 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
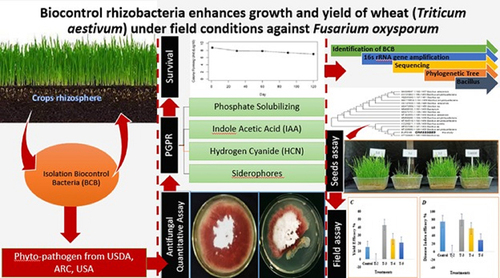
The current study aimed to identify the survival of bio-control bacteria with antifungal activity against Fusarium oxysporum and assess their growth promoting activity in wheat crop field conditions. To evaluate the fungicidal activities of isolated bacteria using the dual culture method, both qualitative and quantitative bioassays were performed. Plant Growth Promoting activities such as Indole 3-Acetic Acid (IAA), phosphate solubilization, Hydrogen cyanide (HCN), and Siderophore production were assessed for three biocontrol bacterial isolates (BCB 07, BCB16, and BCB 83) out of 180 with 70% antagonistic activity against Fusarium oxysporum. Chitinase, protease, and cellulase interaction in isolates was also tested. BCB16 was selected as it had 70% antagonist activity against F. oxysporum but also had the highest PGPR (Plant Growth Promoting Rhizobacteria) traits when compared to the other two isolates. BCB16 was also tested for survival in talc powder and in wheat crop field conditions. Even after 4 months in talc powder, the survival rate remained stable. In a wheat crop field, BCB16 reduced the disease incidence of Fusarium oxysporum by 54.38%. When compared to fungus alone treatment, BCB16 increased average yield by 57% alone and 32% in challenged conditions. BCB16 was identified molecularly using the 16s rRNA gene. Bacillus amyloliquefaciens shared 97% of the deduced sequence. The sequence was submitted to genbank and assigned the accession number OM333889. Bacillus amyloliquefaciens has the potential to be used in the field as an alternative to synthetic fungicides against Fusarium oxysporum.
GRAPHICAL ABSTRACT
HIGHLIGHTS
Isolation and characterization of biocontrol bacteria.
Molecular Identification of efficient biocontrol bacteria.
Survival of biocontrol bacteria in talc powder as carrier material.
The Bacillus amyloliquefaciens has plant growth-promoting characteristics.
B. amyloliquefaciens reduced the disease incidence of F. oxysporum by 57% in field trials.
Introduction
Wheat is the world’s third most important cereal crop, trailing only rice and corn while Wheat was ranked first in Pakistan’s essential diet, followed by rice and maize. Wheat crops are plagued by serious diseases at all stages of development, with nearly 200 different types of wheat diseases reported worldwide [Citation1].
Phytopathogens are threatening agricultural resources, with fungal pathogens being the most serious [Citation2]. Pathogenic fungi play a role in the global loss of agricultural yield. Pathogenic fungi cause a variety of diseases in plants, affecting both the quality and quantity of crop yield [Citation3]. Pathogenic fungi can have an impact on seed germination, plant vigor, and disease in seedlings and plants. One of the deleterious fungi, Fusarium is a soil-borne fungus that belongs to the Ascomycota family. This fungus has a negative impact on the quality and quantity of various crops. They are primarily pathogens that are difficult to control. Fusarium oxysporum, for example, affects the plant in such a way that it produces mycotoxins, rendering the grain harmful to human and animal health [Citation4]. F. oxysporum is known to cause Fusarium wilt diseases in many important crops by entering through the roots and interfering with the plant’s vascular system.
Chemical fungicides are the most commonly used method for controlling fungal pathogens in the field. The use of chemical fungicides for an extended period of time not only harms humans, animals, and the environment, but it also exacerbates the problem of fungicide-resistant pathogens [Citation5]. Nowadays, in the agricultural sector, much more emphasis is placed on using alternative methods of crop protection from phytopathogens. Plant growth promoting bacteria are the most effective bio-fungicides among the various types of bio-fungicides because they not only inhibit the onset of plant pathogen-caused illnesses but also enhance plant development. To avoid the negative effects of synthetic chemicals, bio-fungicides are commonly used as alternatives to synthetic chemicals in the control of diseases caused by phytopathogens. Bio-fungicides are unquestionably more beneficial because they are nonpolluting, less expensive, and biodegradable [Citation6].
Bacillus genus bacteria have been shown to perform well as biocontrol agents (BCAs) due to their ability to produce a wide range of molecules capable of inhibiting fungal growth [Citation7]. Agha et al. [Citation8] demonstrated that B. Velezensis RC 218 bioformulate was effective in reducing Fusarium head blight caused by F. graminearum in green house and field conditions in wheat cultivar. Jangir et al. [Citation9] showed the consortium of B.subtilis and T. harzianum not only suppresses Fusarium wilt in S. lycopersicum but also improves soil health. Parikh et al. [Citation10] demonstrated that use of B. simplex and B. ambifaria significantly reduced the infection of F. graminearum and F. oxysporum in greenhouse of corn, soybean, and wheat crop. When compared to exotic microorganisms, biological control agents may have a better chance of establishment and effective pathogen control if they are native to the soil [Citation11].
The study’s goal was to identify endogenous bacteria that not only effectively control F. oxysporum in laboratory and field conditions, but also have plant-promoting properties and have a longer shelf life in carrier material. F. oxysporum is considered one of the notorious fungal phyto-pathogen.
