ABSTRACT
Edible insects play an important role in human health and food security. Among those, the Giant water bug, Lethocerus indicus (Lep.& Ser.) is a widely used edible insect known for its aroma, flavor, and therapeutic purposes. In the present study, we investigated the nutritional profile, natural habitat, and feeding behavior of L. indicus in aquarium conditions. A comparative analysis of male and female insects’ aroma contents and fatty acid (FA) profiles was also conducted. A dry fried male insect yielded volatile oil of 0.96%/2 g body weight, whereas a dry fried female yielded 0.48%/5.36 g of body weight. In terms of lipids, fresh male insects had 0.15%/5.42 g of body weight and fresh female insects had 0.28%/9.48 g of body weight. There are 24 volatile compounds specific to males, 37 specific to females, and 13 commons to both were identified. 2-Hexen-1-ol, acetate, (Z)- which smells like banana, was prevalently found in males while 4-Octene, 2,6-dimethyl-, [S-(Z)] was prevalently found in female insects. Fatty acids profile analysis detected 32 FA with 12 unique FA from males whereas 22 FA and 3 unique FA were identified from female insects. The SFA percentage present in males and females was 77.44% and 85.21%. Males had 6.78% MUFA content while females have 4.75%. Males have 18% PUFA content enriched with DHA, and EPA, while females had 10.04%. This study revealed that with the presence of a banana-like smell of volatile compound and more MUFA and PUFA in males, the native people of North-East India preferred male over female insects for entomophagy.
GRAPHICAL ABSTRACT
1. Introduction
Giant water bugs are aquatic insects belonging to the Heteroptera suborder. Aquatic insects possess two types of scent glands: one that emerged from the thoracic region during the adult stage and one from the abdominal region during the larval stage [Citation1]. These insects employ the chemical substances they emit to defend themselves from predators and to prevent microbial growth inside their bodies [Citation2]. Lethocerus indicus, (Heteroptera: Belostomatidae) is one of the edible gigantic water bug insects and has long been a crucial flavoring agent in Manipur and Thailand. In contrast to female insects, male insects are well known for their flavor and odor [Citation3,Citation4]. Edible insects have become the source of future feed and food due to their beneficial nutritional characteristics and low environmental impact [Citation5]. It is also safe and healthy for humans to eat insects as food [Citation3]. It was predicted and hoped that malnutrition challenges may be resolved by providing 10% of the protein from insect sources rather than other meat sources. Insects are a good source of minerals, macronutrients, and other nutrients like sodium, calcium, and magnesium [Citation6].
Because of metabolic mechanism variation based on diet, the nutritional component of aquatic insects varies from species to species and is dependent on the feeding habits available in their environmental niche [Citation7]. Nutritional components of lipids present in different sexes of the species also vary due to sex-specific nutritional requirements of the body mechanisms [Citation8]. A potent odorant compound (E)-2-hexenyl acetate which has been responsible for the aroma of the male L. indicus insects was reported in a study conducted in Thailand, but this study did not mention the aroma obtained from female insects [Citation9]. The nine fatty acids identified from the L. indicus insects belonged to saturated and unsaturated fatty acids, but they did not mention the sex of the insect [Citation10]. Lipids are the second largest constituent of insects after proteins [Citation11]. Different fatty acids are utilized as ingredients in the food, pharmaceutical, and cosmetic, industries [Citation12]. Cosmetic and personal care products derived from fatty acids obtained from the insects such as C12:0, C14:0, C16:0, and C18:1, are also available on the market [Citation8]. For example, the insect oil extracted from Tenebrio molitor has positive effects on skin wound treatment [Citation13] whereas fat oil extracted from black soldier fly larvae is used as a replacement for butter for making cookies, waffles, and cakes [Citation14]. These potential properties obtained from the insects hypothesize that L. indicus must possess various properties which are economically important for human being.
Lethocerus indicus has a variety of roles in maintaining ecological balance, human health, and food security [Citation7]. This study aimed to determine the fragrance and fatty acid differences between male and female Lethocerus indicus insects. To understand the adaptation of insects in their natural habitat, ecological conditions, and their feeding behavior a mimicking environment in an aquarium was also created and monitored. The nutritional value of this entomophagy insect has the potential to be very beneficial for bio economy strategies in the North eastern region of India.
