?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
WHO recommends infiltration of rabies immunoglobulins/rabies monoclonal antibodies as anatomically possible, into or close to all category III animal bite wound(s)/exposures for post exposure prophylaxis. The volume required for wound infiltration depending upon the site/size/severity of wound is yet to be defined for guiding the treating physicians. This study aimed to determine the volume of rabies immunoglobulin/rabies monoclonal antibody required for wound infiltration depending upon the site, size, and severity. A prospective cohort study was conducted including category III animal exposures at the anti-rabies clinic, KIMS hospital and Research Center, Bangalore, India. The volume of rabies immunoglobulins/rabies monoclonal antibodies required for wound infiltration, depending on site, severity, and size was determined. All the subjects were followed for 6 months to demonstrate the safety and clinical efficacy of post exposure prophylaxis. The present study included 717 subjects having 1428 bite wounds. There was a significant difference in the median volume required for wound infiltration based on site, size, and severity of bite wounds. However, on pairwise comparison; the median volume among all the pairs for only wound size was found to be statistically significant. Supportively, a strong positive correlation was seen with the size of wound and volume infiltrated. The volume of rabies immunoglobulin/rabies monoclonal antibodies required for wound infiltration shall be determined according to size of wounds, i.e. 1 ml for <1 cm wound, 3 ml for 1–5 cm wound, and 5 ml for >5 cm wound.
Introduction
Animal bites/exposures to humans is a public health problem, both in rural and urban areas and have been documented for more than 4000 years.Citation1,Citation2 A combination of large human and dog population in congested habitable areas combined with widespread poverty has led to more exposures in the World Health Organization (WHO) South-East Asia region, with more than 1.4 billion people at risk.Citation3,Citation4 Therefore, when there is an exposure to an animal that is suspected or confirmed to be rabid or when there is doubt about the factors that led to the exposure, post exposure prophylaxis (PEP) should be initiated immediately.Citation5,Citation6
Early and complete PEP will prevent the disease, even after high-risk exposure to potentially rabid animals. The PEP consists of thorough wound washing with soap/detergent and water, followed by application of virucidal agents to reduce the viral inoculum at the wound site; a complete course of post exposure vaccination to induce antibodies which prevents the risk of virus entering peripheral nerves after an exposure from a rabid animal and timely infiltration of rabies immunoglobulin (RIG)/rabies monoclonal antibodies (RMAb) in all category III exposures to neutralize the virus at the wound site.Citation7–9
Despite the availability of the complete post exposure prophylaxis, there are various barriers to reach the needy, i.e., high cost and injudicious administration of RIG/RMAb as per the body weight. The dose sparing use of RIG/RMAb, based on new evidences available, is an important issue for consideration; as no or very little protection is provided by intramuscular injection of remaining volume of RIG/RMAb at a site away from the bite wound.Citation10
Therefore, WHO recommends infiltration of rabies immunoglobulins/rabies monoclonal antibodies as anatomically possible, into or close to all category III animal bite wound(s)/exposures for post exposure prophylaxis. The volume required for wound infiltration depending upon the site/size/severity of wound is yet to be defined for guiding the treating physicians.
The present study was done to determine the volume of rabies immunoglobulin/rabies monoclonal antibody required for wound infiltration depending upon the site, size, and severity.
This will help in the dose sparing use of RIG/RMAb, which can be useful for the other needy patients.
Materials and methods
A prospective cohort study was conducted at the anti-rabies clinic, Preventive Medicine Unit, Kempegowda Institute of Medical Sciences (KIMS) Hospital and Research Center, Bangalore, India after obtaining the Institutional Ethics Committee clearance.
Sample size calculation
The proportion of category III animal exposure was considered as 63%.Citation11 Assuming a confidence interval of 95% and α = 0.05, precision of 5%; the sample size was calculated as follows:
Using the design effect of 2 = 358.19 × 2 = 716.38 ≈ 717.
The study included 717 category III animal exposures who came for post exposure prophylaxis at the study center and signed the written informed consent to participate in the study. A detailed case history was taken including their socio-demographic profile, details of animal exposure and any history of current or past medical problems, concomitant or past medication, and history of allergy to any medicines.
A detailed clinical examination including general physical examination and systemic examination to know the health status of animal bite victim was done at the study center. All the wounds present in the study subjects were examined in detail to know the site, size and severity of exposures.