Material and methods
1. Isolation of biocontrol rhizobacteria
A total of 180 distinct rhizosphere were gathered from various crops in Baluchistan, Pakistan, including tomatoes, wheat, maize, barley, brassica, and cotton. The samples were aseptically transported to Applied Biotechnolgy Laboratory, BUITEMS (Balochistan University of Information Technology Engineering and Management Sciences) for isolation of biocontrol bacteria. The bacteria from the rhizosphere were isolated according to the method described by Ramzan et al. [Citation12].
2. Screening of biocontrol isolates
The bacteria were chosen based on two criteria: antagonistic activity against F. oxysporum of at least 70% and a high number of plant growth-promoting qualities. The fungal pathogen was available in the Applied Biotechnology Laboratory, BUITEMS. The antifungal activity of bacterial isolates was evaluated quantitatively and qualitatively, and the zone of inhibition was measured using the dual culture technique against F. oxysporum as described by [Citation13]. Briefly, the fungus disc was placed in the center of a petri dish containing LB (Luria-Bertani) medium for the qualitative screening of antagonistic bacteria. The fungus was allowed to grow for 3 days at 25°C. After 3 days, bacterial isolates were placed on the petri dishes periphery and placed in an incubator at 28°C for 3 days.
The percentage inhibition against a fungal pathogen was determined for the bacterial isolates screened in a qualitative assay. The [Citation14] method is used for quantitative evaluation. The bacterial isolates were co-inoculated with Fusarium oxysporum at 2.5 m intervals for 5 days at 28°C. In a control, the pathogen was inoculated in the center of the plate and kept at 28°C for 5 days. The experiment was carried out in triplicate. The percentage inhibition was determined using the following formula:
3. Molecular identification
The BCB16, an isolate that met the selection criteria for further research, was obtained. The isolate was molecularly characterized using 16s rRNA. The genomic DNA was isolated using the DNA purification kit thermo scientific (Catlogue number K0721) according to the manufacturer’s instructions. The primers 1492 R (5′- GGTTACCTTACGACTT −3′) and 27F (5′- AGAGTTTGATCMTGGCTCAG −3′) from the universal set were used. The amplicon was purified and sequenced at the Macrogen sequencing facility in Korea. The resulting sequence was compared to the NCBI Genbank database. In Molecular Evolutionary Genetic Analysis (MEGA X version 10.2.2), the phylogenetic tree was built using the neighbor-joining method.
4. Evaluation of plant growth promoting activity of biocontrol isolates
The Plant Growth Promoting Rhizobacteria activities of antifungal isolates were evaluated. For the solubilization of inorganic phosphate, National Botanical Research Institute Phosphate (NBRIP) medium was used [Citation15]. The colorimetric method was used to calculate the production of Indole 3-Acetic Acid with 100 ppm of L-tryptophan [Citation16]. Chrome azurol S solution was used to identify the siderophore [Citation17]. The Lorck method of picrate test was used to assess Hydrogen cynaide [Citation18].
5. Hydrolytic activity
The antagonistic bacterial isolates were tested for protease, cellulase, and chitinase activity. Protease activity was measured using skimmilk agar plates [Citation19]. Carboxymethyl cellulose was used in Carboxy Methyl Cellulose (CMC) agar medium to test the cellulase activity of the isolates as described by [Citation20], with a slight modification in that Iodine was used as a coloring agent instead of Congo red. Cellulase activity was determined using the dinitrosalicylic acid (DNS) reagent by measuring the amount of reducing sugars released from CMC in a sodium citrate buffer at pH 4.8, as described by [Citation21]. Colloidal chitin was used as a substrate to test the isolates’ chitinase activity using a colorimetric method, and DNS was used to determine the concentration of reducing sugars [Citation22].
6. Survival of biocontrol isolates in talc powder
BCB16, the chosen isolate, was stored in talc powder for 4 months to test its survival. Talc powder was used for survival because it is a low-cost carrier material that can be used in commercial biofungicide production [Citation2]. Talc powder was autoclaved for 15 min at 121°C and 15 psi to remove any contamination. BCB16 isolates were grown in LB broth for 72 h at 32°C in a shaking incubator at 100 rpm. Bacterial LB growth was then combined with autoclaved talc powder for storage. To assess BCB16 survival, the growth of a bacterial isolate in talc powder was monitored every 30 days for 4 months.