2. Materials and methods
2.1. Sample collection and habitat observation
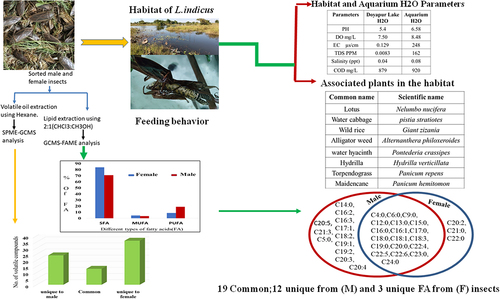
The giant water bugs were collected from the natural habitat of Doyapur Lake at Disagaphu (latitude: 25.7520540, longitude: 93.611874°) (Supplemental Fig. S1(a)) and reared in an aquarium dimension (40X60) cm.The aquatic plants associated with these insects were listed (Supplementary Table S1), and the water from the habitat was stored at 4°C for further analysis.
2.2. Acclimatization of insects in the aquarium
Mass cultivation of L. indicus was performed in an aquarium by keeping the water at room temperature. The water level was 14 cm in the aquarium with the filter and aerators for the constant supply of oxygen. The selected aquatic plants available in the habitat such as Pontederia crassipes (water hyacinth) and Panicum hemitomon (Hub Napi), were added and reared in the aquarium (). The local fish species such as Trichogaster fasciata (Ngapemma), Puntius species (Phabounga), Lata Fish (Ngamu), Amblypharyngodon mola (Mukanga),Hydrophilus piceus (water beetle), Freshwater snails were also added as prey for L. indicus insects. In the aquarium,30% of the water was replaced daily, and dead fish and other unwanted materials were removed.
Figure 1. Acclimatization of Lethocerus indicus in the aquarium with the associated plants. The photo of a) L. indicus insects along with b) giant Zizania (Wild rice) grass and Pontederia crassipes (water hyacinth) aquatic plants inside the aquarium c) photo of L. indicus eggs laid inside the aquarium.

2.3. Water quality analysis
The collected water from the habitat and local pond stored at minus 4°C was brought at room temperature and the water quality parameters such as the potential of Hydrogen (pH), Dissolved Oxygen (DO), Total Dissolved Solids (TDS), Electrical Conductivity (EC), and salinity were tested using Aquaread AP-2000 [Citation15]. The Chemical Oxygen Demand (COD) was analyzed following the protocol described in APHA [Citation16].
2.4. Extraction of volatile oil from L. indicus
The male and female insects are identified based on the genital parts (Supplementary Fig. S1(b & c)).The selected insects were fried in a frying pan above the gas flame without oil as a traditional way roasting method for 10 minutes. The extraction of volatile oil was performed using n-hexane solvents from dry-fried males and females separately. Five fried males (total mass 10 g) and five females (total mass 26 g) were chopped into small pieces using a sterilized scissor and placed into a 500 ml glass bottle with 200 ml of Hexane and kept in the shaker oven at 30°C over night following the oil extraction method with slight modification [Citation17]. The samples had allowed to cool down and filtered using a Whatman filter paper and transferred to the round flask bottles. The sample was concentrated using a rotary evaporator and stored at minus 4°C in an amber vial for further analysis. The total weight of the oil extracted was determined by subtracting the weight of the round flask bottle. The weight of oil obtained per 10 g male fried insect was 0.48 g and 26 g female insects were 0.63 g.
2.5. GC-MS analysis of the essential oil
The essential oil collected from the L. indicus male, and female were diluted in the ratio of 1:100 (essential oil: n-hexane). The 0.5 µl of the sample prepared was injected into the GC-MS. The GC-MS employed have Trace1300 as Gas chromatography and TSQ DUO as Mass Spectrophotometry. The GC have the silica capillary column (30 m X 0.25 mm; 0.25 µm film thickness), TG-5 MS interfaced with the MS.The carrier gas used in the instrument was helium with a flow rate of 1 ml/min for 60 minutes [Citation18,Citation19]. The ionization energy was set at 70 eV, and the mass transfer line temperature at 250°C and the ion source temperature at 280°C. The column temperature was programmed from 40°C for 1 min to 280°C at the rate of 5°C/min by a heating ramp for 20 min and held at 250°C.The inlet injector temperature was set at 240°C with a split mode of 1:20 maintained [Citation18,Citation19]. The mass spectra were filtered from the mass range from 35 to 450 Mw.The identification of the volatile compound was determined by comparing the spectra obtained from the sample with the reference to the mass spectrum given by the National Institute of Standards and Technology (NIST) GC/MS Libraries 2017 [Citation19].