Post exposure prophylaxis (PEP) was provided to all the study subjects at the anti-rabies clinic as per the National Center for Disease Control (NCDC), India guidelines. It included thorough wound wash with soap and running water for 10–15 min, complete course of intramuscular anti-rabies vaccination by Essen regimen, i.e., one dose of vaccine on Days 0, 3, 7, 14 & 28 and simultaneous administration of either rabies immunoglobulin, i.e., human rabies immunoglobulin (HRIG)/equine rabies immunoglobulin (ERIG) or rabies monoclonal antibody (RMAb) to all category III exposures as per the calculated volume according to weight on day “0.” Human rabies immunoglobulin (HRIG) was calculated with the dosage of 20 IU/kg body weight, equine rabies immunoglobulin (ERIG) with the dosage of 40 IU/kg body weight and rabies monoclonal antibody (RMAb) with the dosage of 3.33 IU/kg body weight.
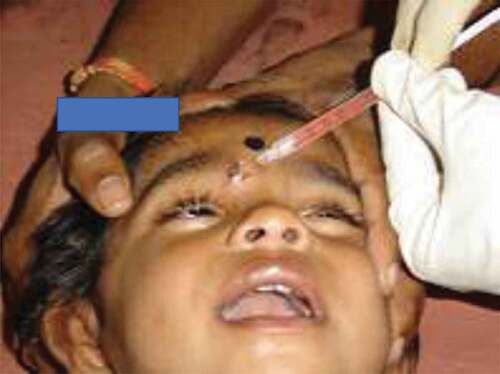
All the wounds were infiltrated with RIG/RMAb to neutralize the virus locally at the wound site; as it was anatomically feasible. The details regarding the volume of RIG/RMAb required for complete wound infiltration, depending on the severity of the bite wounds (abrasion, laceration & punctured wounds), size of wounds (greatest dimension of <1 cm, 1–5 cm & >5 cm), and site of wounds (head, neck & face, trunk & genitals and limbs) were recorded.
All the study subjects were observed for any possible immediate adverse drug events (ADEs), both local and systemic. Likewise, they were given a follow up card with telephonic numbers of the investigators to contact for any delayed adverse events. All the ADEs, if any, were treated free of cost at the anti-rabies clinic. All the study subjects were followed for 6 months to demonstrate the safety and clinical efficacy of post exposure prophylaxis.
The data collected in the study was statistically analyzed using MS Excel and IBM-SPSS statistics software package version 21.0. The normality of the data checked using Shapiro–Wilk test in SPSS 21.0 showed that it was not normally distributed. Hence, non-parametric tests were used for statistical analysis. The results were expressed as median, inter-quartile range, frequency, and percentages. The difference in median volume of rabies immunoglobulin/rabies monoclonal antibody infiltrated locally according to site, size, and severity of bite wound was calculated using Kruskal Wallis test. Post-hoc analysis was done to compare inter-variability between two groups using Dunn Bonferroni test. The Spearman correlation coefficient was calculated to assess the association between the size of wound and the volume of rabies immunoglobulin/rabies monoclonal antibody infiltrated into bite wound.
Results
The study included 717 subjects including all age groups and both the sex. Most of them were adults (56.5%) followed by children (35.3%) and elderly (8.2%). Males were 55.1% and females were 44.9%.
In the present study, 717 subjects with category III animal exposures had 1428 bite wounds. Majority of the wounds were over the limbs followed by wounds over head, neck and face, trunk and in the genital area. The commonest type of wound was abrasion (45.0%) followed by laceration (29.2%) and punctured wounds (25.8%). The wound size varied from as small as <0.1 cm to as large as >10 cm, most of the wounds being of 1–5 cm in size (55.4%); followed by <1 cm (40.8%) and >5 cm (3.8%) ().
Table 1. Characteristics of animal bites among the study subjects (n = 1428)
All the study subjects were provided complete post exposure prophylaxis which included wound wash with soap and water; followed by administration of anti-rabies vaccine by intramuscular route by Essen regimen, using purified verocell rabies vaccine (51.6%) or purified chick embryo cell culture vaccine (48.4%). Rabies immunoglobulin/rabies monoclonal antibody was administered to all the bite wounds; the subjects received either rabies monoclonal antibody (56.9%) or equine rabies immunoglobulin (37.6%) or human rabies immunoglobulin (5.5%); the RIG/RMAb was infiltrated locally into or around the wound site as much as anatomically feasible into the length and depth till it oozes out indicating successful infiltration (). The remaining volume of RIG/RMAb, if any; was injected deep intramuscularly away from the vaccination site as per the Drug Controller General of India guidelines and product insert of the RIG/RMAb.