7. Seed germination assay
Seed germination assay was performed by using the method of Fahsi et al. [Citation23] with slight modification. Wheat seeds (cv. Zarlasht) were surface sterilized with 5% sodium hypochlorite (4 min) and 70% ethanol solution (3 min), rinsed thoroughly 5 times with single sterile distilled water. Inoculum for biocontrol strain (BCB16) was prepared in LB liquid medium - formulated in talc powder and the final concentration of BCB16 in talc powder was 107 CFU/gram and fungal (Fusarium oxysporum) spores were extracted from 7 days old PDA and the final concentrations were maintained 106 Colony Forming Unit (CFU)/ml in sterile distilled water, vortexed for 20s, and used for seed inoculation. A total of 100 seeds were soaked in 50 ml for 30 min under a gentle shaking at 60 rpm, four treatment suspensions were prepared; control-sterile seeds in sterile d/w. Treatment-1 (T1)-talc formulated biocontrol strain (BCB16). T2- phytopathogen (F. oxysporum); T3- talc formulated biocontrol (50%) and phytopathogen (F. oxysporum 50%). For germination, seeds were incubated in a dark space for 72 h in sterile plastic pots containing 4 layers of moist tissue papers, and further left at room temperature in a day/night cycle each group were moist with sterile d/w daily. The disease index was recorded by using the method of [Citation24]. The disease severity index of F. oxysporum on germinated wheat plants was defined as the percentage of leaf area that was in a diseased state, where 0 = no disease symptoms, 1 = 0.1–5%, 2 = 5.1–20%, 3 = 20.1–40%, and 4 = 40.1–100%. The disease severity value per plant was calculated using this formula: Disease severity (%) = {(∑ [number of diseased leaves × disease severity index])/(4 × number of total leaves)} × 100. The disease control value was calculated as follows: Disease control (%) = ([A − B]/A) × 100, where A is the disease severity triggered by pathogen inoculation alone and B is the disease severity after incurring the various treatments. The fresh weight, and dry mass was measured at day 29. The fresh weight, and dry mass was measured at Day 29. The germination rate and vigor index were calculated according to the following equations [Citation25]:
8. Pathogenicity test
According to Dyer and Ingram [Citation26] in vitro pathogenicity tests of bio fungal isolates were done on wheat crops (cultivar Zarlasht) against F. oxysporum. Wheat seeds were rinsed with autoclaved distilled water after being sterilized with 5% sodium hypochlorite. In the lab, the seedlings were cultivated in sterilized pots with filter paper soaked in 10 ml autoclaved distilled water and sealed to maintain a constant relative humidity of 100%. Wheat germinated seeds were transferred to a hydroponic culture containing Hoagland solution [Citation27]. The conidial suspension of a fungal culture grown on Potato Dextrose Agar (PDA) media for 6 days is diluted with water to a concentration of 6 × 106 spores/ml counted by a hemocytometer. The hydroponic culture is uniformly inoculated with the spore suspension. After 25 days of wheat seed germination, the wheat plants were removed from the hydroponic culture and their pathogenicity was determined. The experiment was repeated three times, with the control being uninoculated with fungal spores.
The average of plant height, fresh weight, dry mass, disease index and vigor index was calculated for both control and treatment of hydroponic culture. Vigor index was calculated as Vigor index = (mean root length + mean shot length) germination (%). Along with the disease, symptoms of treatment and control of wheat was also analyzed according to following scale of rating.
Plants rating scale 0= no symptoms, 1 = <25% leaves with symptoms, 2 = 26–50% of leaves with symptoms, 3 = 51–75% of leaves with symptoms, 4 = 76–100% leaves with symptoms. The disease index was calculated accordingly.
Disease index = [∑ (rating x number of plants rated)/Total number of plants x highest rating] x 100
9. Inoculum preparation and seed bacterization
The selected isolate BCB16 was grown overnight in LB broth medium and serially diluted to a concentration of 109 CFU/ml. Seed bacterization was carried out in accordance with Einloft et al. [Citation28], with minor modifications. For each treatment, 100 surface-sterilized wheat seeds were submerged in 250 ml bacterial inoculum (BCB16) in an orbital shaker for 2 h at 120 rpm. In the presence of 1% carboxymethyl cellulose (CMC), the seeds were bacterized. The excess medium was removed, and the bacterized seeds were immediately planted in the field.
10. Field experiment
The bacterized wheat seeds were used for field trial and phytopathogenic fungus F. oxysporum in a wheat as mentioned by Palazzini et al. [Citation29] with slight modifications. Prior to wheat crop cultivation, soil and water analyses were performed at the Agriculture Research Institute on Saryab Road in Quetta. Fertilizers were applied to the wheat crop based on that assessment. The location for the field experiment was chosen as BUITEMS. The wheat was planted in the field in December 2020. For the field trial experiment, 1 × 1-m plots were prepared in randomized complete block design (RCBD) with five replications. The soil in the field chosen for testing was silt loam in texture, with the following characteristics: pH 8.02; EC 7.21 Dsm−1; organic matter 0.67%; total nitrogen 0.03%; available phosphorous 3.18ppm; and available potassium 91ppm.
The cultivated area was designed so that there was total 5 treatments, which are as under, 1) T-1 Control (untreated seeds; negative control); 2) T-2. Fungi (F. oxysporum, treated seeds; positive control); 3) T-3 (BCB16 treated seeds only); 4) T-4 Chemical fungicides (Cabrio top); 5) T-5 Fungus (F. oxysporum) + BCB16 (bacteria treated seeds).
11. Statistical analysis
The data were analyzed statistically by applying one-way ANOVA. Significance was evaluated and compared through Duncan test, with values a, b, c and d, which depicts the significance of treatments with in groups. IBM SPSS Statistics 22 for Windows was used for statistical analysis. The treatment differences were statistically analyzed using analysis of variance (ANOVA) and then by Duncan’s multiple range test at p < 0.05.
Results
The goal of this research was to determine the antifungal activity of a biocontrol isolate under field conditions against one of the most notorious fungus species, Fusarium oxysporum. The selected isolate was further analyzed for PGPR activities, and the biocontrol isolate’s survivability was tested in talc powder, a low-cost carrier medium.
Screening of biocontrol bacteria
The biocontrol bacterial were screened based on two criteria i.e., ≥70% antagonistic activity against F. oxysporum and possessing maximum traits of plant growth promoting activity from tested traits. Initially, 17 bacterial isolates, out of 180, showing some sort of antagonistic against F. oxysporum were selected for further evaluations. From 17 isolates 3 were having at least of 70% antagonistic activity against the fungus were carried forward for further experiments as shown in .