2.6. Total lipid extractions from L. indicus insects
The total lipid content in the fresh Lethocerus indicus of both male and female insects’ was determined using the method developed by Blight and Dyer [Citation20]. The selected five fresh male insects with a total weight of 27.1 g and another five female insects with a total weight of 47.4 g were chopped into pieces and transferred to 200 ml of a mixture of chloroform and methanol solvents (1:2) contained in the 500 ml reagent bottle. The insect samples were kept in the shaking water bath for 4 hours at 30°C for homogenization. The homogenate samples were cooled down and filtered through Whatman No.1 Filter paper on a Coors No.3 Buchner funnel with a slight suction. The extracted lipid sample was transferred to a preweighed round bottle flask and concentrated using a rotary evaporator. The total lipid weight was determined after subtracting the weight of the round bottle flask.
2.7. Thin layer chromatography (TLC) analysis
TLC was performed to identify lipid profile components of lipids extracted from the L. indicus male and female insects. The TLC aluminum sheets coated with 0.2 mm thickness silica 60F254 from E Merck, India was used by spotting a 5 µl sample. The lipid contained in the sample was separated by using a mixture of solvent systems containing hexane, diethyl ether, and acetic acid in the ratio of 80:20:1 [Citation21]. The separation of the lipid was conducted inside a glass chamber, and spots of the samples were visualized with the help of iodine vapors.
2.8. Fatty acids profiling of insect lipid by gas chromatography
Transesterification of extracted lipid from male and female L. indicus insects was conducted using methanol and H2SO4 reagents at 100°C for 1 hr. The fatty acid methyl esters (FAMEs) were dissolved in hexane and allowed to dry at room temperature [Citation21]. The dried FAME samples obtained from male and female insects were analyzed in a GC/MS (6850N, Agilent Technologies) attached to an FID detector along with Agilent DB-225 capillary column. The oven temperature program was set from 160°C for 2 minutes to 230°C at the ramping rate of 5°C/min and held till 230°C for 20 min. The temperature of the injector and detector was maintained at 230°C to 270°C. The sample injection volume was 1 µl and the carrier gas was nitrogen with a flow rate of 1 ml/min. The area percentage for each chromatogram was recorded with a standard HP-chemstation data system. The FAME analysis data were identified by comparing their fragmentation pattern with the internal standard obtained from Sigma Aldrich, India.
2.9. Statistical analysis
Experiments were carried out in triplicate and data were represented as mean ± standard error (SEM) for habitat water parameter analysis.
3. Results
3.1. Observation of the habitat and feeding behavior in the aquarium
The habitat observation for L. indicus aquatic insects was conducted to mimic the natural habitat under aquarium conditions for the large scale production and also for the consistent nutritional profile maintenance. The habitat of Lethocerus indicus Doyapur Lake at Disagaphu has a swampy natural environment, the plentiful aquatic flora in habitat. The water analysis of Doyapur Lake is represented in Supplementary TableS 2. The data showed that water is acidic, with a pH of 5.4, a dissolved oxygen value of 7.5 mg/L, TDS of 0.0083 ppm, EC of 0.129 s/cm, a salinity of 0.04 ppt, and COD of 879 mg/L respectively. The water analysis of habitat help in understanding the adapted environment of L. indicus and mimicking the mass cultivation of insect in the aquarium condition. Under aquarium conditions, L. indicus primarily attacked the caudal peduncle, caudal fin region, head, operculum, lateral line, and spiny region of the local fish preys as in . The disturbance that occurs when fish were attacked in the area mentioned slows down their swimming speed inhibits them from sensing the surrounding water pressure, throws off their swimming balance, and weakens them by interfering with their respiratory systems. All these attacked preferences will help in immobilizing their prey and make it easier to trap them as their food source. These insects were hunting in groups for the larger prey. The freshwater snail was attacked at the region of the digestive gland, stomach, anterior kidney, and testis while the water beetle was assaulted between the head and shoulder and between labrum and labium. Their prey’s tissue was dissolved and sucked the pre-digested food into their proboscis. These insects adapted to the aquarium environment and were able to endure it for six months. Insects began to lay eggs in the adapted aquarium conditions ().The giant predatory aquatic water bug Lethocerus indicus habitat observational study revealed that these insects required a habitat that is swampy and less polluted with specific water parameters. The habitat of this insect’s surrounding area is a thinly populated rural area with less human disturbance and encircled by paddy fields. Such conditions are ideal for their survival in their natural habitat.