The median volume of rabies immunoglobulin/rabies monoclonal antibody infiltrated at different sites, size and severity of wounds are as shown in . The median volume of RIG/RMAb required for local infiltration was compared with respect to site, severity and size of the wound using Kruskal Wallis test which showed that there was a statistically significant difference in the median volume of rabies immunoglobulin/rabies monoclonal antibody required to infiltrate different type of wound ().
Table 2. Volume of RIG/RMAb required for local infiltration based on site, size and severity (n = 1428)
Table 3. Comparison of RIG/RMAb volume required based on wound site, size and severity (n = 1428)
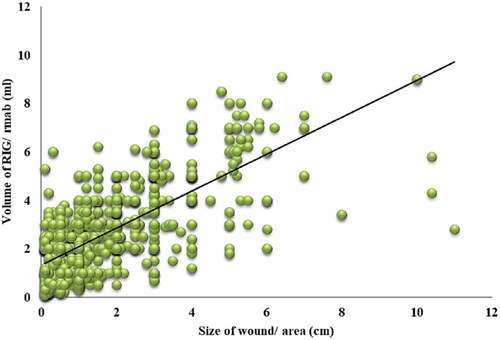
However, the Post-hoc analysis of Kruskal Wallis test by Dunn Bonferroni test for pair wise comparison showed that the different category of wound size viz. <1 cm, 1–5 cm and >5 cm among all pairs was found to be statistically significant (p < .001) (). Supportively, there was also a strong positive correlation between the size of bite wounds and the volume of RIG/RMAb infiltrated to the wounds based on the Spearman’s rho coefficient; which was found to be statistically significant (ρ = +0.602, P < .0001) (Graph 1). Therefore, the size of the wound was considered appropriate to define the volume of RIG/RMAb required for wound infiltration viz. 1.2 ml (≈1 ml) for <1 cm wound; 2.8 ml (≈3 ml) for 1–5 cm wound and about 5.1 ml (≈5 ml) for >5 cm wound at any site/severity depending upon the anatomical feasibility.
Table 4. Post-hoc analysis of pair wise comparison for volume of local RIG/RMAb infiltration (n =1428)
The adverse drug events to rabies PEP were seen in 8.1% of the subjects; the common local ADEs were pain at the injection site, erythema, itching and the systemic ADEs included fever, malaise, headache, and bodyache. All the ADEs were mild in nature and subsided spontaneously/with symptomatic treatment, devoid of any complications. All the subjects were healthy and alive throughout the period of 6 months after receiving post exposure prophylaxis.
Discussion
Rabies immunoglobulin/rabies monoclonal antibody is a life-saving immune-biological for all category III animal exposures. It provides neutralizing antibodies, i.e., passive immunity at the site of exposure and offer immediate protection before patients start producing their own protective levels of neutralizing antibodies as a result of anti rabies vaccination.Citation12–14
RIGs have proved their efficiency when administered in the site of virus entry (wound) in association with rabies vaccine.Citation15–17 Dean and Baer had shown that the local injection of anti rabies serum for rabies virus-contaminated wounds is essential for severe exposure and intramuscular injection at a site away from the wound will not provide a titer of >0.5 IU/mL at systemic level.Citation18 A recent systematic review on RIG also concluded that maximum infiltration of the RIG dose (calculated by body weight) into and around the wound is effective and the benefits from additional intramuscular administration of any remaining RIG at a site distant to the wound are likely to be very limited.Citation19,Citation20
Therefore, WHO recommends that the entire dose of RIG/RMAb or as much as anatomically possible, should be infiltrated carefully into or as close as possible to the wound(s)/exposure sites.Citation21
The present study was done to determine the volume of rabies immunoglobulin/rabies monoclonal antibody required for wound infiltration depending upon the site, size and severity.
