the 4CMenB vaccine may be driven by the secular trends that are observed among meningococcal isolates independently from vaccine use.Citation2 In addition, the number of isolates of CC213 that showed the lower rate of coverage also increased in the year 2013–2014. The expansion of ST-7460 isolates of CC32 was also associated with the decrease in the proportion of isolates with NHBA peptide 3. However, these changes in the composition of CC32 between the two periods did not result in any change in gMATS-based coverage rates.
The clinical impact of lower MATS-predicted coverage of isolates among infants under 1 year of age needs to be interpreted with caution as MATS was reported to substantially underestimate killing in hSBA from infant pooled sera.Citation9,Citation20 Furthermore, MATS requires growth of the isolate, it is time consuming and restricted to a limited number of reference laboratories.
Genetic approaches of predicting coverage were therefore developed, such as gMATS and MenDeVAR. These approaches provide an acceptable alternative to MATS and allow generating data from large collections of isolates, while SBA assays may not be feasible on all isolates in particular on non-cultivable isolates. Our data clearly show the ability of genetic approaches in predicting isolate coverage confirming previously published results.Citation9 gMATS predicted lower coverage for the year 2018–2019 compared to the year 2013–2014, with an overall coverage of 70.7% (LL-UL: 61.5–80.0%) and 77.2% (LL-UL: 66.7–87.7%) for the two periods, respectively. The lower predicted coverage in 2018–2019 was driven by the reduction of the CC41/44 that accounted only for 13.3% of the isolates versus 29.8% in the year 2013–2014. In addition, new alleles of fhbp and nhba appeared for which no MATS data are available, making the corresponding isolates unpredictable. On the other hand, coverage rate among infants under the age of 1 year increased from 61.4% for the year 2013–2014 to 69.1% for the year 2018–2019 in line with the periodic changes in the genotypes of the isolates with more isolates belonging to the CC162 and ST-9316 that were shown to be highly covered by the 4CMenBCitation21,Citation22 (Supplementary Tables 1 and 2). Country-wide and age-related consistent predictions were also recently reported between MATS and gMATS in Australia.Citation23 Real-world effectiveness data from England, 3 years post-vaccine implementation using a 2 + 1 schedule, showed a positive vaccine impact (75%) with a similar strain coverage, around 70%, as in France.Citation24
MenDeVAR-based prediction showed a strong correlation with MATS and gMATS for the predictable isolates. However, MenDeVAR showed a lower predictability for the two study periods compared to gMATS predictions. This was due to a higher proportion of isolates with insufficient data, particularly for the year 2018–2019. As for gMATS, this is driven by the appearance of new alleles of fhbp and nhba genes. A recent study from Greece showed lower MenDeVAR- and gMATS-based coverage predictions for the period 2010–2017 compared to that of 2008–2010, also suggesting modifications in vaccine antigens in the more recent isolates.Citation25
MenDeVAR uses higher proportion of isolates with positive MATS prediction (75%)Citation10 than the gMATS (60%),Citation9 as a threshold to consider NHBA and fHbp peptides as covered. Our results suggest that there are not enough available MATS data for several alleles. For example, the fHbp variant 1 peptide 510 (that is found mainly among the expanding ST-7460 isolates) was indicated as “insufficient data” using the MenDeVAR approach but covered by gMATS. MATS results generated from the 14 strains carrying the fHbp variant 1 peptide 510, isolated in the year 2013–2014 showed RP values above the PBT threshold (ranging between 0.014 and 0.048), thus predicting coverage for all the isolates harboring the corresponding fhbp 510 allele. It is therefore important to keep generating MATS for MenB isolates harboring new alleles of fhbp and nhba and harmonization between gMATS and MenDeVAR is also required. Reaching a unified and updated approach is mandatory to provide reliable prediction for decision-making in vaccination strategies against MenB. Thanks to the increased accessibility to bacterial genome sequences, the prospective use of genomic data for the benefit of public health has become a reality.
Authors’ contribution
All authors participated in the design or implementation of the study and were involved in the analysis and interpretation of the results and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Data availability
All the data on isolates are available in supplementary materials as anonymized case by case data. Other unlisted data but without patient-level data can be provided through an inquiry to the corresponding Author.
Trademark statement
Bexsero is a trademark owned by or licensed to the GSK group of companies.
Acknowledgments
We would like to thank all members of the National Reference centre for meningococci and Haemophilus influenzae for their help and support. Many thanks to Maria Giuliani (GSK, Siena, Italy), for the curation of the MenB strains database.
Disclosure statement
MKT reported his institution received fees from the GSK group of companies for the work presented here and from the GSK group of companies, Pfizer, Sanofi-Pasteur for activities outside the presented work. EH, AED and MKT reported a patent 630133 issued. AM, RDP, GB, RLG, MP and LS are employed by the GSK group of companies. RLG, MP and LS hold shares in the GSK group of companies.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.2004055
Additional information
Funding
References
- Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19(4):566–73. doi:10.3201/eid1904.111799.
- Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, et al. The global meningococcal initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18(1):15–30. doi:10.1080/14760584.2019.1557520.
- Jolley KA, Chan M-S, Maiden MC. mlstdbNet - distributed multi-locus sequence typing (MLST) databases. BMC Bioinform. 2004;5(1):86. doi:10.1186/1471-2105-5-86.
- Borrow R, Taha M-K, Giuliani MM, Pizza M, Banzhoff A, Bekkat-Berkani R. Methods to evaluate serogroup B meningococcal vaccines: from predictions to real-world evidence. J Infect. 2020;81(6):862–72. doi:10.1016/j.jinf.2020.07.034.
- Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381(9869):825–35. doi:10.1016/S0140-6736(12)61961-8.
- Santolaya ME, O’Ryan ML, Valenzuela MT, Prado V, Vergara R, Munoz A, Toneatto D, Grana G, Wang H, Clemens R, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet. 2012;379(9816):617–24. doi:10.1016/S0140-6736(11)61713-3.
- Vogel U, Taha M-K, Vazquez JA, Findlow J, Claus H, Stefanelli P, Caugant DA, Kriz P, Abad R, Bambini S, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13(5):416–25. doi:10.1016/S1473-3099(13)70006-9.
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, Moxon ER, Stella M, Comanducci M, Bambini S, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A. 2010;107(45):19490–95. doi:10.1073/pnas.1013758107.
- Muzzi A, Brozzi A, Serino L, Bodini M, Abad R, Caugant D, Comanducci M, Lemos AP, Gorla MC, Krizova P, et al. Genetic meningococcal antigen typing system (gMATS): a genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine. 2019;37(7):991–1000. doi:10.1016/j.vaccine.2018.12.061.
- Rodrigues CMC, Jolley KA, Smith A, Cameron JC, Feavers IM, Maiden MCJ. Meningococcal deduced vaccine antigen reactivity (MenDeVAR) Index: a rapid and accessible tool that exploits genomic data in public health and clinical microbiology applications. J Clin Microbiol. 2020;59(1). doi:10.1128/JCM.02161-20.
- HCSP. Avis du HCSP relatif à l’utilisation du vaccin Bexsero (Novartis Vaccines and Diagnostics). 2013.
- Taha MK, Gaudelus J, Deghmane AE, and Caron F. Recent changes of invasive meningococcal disease in France: arguments to revise the vaccination strategy in view of those of other countries. Hum Vaccin Immunother . 2020;16(10):1–6.
- Haute Autorité de Santé. Stratégie de vaccination pour la prévention des infections invasives à méningocoques: le sérogroupe B et la place de Bexsero®. 2021. http://www.has-sante.fr/upload/docs/application/pdf/2021-06/strategie_de_vaccination_pour_la_prevention_des_infections_invasives_a_meningocoques_le_serogroupe_b_et_la_place_de_bexsero.pdf.
- Parent Du Chatelet I, Deghmane AE, Antona D, Hong E, Fonteneau L, Taha MK, Levy-Bruhl D. Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006-2015. J Infect. 2017;74(6):564–74. doi:10.1016/j.jinf.2017.02.011.
- Hong E, Bakhalek Y, Taha MK. Identification of Neisseria meningitidis by MALDI-TOF MS may not be reliable. Clin Microbiol Infect. 2019;25(6):717–22. doi:10.1016/j.cmi.2018.09.015.
- Jolley KA, Maiden MC. Automated extraction of typing information for bacterial pathogens from whole genome sequence data: Neisseria meningitidis as an exemplar. Euro Surveill. 2013;18(4):20379. doi:10.2807/ese.18.04.20379-en.
- Plikaytis BD, Stella M, Boccadifuoco G, Detora LM, Agnusdei M, Santini L, Brunelli B, Orlandi L, Simmini I, Giuliani M, et al. Interlaboratory standardization of the sandwich enzyme-linked immunosorbent assay designed for MATS, a rapid, reproducible method for estimating the strain coverage of investigational vaccines. Clin Vaccine Immunol. 2012;19(10):1609–17. doi:10.1128/CVI.00202-12.
- Cohen J. A coefficient of agreement for nominal scales. Edu Psychol Measur. 1960;20(1):37–46. doi:10.1177/001316446002000104.
- Caron F, Du Chatelet IP, Leroy JP, Ruckly C, Blanchard M, Bohic N, Massy N, Morer I, Floret D, Delbos V, et al. From tailor-made to ready-to-wear meningococcal B vaccines: longitudinal study of a clonal meningococcal B outbreak. Lancet Infect Dis. 2011;11(6):455–63. doi:10.1016/S1473-3099(11)70027-5.
- Frosi G, Biolchi A, Lo Sapio M, Rigat F, Gilchrist S, Lucidarme J, Findlow J, Borrow R, Pizza M, Giuliani MM, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31(43):4968–74. doi:10.1016/j.vaccine.2013.08.006.
- Tzanakaki G, Hong E, Kesanopoulos K, Xirogianni A, Bambini S, Orlandi L, Comanducci M, Muzzi A, Taha M-K. Diversity of Greek meningococcal serogroup B isolates and estimated coverage of the 4CMenB meningococcal vaccine. BMC Microbiol. 2014;14(1):111. doi:10.1186/1471-2180-14-111.
- Deghmane AE, Haeghebaert S, Hong E, Jousset A, Barret AS, Taha MK. Emergence of new genetic lineage, ST-9316, of Neisseria meningitidis group W in Hauts-de-France region, France 2013-2018. J Infect. 2020;80(5):519–26. doi:10.1016/j.jinf.2020.01.020.
- Tozer SJ, Smith HV, Whiley DM, Borrow R, Boccadifuoco G, Medini D, Serruto D, Giuliani MM, Stella M, and De Paola R, et al. High coverage of diverse invasive meningococcal serogroup B strains by the 4-component vaccine 4CMenB in Australia, 2007-2011: concordant predictions between MATS and genetic MATS. Hum Vaccin Immunother. 2021;17(9):1–9.
- Ladhani SN, Andrews N, Parikh SR, Campbell H, White J, Edelstein M, Bai X, Lucidarme J, Borrow R, Ramsay ME. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–17. doi:10.1056/NEJMoa1901229.
- Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81(4):483–98. doi:10.1016/j.jinf.2020.05.079.