Figure 2. Feeding behavior observation in the aquarium. a) attacked at the caudal peduncle and caudal fin region of Trichogaster fasciata. b) attacked at the head region, operculum as well as a lateral line of Puntius species. c) attacked in between the shoulder and head part of Hydrophilus piceus. d) attacked at the caudal fin and lateral portion close to the intestine of Amblypharyngodon mola; e) attacked at the caudal fin, head, and spiny region of Lata fish. f) feeding on freshwater snails by piercing at digestive gland, stomach, anterior kidney, and testis portion.

3.2. Characterization and comparative analysis of the volatile compound from L. indicus insects
L. indicus male insects are preferable entomophagy insects for their aroma and flavor by the local people of northeast India, Manipur. We aim to compare the volatile compound present in both male and female insects. The male and female insects acclimatized in the aquarium conditions were used for the volatile oil extraction. The amount of volatile oil extracted from fried insects was 0.96%/2 g male body weight and 0.47%/5.36 g female body weight. 50 volatile compounds from female insects () and 37 from male insects () were identified using GC-MS analysis. Thirteen of these chemicals are common to both male and female insects, whereas 37 of these compounds are specific to female insects, and 24 of these compounds are specific to male insects (Supplementary Fig. S2). We also identified 13 functionally uncharacterized volatile compounds from male and 19 functionally uncharacterized volatile compounds from female insects. The functionally characterized volatile compounds of male L. indicus were 64.9% and their respective functions are represented in the (Supplementary Fig. S3) whereas 62% of the female volatile compounds are functionally characterized as represented in the (Supplementary Fig. S4). The functionally characterized volatile compounds obtained from male and female insects are used as an ingredient in food as well as cosmetic industry. Some of them are used in the pharmaceutical industry and some other compounds are used as Surfactant, Refrigerant, Heat and cooking etc.
Table 1. Volatile compounds detected from the female L. indicus insects.
Table 2. Volatile compounds detected from the male L. indicus insects.
3.3. Comparative analysis of volatile compounds obtained from L. indicus male and female insects
The most prevalent volatile substances found in the female insects were 4-Octene, 2,6-dimethyl-, [S-(Z)]-with 42.28%, 1-Undecanol (21.6%), 1-Hexadecanol (10.2%) à, à-dimethyl-Palmitoleamide (6.96%). Other volatile substances from female insects were found in the range of 0.06 to 1.73% (). The most prevalent volatile substance discovered in male insects was 2-Hexen-1-ol, acetate, (Z)- with an area percentage of 54.86, followed by cholesterol with 7.83%, 10-Pentadecen-5-yn-1-ol, (E)- with 6.39%, 1-Hexyl-2-nitrocyclohexane with 3.19%, and heptacos-1-ene with 2.55%. Other volatile substances from male insects were found in the range of 0.06 to 1.6% (). The comparative analysis of abundant volatile compounds illustrates that male has higher composition of 2-Hexen-1-ol, acetate, (Z) volatile substance which has been used as ingredient in food and cosmetic industry due to its fruity, apple & waxy oily aroma. The most abundant substance present in female insect 4-Octene, 2,6-dimethyl-, [S-(Z)] function is not reported in the industry whereas its second abundant volatile compounds (1-Undecanol) are used in flavor and cosmetic industry with Citrus, apple & banana aroma.
3.4. Characterization of fatty acid from L. indicus insects
The male L. indicus is more palatable than the female insects. We aimed to compare the Fatty acids present in both male and female insects. The total amount of lipid extracted from the acclimatized fresh L. indicus insects were 0.28%/5.42 g from males and 0.15%/9.48 g from female insects. The extracted lipids profile contained TAG (triacyl glycerides), FFA (free fatty acids), DAG (diacyl glycerides), and MAG (monoacyl glycerides) were observed in the TLC plate (Supplemental Fig. S5). Male and female L. indicus insect’s fatty acid methyl ester (FAME) analysis showed 31 fatty acids from the male and 15 fatty acids from the female. The fatty acids profiles of both male and female insects are represented in (). Insect fatty acids are enriched with saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) containing omega 3 and 6. Both male and female insects detected all four types of saturated fatty acids although only short-chain MUFAs were present whereas PUFA detected from these insects fall into two categories: short-chain and long-chain fatty acids (PUFAs). The important omega −3 fatty acids found among PUFAs in insects are alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), which are essential fatty acids required for humans and animals.