717 subjects having 1428 bite wounds were included in the study; most of the wounds were over the limbs (85.6%); followed by wounds over head, neck and face, trunk and in the genital area. The commonest type of wound was abrasion (45%) followed by laceration (29.2%) and punctured wound (25.8%). Most of the wounds were 1–5 cm in size (55.4%); followed by <1 cm (40.8%) and >5 cm (3.8%).
Likewise, a multicentric study conducted in six selected states of India namely Himachal Pradesh and Bihar (North), West Bengal (East), Kerala (South), Madhya Pradesh (Central), and Gujarat (West) by WHO-APCRI including 529 animal bite cases from 18 health- care facilities, showed that lacerations (51.9%) were most common type of wounds followed by abrasions (42.3%). The bite wounds were commonly found on lower limbs (60.5%) and upper limbs (29.7%); other areas include the head, neck, face, and genitals.Citation22 Similarly, an epidemiological study including 69 animal bites of rural population in Tamil Nadu showed that majority of them had superficial bites (76.81%) and most of the bite wounds were on legs (60.87%), followed by hands (26.08%), face/neck (2.9%), body (7.25%), and trunk (2.9%).Citation23 A multicentre cross-sectional study conducted in Korea on 9966 animal exposure subjects showed that the most common anatomical site of bites was the upper extremity (33.3%), followed by head and neck (21.9%), lower extremity (15.7%), and multiple sites (3.2%) and the torso (0.9%).Citation24 All these studies showed that the severity/size/site of bite wounds varies among the animal bite victims and the PEP has to be provided accordingly.
In the present study, the volume of RIG/RMAb required for local infiltration of wounds depending upon various sites, severity and size of exposures was determined. Although the volume of RMAb required was less as its requirement was 3.33 IU/kg body weight, it was not diluted to maintain the uniformity of determining the volume to be infiltrated into the wound.
It was seen that, in the head, neck, and face regions, the median volume of RIG/RMAb infiltrated for <1 cm wound was 0.8 ml (abrasions) and 1.2 ml (lacerations/puncture wound); in trunk region it is 1 ml (abrasions/puncture wounds) and 1.5 ml (lacerations); and in genital area 0.2–0.9 ml for all types of wounds <1 cm. Likewise, the median volume of RIG/RMAb required for 1–5 cm wound in head, neck & face region was 2.1 ml (abrasion and lacerations) and 1.3 ml (puncture wounds); in trunk region it is 3 ml (abrasion/lacerations) and 1 ml (puncture wounds); in upper limbs it is 2.5 ml (abrasion and lacerations) and 2.1 ml (puncture wounds); and in lower limbs 3 ml (abrasion and lacerations) and 2.5 ml (puncture wounds). Likewise, for >5 cm wounds, the median volume of RIG/RMAb infiltrated ranged from 2.8 ml in head, neck & face area, 4–7 ml in upper limbs, and 6 ml in lower limbs. The volume of RIG/RMAb infiltrated also considered the depth of the wound, infiltrating deep into the tissues which were exposed, till the RIG/RMAb oozes out from the tissues indicating successful infiltration.
The study showed a significant difference in the median volume of RIG/RMAb required for local infiltration of bite wounds based on various sites, sizes and severity of wounds. However, on pairwise comparison of different category of wound size viz. <1 cm, 1–5 cm and >5 cm; the difference in median volume of RIG/RMAb infiltrated among all pairs was found to be statistically significant (p < .0001), which implies that the volume of RIG/RMAb required for wound infiltration varies significantly for different size of the wound. Further, the present study also showed a strongly positive correlation between the size of bite wounds and the volume of rabies immunoglobulin/rabies monoclonal antibody infiltrated to the wounds based on the Spearman’s rho coefficient; which was found to be statistically significant. Therefore, the volume of rabies immunoglobulin/rabies monoclonal antibodies required for wound infiltration shall be determined according to the size of wounds, i.e., 1 ml for <1 cm wound; 3 ml for 1–5 cm wound and 5 ml for >5 cm wound. There is hardly any published data present on the volume required for infiltration of bite wounds based on its size, site, and severity of wounds.