Figure 1. The antifungal activity of selected isolated strains a) qualitative assay of biocontrol isolates against F. oxysporum; b) graphical representation of a quantitative biocontrol isolate inhibiting F. oxysporum by at least 70%. All experiments were carried out in triplicate. (BCB07: Bacillus velezensis), (BCB16: Bacillus amyloliquefaciens) and (BCB83: Bacillus subtilis).

Plant growth promoting properties
Plant growth promoting (PGP) properties such as in-organic phosphate solubilization, indole 3-acetic acid, hydrogen cyanide, and siderophore production were assessed in the selected bacterial isolates. None of the three isolates showed a clear zone on NBRIP plates for the P-solubilzation assay. In the presence of L-tryptophan, two isolates (BCB16 and BCB 83) produced indole 3-acetic acid by changing the color of the reaction to red in the presence of Salkowski reagent while BCB 83 had no effect on the reagent’s color. For screening siderophore producing biocontrol isolates, chrome azurol S (CAS) medium was used. Only one isolate (BCB16) produced siderophore, as evidenced by the formation of an orange color zone around the colonies. All three of the isolates tested positive for HCN production. The evaluation of selected biocontrol isolates for Plant Growth Promoting activities is summarized in .
Table 1. Plant growth-promoting activity of the selected biocontrol isolates. PS: phosphate solubilization; IAA: indole 3-acetic acid; HCN: hydrogen cyanide. Positive activity is shown by (+) sign and negative activity by (-) sign.
Hydrolytic enzyme activity
The hydrolytic enzyme activity of the three biocontrol strains was measured, including cellulase, chitinase, and protease. The hydrolytic enzyme activity of the tested biocontrol strains is depicted in . Cellulase was produced by all of the tested strains, but chitinase activity was not detected in any of them when tested on colloidal chitin. To confirm the protease activity, a clear zone was detected in two isolates (BCB 07 and BCB16) in skim milk medium. shows that the clear zone of BCB16 for cellulase and protease activity is significantly better than that of other biocontrol isolates.
Molecular identification
The molecular identification of the selected biocontrol isolates was carried out using 16srRNA gene amplification. The resultant sequences were compared for homology using BLASTn of NCBI program (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). Phylogenetic dendrograms were prepared using MEGA X version 10.2.2 Neighbor-joining method. After BLASTn, the BCB16 16srRNA sequence showed 97% homology with Bacillus amyloliquefaciens. The BCB16 sequence was deposited in Genbank NCBI and assigned the accession number OM333889. The phylogenetic tree of BCB16 is shown in .
Survival of biocontrol bacteria in talc powder
The survival of the biocontrol bacterial isolate is critical to the product’s viability. Different cost-effective carrier materials can be used in industrial production. We used talcum powder because it is inexpensive and widely available. The autoclaved talcum powder was mixed with 40% overnight biocontrol isolate (BCB16) and serially diluted for 120 days. As shown in , the number of bacteria dropped slightly after 120 days, i.e., 7 × 107 CFU/g compared to day zero, 8.3 × 108 CFU/g.
Seed germination assay
BCB16 is the most effective inducer of seed germination and phytopathogen inhibitor (F. oxysporum). We investigated the effect of strain BCB16 on wheat seed germination in this section. In comparison to non-inoculated seeds and phytopathogen-inoculated treatment (F. oxysporum), bacterization of wheat seeds by the strains significantly increased germination rate, vigor index, fresh and dry mass, and inhibited the effect of phytopathogen (). At Day 29, a significant difference in shoot length, root length and root numbers were observed and no significant difference in leaf number was recorded by ANOVA at p < 0.05. In addition, a significance difference effectiveness (84.7%) was noted by controlling the disease severity with comparison to phytopathogen inoculate seeds. When compared with positive control (fungi) the mean efficacy was recorded 84.7% for the strain BCB16 and 72.5% effective with comparison to negative control .
Figure 5. a. Seed germination (wheat cv. Zarlasht). (T-1) seed treated with strain BCB16; (T-2) seeds treated with phytopathogen F.Oxysporum spores; (T-3) seeds treated with strain BCB16 and F. oxysporum; (control) seeds treated with sterile distilled water. b) (i)vigor index; (ii) fresh weight and dry mass; (iii) leaves and root numbers; (iv) disease index; (v) mean efficacy. Letter on the bar indicate significance differences between treatments using analysis of variance (ANOVA) and subsequently by Duncan’s multiple range test at p < 0.05.

Pathogenicity test
In vitro pathogenicity test of bio fungal isolates was performed on wheat against F. oxysporum. Wheat seeds were germinated and transferred to hydroponic containing Hoagland solution. Control treatment was kept untreated in separate hydroponic container while the F. oxysporum treated wheat sees were allowed to grow for 25 days and later fresh and dry weight, number of leaves and roots vigor index and disease index were noted down. shows the pathogenicity test of F. oxysporum on wheat, while shows quantitative data of pathogenicity test.
Figure 6. Pathogenicity test of effect of Fusarium oxysporum on wheat cv. Zarlasht. a) control wheat plants without fungus treatment and b) pathogen (F.Oxysporum) at 106 spores/ml treated plants.