Table 3. Fatty acid composition obtained from L. indicus.
3.5. Comparative analysis of fatty acids obtained from L. indicus male and female insects
FAME-GCMS analysis of female insects reveals a higher concentration of saturated fatty acids than unsaturated fatty acids (). The male and female insects contain 12 common fatty acids with 19 unique fatty acids in male insects and 3 unique fatty acids in female insects (). The abundance of SFA fatty acids () in male and female insects have palmitic acid (C16) whereas elaidic acid(C18:1) MUFA and the PUFA, omega-6 (C22:5) docosapentaenoic acid (DHA) are present in female insects. The MUFA (C16:1) palmitoleic acid and PUFA omega-6 (18:2), linoleic acid, and omega −3 (C21:3).Docosapentaenoic acids are the abundant fatty acids present in male insects. The abundant fatty acids MUFA and PUFA fatty acids detected in male insects are not present in female insects.
4. Discussion
The volatile compounds found in L. indicus are employed as a component in the manufacture of lubricants, emulsifiers, solvents, rubber surfactants, paints, coatings, household detergents, and products for the food, cosmetics, and pharmaceutical industries [Citation22,Citation23]. The chemical 1-Hexyl-2-nitrocyclohexane, which has neuroactive, anti-inflammatory, and analgesic properties, is also present in both male and female insects [Citation22,Citation23]. Nonacos-1-ene, a female-specific chemical, is one of the pheromone elements utilized for insect communication [Citation24], and 2-Heptadecenal, a substance with lipophilic characteristics aids in the production of propolis bee glue is also detected [Citation25]. Among the unique volatile compounds of male insects, indole has been used as a promising agent for the treatment of antimicrobial activity, malaria, diabetes, cancer, migraines, convulsions, and hypertension [Citation26–28]. 2-Methyl-3-(2,2-dimethyl propyl)-butadiene, present in the insect is reported as having antioxidant and antibacterial properties [Citation29].
The most pungent volatile chemicals detected from Thailand male L. indicus insects were E-2- (E)-2-hexenyl acetate and (E)-2-hexenyl butanoate [Citation9]. Among these two volatile compounds (E)-2-hexenyl acetate has been used as a flavoring agent commercially in Thailand [Citation30]. The major volatile compound detected from the Indian male insect sample was 2-Hexen-1-ol, acetate whereas the female has 4-Octene, 2,6-dimethyl-, [S-(Z)]. The number of volatile compounds detected in the present study was 50 for females and 37 for male L. indicus insects, whereas the Thailand study reported the identification of only 23 volatile compounds.
Previous studies on L. indicus insects from Thailand showed the presence of four SFA, two MUFA fatty acids and three PUFA fatty acids [Citation10] (Supplemental Table S3) whereas the FAME analysis performed on L. indicus collected from North-east India, obtained 17 saturated fatty acids, 4 MUFA fatty acids, and 13 PUFA fatty acids from both male and female insects. Fatty acids obtained from L. indicus could be bioaccumulated from their diet available in their aquatic ecosystem [Citation31]. C18:2 Fatty acids might have synthesize in their body as insect posses Δ12 desaturase which convert (C18:1) fatty acid to (C18:2) fatty acids, which is essential for the entire animal [Citation31,Citation32].
The fatty acids derived from insects have been used in cosmetic and personal care products. Lauric acid (C12:0) has been used in 92 products; myristic acid has been used in 45 personal care products; palmitic acids (C16) have 255 products and oleic acid (C18:1) has been used in 367 cosmetic and personal care products [Citation8]. In the present scenario, the insect as a source of fatty acids has become the most promising solution for use in cosmetic and personal care applications [Citation33]. Due to ethical issues, animal-source fatty acids were replaced by plant-source fatty acids, but there were some drawbacks, such as the use of valuable land for non-food use, transport cost, and the release of pollutants throughout the process. So, insects can be an alternative resource for industries [Citation33].
The saturated and unsaturated fatty acids used in the cosmetic industry were detected in male insect samples collected from India whereas myristic acid is not found in female insects and samples from Thailand present only C16 and C18:1 fatty acids [Citation10]. We have demonstrated that male from various locations possesses different fatty acid and volatile chemical compositions. Another finding is that females synthesize unique volatile fatty acids compared to male insects.
L. indicus insects also posses fatty acids such as EPA and DHA which have anticancer characteristics [Citation34]. ALA supports the heart, immunological system, and nervous systems. Additionally, EPA has anti-inflammatory, anti-atherosclerotic, and anti-depressive effects [Citation35]. DHA makes up 8% of the brain weight and supports brain function and helps in brain development. This fatty acid also supports the prevention of type 2X-linked adrenoleukodystrophy [Citation35,Citation36]. Both male and female insects contain omega-6 fatty acids, which also have anti-inflammatory, anti-cancer, and immune-regulatory effects and support brain development [Citation35,Citation36]. All these important fatty acids found in L. indicus suggest that these insects are medically important entomophagy insects.
The consumption of aquatic species from the polluted habitat causes harm to human health due to the intake of heavy metals (Pb, and Cd) that are accumulated by the insects [Citation37]. The long-term accumulation of Cd causes cancer and is harmful to the skeletal and respiratory systems of the human body whereas Pb causes harm to the gastrointestinal, nervous, hematologic, cardiovascular, and renal systems. Arsenic (As) is also hazardous to the neurological and pulmonary systems and has been linked to skin, lung, liver, and bladder cancer. However, it is important to prevent water pollution to protect aquatic species [Citation38]. The population of L. indicus is also disappearing from the habitats of Manipur Lake has an impact on the ecosystem and food culture of the people which is inherited from the old-age tradition of consuming giant water bugs as their food. Aquaculture has been contributing to the supply of fish and shellfish in the market with a consistent nutritional profile than the wild fishes [Citation39]. Mass cultivation under controlled conditions will also be safer for human health as compared to insects collected from polluted areas that may accumulate heavy metals which are hazardous to human health. The observational study data for L. indicus under aquarium condition showed that this insect can be cultivated in aquariums safely without any contamination from harsh pollutants.
Another significance of this insect is its economic value to local ladies who harvest them and sell at the market at Rs.30 per insect. It became a livelihood during the season from January to April. Once the rainy season arises in June, this insect are not able to be caught due to the increase in water level in its habitat. Furthermore, the Lethocerus genus used to fly from August to November. It is a highly demanded insect on the market because of its aroma, flavor, and therapeutic purposes. However, mass production of these insects in pollutant-free water is important to fulfill the demand for these insects in the market.
5. Conclusion
The present study highlighted that the volatile oil extracted from male insects is rich in volatile flavoring compounds 2-Hexen-1-ol, acetate with 54.86% a banana-like odor, Monounsaturated fatty acids, and Polyunsaturated fatty acids unique to male insects. The most abundant volatile compound found in female insects was 4-octene, 6, dimethyl with 42.28% whereas 2-Hexen-1-ol, acetate with 0.94% was detected. The pharmaceutical, cosmetic, and personal care industries, as well as the detergent and surfactant industries, use saturated and unsaturated fatty acids. The functional characterization of 32 volatile compounds is another area for future research, to aid the scientific community and better comprehend the vital roles these compounds play in nature. The mass cultivation of these entomophagy insects has the potential to be a sustainable bioresource that is very valuable for the bioeconomy approaches of NER regions and the conservation of this species.
Credit authorship contribution statement
RM: Writing – original draft and experimental design of the research work, SBU: Review, editing, and supervision of experimental work, AB: review& editing, supervision&guiding the feeding behavior assay in the aquarium. VR: Supervision. YR: Review & editing, supervision& guiding on volatile compound work.
Supplemental Material
Download MS Word (3 MB)Acknowledgments
The authors would like to thank the Director, Institute of Bioresources and Sustainable Development for providing support and Dr. Muthu Arumugam, Microbial Process Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology, Trivandrum for extensive support for GC-MS analysis of the fatty acid profile of insect oil and IBSD manuscript No: IBSD/MS/2020/01/112.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21655979.2023.2252669
Additional information
Funding
References
- Dettner K. Defenses of water insects. In: Del-Claro K Guillermo R, editors. Aquatic insects. Springer, Cham: Springer International Publishing; 2019. p. 191–468. doi: 10.1007/978-3-030-16327-3_9
- KovacD M, Maschwitz U. Secretion-grooming in the water bug plea minutissima: a chemical defence against microorganisms interfering with the hydrofuge properties of the respiratory region. Ecol Entomol. 1989;14(4):403–411. doi: 10.1111/j.1365-2311.1989.tb00942.x
- Melo-ruizVE. Macronutrient composition of giant water bug (Lethocerus sp.) edible insects in Mexico and Thailand. JAgric Sci Technol. 2016:349–354. doi: 10.17265/2161-6256/2016.05.007
- Shantibala T, Lokeshwari RK, Devaraj H. Nutritional and antinutritional composition of the five species of aquatic edible insects consumed in Manipur, India. JInsectSci. 2014;14(1):1–10. doi: 10.1093/jis/14.1.14
- Kapesa K, Devi DW, Bonysana KR. Rajashekar. Anthropo-entomophagy and ethno-entomology among the ethnic mao-naga and poumai-naga tribes of Manipur, Northeast India. JInsectsFood Feed. 2020;6(5):507–514. doi: 10.3920/JIFF2020.0012
- Mishra N, Hazarika NC, Narain K, et al. Nutritive value of non-mulberry and mulberry silkworm pupae and consumption pattern in Assam. India Nutr Res. 2003;23(10):1303–1311. doi: 10.1016/S0271-5317(03)00132-5
- Zhao M, Wang CY, Sun L, et al. Edible aquatic insects: diversities, nutrition, and safety. Foods. 2021;10(12):3033. doi: 10.3390/foods10123033
- Franco A, Salvia R, Scieuzo C, et al. Lipids from insects in cosmetics and for personal care products. Insects. 2021;13(1):41. doi: 10.3390/insects13010041
- Kiatbenjakul P, Intarapichet KO, Cadwallader K. Characterization of potent odorants in male giant water bug (Lethocerus indicus lep. and serv.), an important edible insect of Southeast Asia. Food Chem. 2015;168:639–647. doi: 10.1016/j.foodchem.2014.07.108
- Siriamornpun S, Li DUO, LI D. The polyunsaturated fatty acid content of edible insects in Thailand. J Food Lipids. 2006;13(3):277–285. doi: 10.1111/j.1745-4522.2006.00051.x
- Lorrette B, Sanchez L. New lipid sources in the insect industry, regulatory aspects, and applications. Oilseeds Fats, Crops Lipids (OCL). 2022;29(22):7. doi: 10.1051/ocl/2022017
- Chown SL, Terblanche JS. Physiological diversity in insects: Ecological and evolutionary contexts. Adv In Insect Physiol. 2006;33:50–152. doi: 10.1016/S0065-2806(06)33002-0
- Kim JH, Kim EY, Chung KJ. Mealworm oil (MWO) enhances wound healing potential through the activation of fibroblast and endothelial cells. Molecules. 2021;26(4):779–790. doi: 10.3390/molecules26040779
- Delicato C, Schouteten JJ, Dewettinck K, et al. Consumers’ perception of bakery products with insect fat as a partial butter replacement. Food Qual Prefer. 2020;79:0950–3293. doi: 10.1016/j.foodqual.2019.103755
- Ummalyma SB, Sirohi R, Udayan A, et al. Sustainable microalgal biomass production in food industry wastewater for low-cost biorefinery products: a review. Phytochem Rev. 2022. doi: 10.1007/s11101-022-09814-3
- APHA. Standard methods for the examination of water and wastewater. 23rd ed. Washington DC: American Public Health Association; 2017.
- Koubaa M, Mhemdi H, Barba FJ, et al. Seed oil extraction from red prickly pear using hexane and supercritical CO 2: assessment of phenolic compound composition, antioxidant and antibacterial activities. J Sci Food Agric. 2017;97(2):613–620. doi: 10.1002/jsfa.7774. PMID: 27106858.
- Singh NB, Devi ML, Biona T, et al. Phytochemical composition and antimicrobial activity of essential oil from the leaves of Artemisiavulgaris L. Molecules. 2023;28(5):2279. doi: 10.3390/molecules28052279
- Mayanglambam S, Raghavendra A, Rajashekar Y. Use of ageratina adenophora (Spreng.) essential oil as insecticidal and antifeedant agents against diamondback moth, plutella xylostella (L.). J Plant Dis Prot. 2022;129:439–448. doi: 10.1007/s41348-022-00573-z
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/y59-099
- Ummalyma SB, Sukumaran KR. Cultivation of the freshwater microalga chlorococcum sp. RAP13 in seawater for producing oil suitable for biodiesel. J ApplPhycol. 2015;27(1):141–147. doi: 10.1007/s10811-014-0340-4
- Rathinavel T, Ammashi S, Muthusamy G. Screening of anti-inflammatory phytocompounds from crateva adansonii leaf extracts and its validation by in silico modeling. J Genet Eng Biotechnol. 2018;16(2):711–719. doi: 10.1016/j.jgeb.2018.03.004
- Sarmah M, Bhattacharyya B, Bhagawati S, et al. Nutritional composition of some commonly available aquatic edible insects of Assam, India. Insects. 2022;13:976. doi: 10.3390/insects13110976
- Brei B, Edman JD, Gerade B, et al. Relative abundance of two cuticular hydrocarbons indicates whether a mosquito is old enough to transmit malaria parasites. J Med Entomol. 2004;41(4):807–809. doi: 10.1603/0022-2585-41.4.807
- Ucar AKD, Gecibesler HI, Sudagidan HI, et al. Bingöl Propolis İzolatlarının Biyolojik Aktivitesinin, Lipofilik ve Uçucu Organik Bileşenlerinin Belierlenmesi. Türk Doğa ve Fen Dergisi. 2020;9(Özel Sayı):92–102. doi: 10.46810/tdfd.776424
- Ramesh D, Joji A, Vijayakumar BG, Sethumadhavan A, Mani M. Indole chalcones: Design, synthesis, in vitro and in silico evaluation against mycobacterium tuberculosis. Eur J Med Chem. 2020;198:112358. doi: 10.1016/j.ejmech.2020.112358
- Qin R, You FM, Zhao Q, et al. Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: from molecular mechanisms to potential therapeutic targets. J Hematol Oncol. 2022;15(1):133. doi: 10.1186/s13045-022-01350-z
- Kumari A, Singh RK. Medicinal chemistry of indole derivatives: Current to future therapeutic perspective. Bioorg Chem. 2019;89:0045–2068. doi: 10.1016/j.bioorg.2019.103021
- Bhardwaj K, Sharma R, Cruz-Martins N, et al. Studies of phytochemicals, antioxidant, and antibacterial activities of Pinus gerardiana and Pinus roxburghii seed extracts. BioMed Res Int. 2022;10:1–10. doi: 10.1155/2022/5938610
- Mahattanatawee K, Luanphaisarnnont T, Rousseff R. Comparison of aroma character impact volatiles of thummong leaves (Litsea petiolata hook. f.), mangdana water beetle (Lethocerus indicus), and a commercial product as flavoring agents in Thai traditional cooking. J Agric Food Chem. 2018;66(10):2480–2484. doi: 10.1021/acs.jafc.7b01499
- Blomquist GJ, Borgeson EC, Vundla M. Polyunsaturated fatty acids and eicosanoids in insects. Insect Biochem. 1991;21(1):99–106. doi: 10.1016/0020-1790(91)90069-Q
- Scharnweber K, Chaguaceda F, Dalman E, et al. The emergence of fatty acids—aquatic insects as vectors along a productivity gradient. Fresh Water Biol. 2020;65(3):565–578. doi: 10.1111/fwb.13454
- Verheyen GR, Ooms T, Vogels L, et al. Insects as an alternative source for the production of fats for cosmetics. J Cosmet Sci. 2018;69(3):187–202.
- Calder PC. ‘Polyunsaturated fatty acids and inflammation’. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):197–202. doi: 10.1016/j.plefa.2006.05.012. PMID: 16828270.
- Nowak JZ. Anti-inflammatory pro-resolving derivatives of omega-3 and omega-6 polyunsaturated fatty acids. Postepy Hig Med Dosw. 2010;64:115–132. PMID: 20354260.
- Hwang JK, Yu HN, Noh EM, et al. DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-γ/NF-κB pathway in MCF-7 cells. Oncol Lett. 2017;13(1):243–249. doi: 10.3892/ol.2016.5382
- Maneechan W, Prommi TO, Campus KS, et al. Nutrient composition and bioaccumulation of an edible aquatic insect. bioRxiv Preprint. 2021. doi: 10.1101/2021.12.26.474203
- Hung DQ, Nekrassova O, Compton RG. Analytical methods for inorganic arsenic in water: a review. Talanta. 2004;64(2):269–277. doi: 10.1016/j.talanta.2004.01.027
- Nettleton JA, Xler J. Nutrients in wild and farmed fish and shellfish. J Food Sci. 2017;57(2):257. doi: 10.1111/j.1365-2621.1992.tb05470.x