The present study also showed that the adverse drug events to PEP were seen in 8.1% of the subjects; the local ADEs were pain at the injection site, erythema, itching and the systemic ADEs included fever, malaise, headache, and bodyache. All ADEs were mild in nature and subsided spontaneously/with symptomatic treatment without leading to any complications. All the subjects were healthy and alive throughout the period of 6 months after receiving post exposure prophylaxis. The arbitrary period of 6 months was kept based on the maximum incubation period of the disease, as found in the National multi-center epidemiological survey conducted in India by APCRI supported by WHO.Citation25
Similarly, a study conducted in Bangalore among 550 animal bite victims attending the anti- rabies clinic, showed that overall 7.1% ADEs were reported among the study subjects, the local adverse events reported were pain (8.5%), erythema (7.3), and itching (5.8%); while, systemic adverse reactions were headache, bodyache, fever, malaise, and nausea.Citation26 All the studies showed the ADEs to PEP were minimal and mild in nature, as they subsided spontaneously/with symptomatic treatment without any complications; showing that PEP is safe for all animal bite victims.
The limitation of the study was that the biting animals could not be followed up to know whether the patients were bitten by lab-confirmed rabid dogs because of the logistical issues. Likewise, the RVNA analysis to know the immunogenicity for the anti rabies vaccine having titers >0.5 IU/ml of serum was not done for the cost involved. However, since the maximum incubation period of the disease in India was 6 months, the survival status of all the study subjects beyond 6 months was considered as the clinical effectiveness of the PEP provided.
In conclusion, the present study defined the volume of RIG/RMAb required for wound infiltration as 1 ml for <1 cm wound; 3 ml for 1–5 cm wound and 5 ml for >5 cm wound for any site/severity depending upon the anatomical feasibility, irrespective of the age or any RIG/RMAb usage; not exceeding the volume recommended as per body weight in multiple wounds. This sequentially may bring about advocacy change in the country for infiltrating RIG/RMAb only into the wounds as per the WHO position for PEP and will help in the dose sparing use of RIG/RMAb, which can be useful for the other needy patients to achieve Universal Health Coverage; ultimately supporting elimination of dog mediated human rabies by 2030.Citation27,Citation28
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Weekly Epidemiological Record. Rabies Vaccines: WHO Position Paper No 16. World Health Organization; 2018. Vol. 93, p. 201–20.
- Tarantola A. Four thousand years of concepts relating to rabies in animals and humans, its prevention and its cure. Trop Med Infect Dis. 2017; 2:E5. doi:10.3390/tropicalmed2020005.
- World Health Organization. WHO South East Asia region strategic framework for elimination of human rabies transmitted by dogs in the South-East Asia region. Geneva (Switzerland): World Health Organization, Regional office for South East Asia; 2012.
- Wilde H, Khawplod P, Khamoltham T, Hemachudha T, Tepsumethanon V, Lumlerdacha B, et al., Rabies control in South and Southeast Asia. Vaccine. 2005;23:2284–89. doi:10.1016/j.vaccine.2005.01.030.
- Gongal G. Introduction of intradermal rabies vaccination–a paradigm shift in improving post-exposure prophylaxis in Asia. Vaccine. 2018;37(1):94–98. doi:10.1016/j.vaccine.2018.08.034.
- World Health Organisation, Geneva. Zero by 30: the global strategic plan to end human deaths from dog mediated rabies by 2030. Geneva (Switzerland): Food and Agriculture Organization of the United Nations, World Organisation for Animal Health, World Health Organization and Global Alliance for Rabies Control; 2018.
- National guidelines for rabies prophylaxis 2019, National rabies control programme. National centre for diseases control. New Delhi (India): National Centre for Disease Control; 2019. p. 6–18.
- World Health Organization. WHO expert consultation on rabies. Geneva (Switzerland): World Health Organization; 2018. 3rd Report, Technical Report Series 1012.
- Sudarshan MK, Ravish HS. Facilities and services of post exposure prophylaxis in anti rabies clinics: a national assessment in India. Indian J Public Health. 2019;63(1):s26–30. doi:10.4103/ijph.IJPH_367_19.
- Bharti OK, Madhusudana SN, Gaunta PL, Belludi AY. Local infiltration of rabies immunoglobulins without systemic intramuscular administration: an alternative cost-effective approach for passive immunization against rabies. Hum Vaccin Immunother. 2016;12(3):837–42. doi:10.1080/21645515.2015.1085142.
- Ichhpujani RL, Mala C, Veena M, Singh J, Bhardwaj M, Bhattacharya D, et al. Epidemiology of animal bites and rabies cases in India. A multicentric study. J Commun Dis. 2008;40:27–36.