Table 2. The data after pathogenicity test was analyzed statistically by applying one-way ANOVA, average values of each treatment. Significance was evaluated to compare significance, through Duncan test. Different letters indicate significant difference among the groups (control vs fungus treated).
Field trial of biocontrol bacteria against pathogenic fungi on wheat crop
The experiment was conducted to see the effect of biocontrol isolate BCB16 in field condition against F. oxysporum on wheat crop. The bacterized seeds with 1% CMC were planted in treatments with bacterial inoculation while for all other treatment surface-sterilized seeds were placed on sterile water for the same amount of time as for bacterial treatments. In the wheat field, the isolated bacteria were treated with the phytopathogenic fungus F. oxysporum. The fungal spores were adjusted to 106 spores/ml and sprayed on plantlets after few days of germination. Prior to wheat crop cultivation, soil and water analyses were performed at the Agriculture Research Institute Saryab road in Quetta, Pakistan. The isolated bacteria BCB16 were applied to wheat in a field experiment to combat the phytopathogenic fungus F. oxysporum as mentioned in .
Figure 7. The efficacy of biocontrol bacteria (BCB16) against phytopathogenic fungi in a field trial. (a) efficacy of vigor index; (b) efficacy of dry mass; (c) yield efficacy and (d) disease index efficacy. The experiments is performed in triplicate.

In field experiment, the effects of phytopathogenic fungi and chemical fungicides were compared. In field studies, the following results show that bacterial isolates have a strong inhibitory effect on F. oxysporum. shows the field data of BCB16 on F. oxysporum on wheat crops.
Table 3. Field data from BCB16 on wheat crops against F. oxysporum show a significant reduction in disease index when compared to the positive control. The similarity of the letters between the groups indicates a non-significant difference.
The shoot length, root length, and germination percentage are all included in the vigor index. Barik et al. [Citation30] formula is used to calculate the vigor index. The vigor index at alpha p < 0.05 was significantly better of biocontrol treated plots compared to control. BCB16 challenged with F. oxysporum has a significant effect on all agronomical and pathological traits, including vigor index, dry mass, yield, and disease index. The highest yield was obtained from a BCB16 treated field. In vitro studies revealed that BCB16 not only has strong antifungal activity but also produces plant growth promoting hormones such as indole 3-acetic acid, which has an effect on plant vegetative growth and, ultimately, yield.
Discussion
Fungal pathogens are emerging crop-destroying agent [Citation31]. A number of important fungal agents harm crop species while also producing toxins that are harmful to human health, such as ergot or aflatoxin [Citation32]. Fusarium oxysporum is a lethal fungal pathogen that affects all stages of plant development [Citation1]. Treatment with synthetic fungicides is ineffective and is considered uneconomical, as well as an environmental and health hazard. The use of microbes, bio fungicides, as alternative to synthetic chemicals are getting popularity among scientific community. It has previously been demonstrated that in vitro antagonistic activity is cost-effective, but the results have not been replicated in field conditions [Citation33].
In this study, we demonstrated that biocontrol microbes can be used as alternative to chemical fungicide to control F. oxysporum. We isolated about 180 bacteria from rhizosphere of different crops and tested it for antagonistic activity against F. oxysporum using dual culture plate assay. The dual culture plate assay is a popular method for testing biocontrol activity against fungi [Citation8,Citation34,Citation35]. The threshold activity was maintained at 70% antagonism. The isolates that inhibited the fungus 70% were tested for plant growth promoting activity. The phosphorus solubilization and Indole 3-Acetic Acid levels of the selected isolates were determined. Three bacterial isolates demonstrated 70% antifungal activity, two of which produced IAA and none of which were P-solubilization positive [Citation36]. discovered that 80% of rhizospheric bacterial isolates can produce auxins [Citation37]. investigated biocontrol agents with 50% growth inhibition against Aspergillus Niger and Indole 3-Acetic Acid activity. Ali et al. [Citation38] demonstrated that the isolated biocontrol bacteria inhibited 70% of Fusarium species and 80% of F. oxysporum growth inhibition with strain LB1. In addition to a high percentage of inhibition, the LB1 strain had IAA acetic acid and no phosphate solubilization activity, which was consistent with our findings. Xia et al. [Citation39] tested Bacillus xiamenensis against various phytopathogenic fungi and found that it inhibited F. oxysporum by 68.24%. Similar to our study, the biocontrolled bacterium was positive for IAA and siderophore production, but unlike our study, B. xiamenensis was also positive for phosphate solubilization.
Understanding the mechanism underlying pathogen suppression is required for the selection of promising biocontrol agents [Citation40]. We chose bacteria with siderophore, HCN, cellulase, and protease activity. Unfortunately, we were unable to detect chitinase activity. HCN gas inhibits pathogen growth by interfering with the pathogen’s respiratory mechanism [Citation41], whereas protease breaks down the glycoprotein bond of the pathogen’s cell wall [Citation42], and siderophore is an iron-binding protein that creates competition between biocontrol bacteria and pathogens.
One isolate, BCB16, was chosen for a survival assay in carrier material and a wheat crop field trial. When compared to other isolates, the BCB16 had the most PGPR traits such as IAA, HCN, and siderophore. It is well known that while microbial fungicides perform well in the laboratory, their activity drops significantly in the field. According to Tabassum et al. [Citation6], the carrier material is the major limitation for the reduced activity of microbes in field conditions. The carrier material must be capable of supporting and sustaining a microbe population over time. In this study, talc powder was used. 40% of the 8.3 × 108 CFU/g BCB16 were added to the autoclaved talc powder, and the number of microbes were counted using serial dilution method every 30 days for 4 months. Novinscak and Filion [Citation43] found that using talc powder as a carrier material for the Pseudomonas strain resulted in a stable microbe population. Tamreihao et al. [Citation44] compared corn starch and talc powder as biofertilizer formulations and concluded that the talc formulation has a higher cell count than corn starch.
Our findings show that using PGPR strains as a seed treatment significantly reduced Fusarium oxysporum disease severity, increased wheat germination rate, induced a high seedling vigor index, and increased wheat plant length and weight. PGPR promotes plant growth by increasing nutrient uptake, such as N, through nitrogen fixation and IAA biosynthesis [Citation45]. Plants and a few microbes involved in root initiation and cell division synthesize the IAA phytohormone [Citation46]. Ammonia is a chemical compound that provides numerous plant health benefits, primarily by acting as metabolic inhibitors against phytopathogens [Citation47]. Strain BCB16 produced IAA, HCN, and siderophore and has the ability to significantly inhibit F. oxysporum, which may explain the improved absorption by wheat plant.
To test its efficacy against F. oxysporum, BCB16 was applied to a wheat field. When compared to the pathogenic treatment, the disease index treatment containing BCB16 was significantly lower. In BCB16 challenged with pathogenic fungus and F. oxysporum alone, an estimated 54.38% disease reduction of F. oxysporum was calculated. BCB16 not only increased wheat yield in the field, even in pathogen-infested conditions, but it also increased the vigor index. Similarly, Fallahzadeh-Mamaghani et al. [Citation48] concluded that P. polymixa N179 improved wheat crop growth and yield [Citation49]; Maheshwari [Citation50] obtained similar results by using biocontrol agents to successfully control early blight disease against tomato. Einloft et al. [Citation28] discovered that using a consortium of bacillus biocontrol agents can reduce the impact of F. verticillioides on maize seedlings. According to [Citation51], in field conditions, Bacillus subtilis significantly reduced the disease incidence against F. graminearum.
Conclusion
The goal of this study was to characterize biocontrol isolates against Fusarium oxysporum in wheat under controlled and field conditions. The BCB16 not only significantly reduced the disease incidence of F. oxysporum, but it also had a high survival potential in talc powder, which was used as a carrier material. B. amyloliquefaciens, BCB16, can be used as an alternative to chemical fungicides against F. oxysporum.
List of abbrevations
| Abbreviation | = | Full form |
| % | = | Percentage |
| °C | = | Degree centigrade |
| µg | = | Micro gram |
| µl | = | Micro liter |
| ANOVA | = | Analysis of variance |
| BCB | = | Biocontrol bacterial isolate |
| BLAST | = | Basic local allignment seracg tool |
| bp | = | base pairs |
| BUITEMS | = | Balochistan University Of Information Technology Engineering And Management Sciences |
| CFU | = | Colony forming unit |
| CMC | = | Carboxymethyl cellulose |
| DNA | = | Deoxyribonucleic acid |
| DNS | = | Dinitrosylicyclic acid |
| dNTPs | = | Di Nucleotide Tri Phosphate |
| HCN | = | Hydrogen cyanide |
| IAA | = | Indole acetic acid |
| IM | = | Isomaltuose |
| KB | = | Kilo base |
| Kg/m2 | = | Killo gram per meter square |
| L | = | Litre |
| LB | = | Luria Bertani |
| MEGA | = | Molecular evolutionary genetic analysis |
| mg | = | Milli-gram |
| ml | = | Milli liter |
| mm | = | Milli Molar |
| NaCl | = | Sodium Chloride |
| NBRIP | = | National Botanical Research Indian Phosphate |
| PCR | = | Polymerase Chain Reaction |
| PGP | = | Plant growth promoting |
| PGPR | = | Plant growth promoting rhizobacteria |
| rpm | = | Revolutions per minutes |
| Taq | = | Thermus aquatics |
| UV | = | Ultra Violet |
| V | = | Volts |
Authors contribution
This manuscript is part of the Ph.D. thesis of SIA under the supervision of AK. SIA and MU completed the research; Conceptualization: AH, ZR, BT and AK; SP and ZR: Molecular study; MU, SA and SMU: Data curation and Statistics: SIA, NJ and BT; Field experiment: SMU, MU, MHH and SP; Writing – review and editing: NJ and SP; Funding acquisition: AK
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials and the accession number of bacterial strain available in Genbank.
Additional information
Funding
References
- Wang X, Ji C, Song X, et al. Biocontrol of two bacterial inoculant strains and their effects on the rhizosphere microbial community of field-grown wheat. Bio Med Res Int. 2021;2021:1–14. doi: 10.1155/2021/8835275
- Azeem S, Agha SI, Jamil N, et al. Characterization and survival of broad-spectrum biocontrol agents against phytopathogenic fungi. Revista Argentina de Microbiología. 2022;54(3):233–242. doi: 10.1016/j.ram.2021.10.005
- Nazarov PA, Baleev DN, Ivanova MI, et al. Infectious plant diseases: etiology, current status, problems and prospects in plant protection. Acta Naturae. 2020;12(3):46. doi: 10.32607/actanaturae.11026
- Bhagat S, Bolton B, Romano R. The promise and peril of corporate governance indices. Colum L Rev. 2008;108:1803. doi: 10.2139/ssrn.1019921
- Yi Y, Luan P, Liu S, et al. Efficacy of Bacillus subtilis XZ18-3 as a biocontrol agent against rhizoctonia cerealis on wheat. Agriculture. 2022;12(2):258. doi: 10.3390/agriculture12020258
- Tabassum B, Khan A, Tariq M, et al. Bottlenecks in commercialisation and future prospects of PGPR. Appl Soil Ecol. 2017;121:102–117. doi: 10.1016/j.apsoil.2017.09.030
- Palazzini J, Roncallo P, Cantoro R, et al. Biocontrol of Fusarium graminearum sensu stricto, reduction of deoxynivalenol accumulation and phytohormone induction by two selected antagonists. Toxins (Basel). 2018;10(2):88. doi: 10.3390/toxins10020088
- Agha SI, Jahan N, Azeem S, et al. Research article characterization of broad-spectrum biocontrol efficacy of Bacillus velezensis against Fusarium oxysporum in triticum aestivum L. Not Bot Horti Agrobo Cluj-Napoca. 2022;50(1):12590–12590. doi: 10.15835/nbha50112590
- Jangir M, Sharma S, Sharma S. Target and non-target effects of dual inoculation of biocontrol agents against Fusarium wilt in solanum lycopersicum. Biol Control. 2019;138:104069. doi: 10.1016/j.biocontrol.2019.104069
- Parikh L, Eskelson M, Adesemoye A. Relationship of in vitro and in planta screening: improving the selection process for biological control agents against Fusarium root rot in row crops. Arch Phytopathol Plant Prot. 2018;51(3–4):156–169. doi: 10.1080/03235408.2018.1441098
- Khalil MMR, Fierro-Coronado RA, Peñuelas-Rubio O, et al. Rhizospheric bacteria as potential biocontrol agents against Fusarium wilt and crown and root rot diseases in tomato. Saudi J Biol Sci. 2021;28(12):7460–7471. doi: 10.1016/j.sjbs.2021.08.043
- Ramzan M, Tabassum B, Nasir IA, et al. Identification and application of biocontrol agents against cotton leaf curl virus disease in gossypium hirsutum under greenhouse conditions. Biotechnol Biotechnol Equip. 2016;30(3):469–478. doi: 10.1080/13102818.2016.1148634
- Awais M, Tariq M, Ali Q, et al. Isolation, characterization and association among phosphate solubilizing bacteria from sugarcane rhizosphere. Cytol Genet. 2019;53(1):86–95. doi: 10.3103/S0095452719010031
- Haider E, Khan MA, Atiq M, et al. Phytoextracts as management tool against fungal diseases of vegetables. Int J Biosci. 2020;16:303–314.
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170(1):265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x
- Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl environ microbiol. 2002;68(8):3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002
- Schwyn B, Neilands J. Siderophores from agronomically important species of the rhizobiacae. Comments Agric Food Chem. 1987;1:95–114.
- Lorck H. Production of hydrocyanic acid by bacteria. Physiol Plant. 1948;1(2):142–146. doi: 10.1111/j.1399-3054.1948.tb07118.x
- Kumar RS, Ayyadurai N, Pandiaraja P, et al. Characterization of antifungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad‐spectrum antifungal activity and biofertilizing traits. J Appl Microbiol. 2005;98(1):145–154. doi: 10.1111/j.1365-2672.2004.02435.x
- Hussain AA, Abdel-Salam MS, Abo-Ghalia HH, et al. Optimization and molecular identification of novel cellulose degrading bacteria isolated from Egyptian environment. J Genet Eng Biotechnol. 2017;15(1):77–85. doi: 10.1016/j.jgeb.2017.02.007
- Behera SS, Ray RC. Solid state fermentation for production of microbial cellulases: recent advances and improvement strategies. Int j biol macromol. 2016;86:656–669. doi: 10.1016/j.ijbiomac.2015.10.090
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030
- Fahsi N, Mahdi I, Mesfioui A, et al. Plant growth-promoting rhizobacteria isolated from the jujube (ziziphus lotus) plant enhance wheat growth, zn uptake, and heavy metal tolerance. Agriculture. 2021;11(4):316. doi: 10.3390/agriculture11040316
- Chen X, Huang H, Zhang S, et al. Bacillus velezensis WZ-37, a New broad-spectrum biocontrol strain, promotes the growth of tomato seedlings. Agriculture. 2021;11(7):581. doi: 10.3390/agriculture11070581
- Islam S, Akanda AM, Prova A, et al. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 2016;6:1360. doi: 10.3389/fmicb.2015.01360
- Dyer P, Ingram D. An improved test for evaluating the pathogenicity of isolates of Fusarium solani f. sp. pisi on pea. Ann Appl Biol. 1990;117(2):469–472. doi: 10.1111/j.1744-7348.1990.tb04234.x
- Hoagland R. The water culture methods for growing plants without soil. Cal Agric Exp St Circ. 1950;347:1–32.
- Einloft TC, Hartke S, de Oliveira PB, et al. Selection of rhizobacteria for biocontrol of Fusarium verticillioides on non-rhizospheric soil and maize seedlings roots. Eur J Plant Pathol. 2021;160(3):503–518. doi: 10.1007/s10658-021-02259-y
- Palazzini J, Alberione E, Torres A, et al. Biological control of Fusarium graminearum sensu stricto, causal agent of Fusarium head blight of wheat, using formulated antagonists under field conditions in Argentina. Biol Control. 2016;94:56–61. doi: 10.1016/j.biocontrol.2015.12.009
- Barik SR, Pandit E, Sanghamitra P, et al. Unraveling the genomic regions controlling the seed vigour index, root growth parameters and germination per cent in rice. PLoS One. 2022;17(7):e0267303. doi: 10.1371/journal.pone.0267303
- Bebber DP, Gurr SJ. Crop-destroying fungal and oomycete pathogens challenge food security. Fungal Genet Biol. 2015;74:62–64. doi: 10.1016/j.fgb.2014.10.012
- Hydrick A. Agricultural emergencies: factors and impacts in the spread of transboundary diseases in, and adjacent to, Agriculture. In: Global health security. Springer International Publishing; 2020. pp. 13–31. doi:10.1007/978-3-030-23491-1_2
- Kamilova F, Leveau JH, Lugtenberg B. Collimonas fungivorans, an unpredicted in vitro but efficient in vivo biocontrol agent for the suppression of tomato foot and root rot. Environ Microbiol. 2007;9(6):1597–1603. doi: 10.1111/j.1462-2920.2007.01263.x
- Bektas I, Kusek M. Biological control of onion basal rot disease using phosphate solubilising rhizobacteria. Biocontrol Sci Technol. 2021;31(2):190–205. doi: 10.1080/09583157.2020.1839381
- Senthilkumar M, Amaresan N, Sankaranarayanan A. Isolation of bacteria with biocontrol activity against phytopathogens: dual plate assay. In: Plant-microbe interactions. Springer US; 2021. pp. 167–169. doi:10.1007/978-1-0716-1080-0_44
- Katiyar D, Hemantaranjan A, Singh B. Application of plant growth promoting rhizobacteria in promising Agriculture: an appraisal. J Plant Physiol Pathol. 2017;5(4):2. doi: 10.4172/2329-955X.1000168
- Yuttavanichakul W, Lawongsa P, Wongkaew S, et al. Improvement of peanut rhizobial inoculant by incorporation of plant growth promoting rhizobacteria (PGPR) as biocontrol against the seed borne fungus, Aspergillus niger. Biol Control. 2012;63(2):87–97. doi: 10.1016/j.biocontrol.2012.06.008
- Ali S, Hameed S, Shahid M, et al. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol Res. 2020;232:126389. doi: 10.1016/j.micres.2019.126389
- Xia A, Xia Y, Farooq MA, et al. Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol Biochem. 2020;151:640–649. doi: 10.1016/j.plaphy.2020.04.016
- Zaim S, Belabid L, Bayaa B, et al. Biological control of chickpea Fusarium wilts using rhizobacteria “PGPR. In: Microbial-mediated induced systemic resistance in plants. Springer; 2016. pp. 147–162. doi:10.1007/978-981-10-0388-2_10
- Nandi M, Selin C, Brawerman G, et al. Hydrogen cyanide, which contributes to Pseudomonas chlororaphis strain PA23 biocontrol, is upregulated in the presence of glycine. Biol Control. 2017;108:47–54. doi: 10.1016/j.biocontrol.2017.02.008
- Banani H, Spadaro D, Zhang D, et al. Biocontrol activity of an alkaline serine protease from aureobasidium pullulans expressed in Pichia pastoris against four postharvest pathogens on apple. Int J Food Microbiol. 2014;182:1–8. doi: 10.1016/j.ijfoodmicro.2014.05.001
- Novinscak A, Filion M. Long term comparison of talc-and peat-based phytobeneficial Pseudomonas fluorescens and Pseudomonas synxantha bioformulations for promoting plant growth. Front Sustain Food Syst. 2020;4:602911. doi: 10.3389/fsufs.2020.602911
- Tamreihao K, Ningthoujam DS, Nimaichand S, et al. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol Res. 2016;192:260–270. doi: 10.1016/j.micres.2016.08.005
- Mittal V, Singh O, Nayyar H, et al. Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biol Biochem. 2008;40(3):718–727. doi: 10.1016/j.soilbio.2007.10.008
- Pérez-Flores P, Valencia-Cantero E, Altamirano-Hernández J, et al. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma. 2017;254(6):2201–2213. doi: 10.1007/s00709-017-1109-9
- Mahdi I, Fahsi N, Hafidi M, et al. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from chenopodium quinoa willd. Microorganisms. 2020;8(6):948. doi: 10.3390/microorganisms8060948
- Fallahzadeh-Mamaghani V, Golchin S, Shirzad A, et al. Characterization of Paenibacillus polymixa N179 as a robust and multifunctional biocontrol agent. Biol Control. 2021;154:104505. doi: 10.1016/j.biocontrol.2020.104505
- Ramakrishna A, Desai S, Uma Devi GT, et al. Biocontrol activity and PGPR ability of different isolates of Pseudomonas and Bacillus on tomato. Int J Pure App Biosci. 2018;6(6):728–735. doi: 10.18782/2320-7051.6943
- Maheshwari DK. Bacteria in agrobiology: plant growth responses. Springer Science & Business Media: 2011. doi: 10.1007/978-3-642-20332-9
- Zhao Y, Selvaraj JN, Xing F, et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS One. 2014;9(3):e92486. doi: 10.1371/journal.pone.0092486