- Ashwath Narayana DH, Ravish HS, Ramesh H. Clinical evaluation of safety of equine rabies immunoglobulin. APCRI J. 2011;12:12–15.
- Kim PK, Keum SJ, Osinubi MOV, Franka R, Shin JY, Park ST, et al, et al. Development and characterization of novel chimeric monoclonal antibodies for broad spectrum neutralization of rabies virus. PLoS ONE. 2017;12(10):e0186380. doi:10.1371/journal.pone.0186380.
- Gogtay NJ, Munshi R, Ashwath Narayana DH, Mahendra BJ, Kshirsagar V, Gunale B, et al, et al. Comparison of a novel human rabies monoclonal antibody to human rabies immunoglobulin for post-exposure prophylaxis: a phase 2/3 randomized, single blind, non-inferiority, controlled study. Clin Infect Dis. 2018;66(3):387–95. doi:10.1093/cid/cix791.
- Habel K, Koprowski H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull World Health Organ. 1955;13:773–79.
- Hobart-Porter N, Stein M, Toh N, Amega N, Nguyen H-B, Linakis J. Safety and efficacy of rabies immunoglobulin in pediatric patients with suspected exposure. Hum Vaccin Immunother. 2021;17:7, 2090–96. doi:10.1080/21645515.2020.1854000.
- Ravish H, Krishna C, Kumar P, Siddareddy I, Annadani R. Safety, immunogenicity and clinical efficacy of post exposure prophylaxis in confirmed rabies exposures. Global Vaccines Immunol. 2016;1:56–59. doi:10.15761/GVI.1000116.
- Dean DJ, Bear GM, Thompson WR. Studies on the local treatment of rabies infected wounds. Bull World Health Organ. 1963;28:477–84.
- Madhusudana SN, Ashwin BY, Sudarshan S. Feasibility of reducing rabies immunoglobulin dosage for passive immunization against rabies: results of In vitro and In vivo studies. Hum Vaccin Immunother. 2013;9(9):1914–17. doi:10.4161/hv.25431.
- Bharti OK, Madhusudana SN, Wilde H. Injecting rabies immunoglobulin (RIG) into wounds only: a significant saving of lives and costly RIG. Hum Vaccin Immunother. 2017;13(4):762–65. doi:10.1080/21645515.2016.1255834.
- Bharti OK, Thakur B, Rao R. Wound-only injection of rabies immunoglobulin (RIG) saves lives and costs less than a dollar per patient by “pooling strategy.” Vaccine. 2019;37:A128–A131. doi:10.1016/j.vaccine.2019.07.087.
- Haradanhalli RS, Anwith HS, Pradeep BS, Isloor S, Bilagumba G. Health-seeking behavior and compliance to post exposure prophylaxis among animal bite victims in India. Indian J Public Health. 2019;63:S20–5. doi:10.4103/ijph.IJPH_364_19.
- Sangeetha S, Sujatha K, William RF. An epidemiological study of animal bites among rural population in Tamil Nadu, India. Int J Community Med Public Health. 2016;3:1413–18. doi:10.18203/2394-6040.ijcmph20161603.
- Park JW, Kim DK, Jung JY, Lee SU, Chang I, Kwak YH, et al. Dog-bite injuries in Korea and risk factors for significant dog-bite injuries: a 6-year cross-sectional study. PLoS ONE. 2019;14(2):e0210541. doi:10.1371/journal.pone.0210541.
- Sudarshan MK, Madhusudana SN, Mahendra BJ, Rao NS, Ashwath Narayana DH, Abdul Rahman S, et al. Assessing the burden of human rabies in India: results of a national multi-center epidemiological survey. Int J Infect Dis. 2007;11(1):29–35. doi:10.1016/j.ijid.2005.10.007.
- Ravish HS, Kumari N, Ramya MP, Surendran J. Safety of rabies immunoglobulin (RIG)/ rabies monoclonal antibody (RMAb) for post-exposure prophylaxis in patients with potential rabies exposure. APCRI J. 2019;21:22–27.
- World Health Organization. New global strategic plan to eliminate dog-mediated rabies by 2030. Geneva (Switzerland): World Health Organization; 2018.
- Universal health Coverage. Geneva (Switzerland): World Health Organization. [accessed 2021 Aug 23]. https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc).