ABSTRACT
Omicron and its sublineages are currently predominant and have triggered epidemiological waves of SARS-CoV-2 around the world due to their high transmissibility and strong immune escape ability. Vaccines are key measures to control the COVID-19 burden. Omicron BA.2 caused a large-scale outbreak in Shanghai since March 2022 and resulted in over 0.6 million laboratory-confirmed infections. The vaccine coverage of primary immunization among residents aged 3 years and older in Shanghai exceeded 90%, and inactivated COVID-19 vaccines were mainly delivered. In the context of high vaccine coverage, we conducted a cohort study to assess vaccine effects on reducing the probability of developing symptoms or severity of disease in infections or nonsevere cases. A total of 48,243 eligible participants were included in this study, the majority of whom had asymptomatic infections (31.0%) and mild-to-moderate illness (67.9%). Domestically developed COVID-19 vaccines provide limited protection to prevent asymptomatic infection from developing into mild-to-moderate illness and durable protection to prevent nonsevere illness from progressing to severe illness caused by Omicron BA.2. Partial vaccination fails to provide effective protection in any situation. The level of vaccine effects on disease progression in the elderly over 80 years old was relatively lower compared with other age groups. Our study results added robust evidence for the vaccine performance against Omicron infection and could improve vaccine confidence.
Introduction
The lineages of the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are currently predominant around the world. In addition, regarding a set of nonpharmaceutical interventions (NPIs) with adjusted intensities, medical interventions, including SARS-CoV-2 vaccines and antiviral drugs, will become the main strategies to curb coronavirus disease 2019 (COVID-19) in the next stage.
The local outbreak of the Omicron sublineage BA.2 in Shanghai since March 2022 resulted in over 0.6 million laboratory confirmed infections in early June [Citation1], which was the largest-scale local outbreak in mainland China since 2020 and was contained with the measures implemented in a subregional and phased manner. With a largely infection-naïve population and with inactivated COVID-19 vaccines in widespread use, Shanghai represents a unique environment for monitoring inactivated vaccine performance against Omicron BA.2. A recent study in Hong Kong using surveillance data indicated that two doses or three doses of inactivated CoronaVac vaccine could provide strong and durable protection against severe disease and death [Citation2]. However, the overall primary vaccination coverage among the population aged 3 years and older in Shanghai was much higher than that in Hong Kong and exceeded 90% at the beginning of the outbreak [Citation3]. Booster doses are only recommended for adults aged 18 years and older in Shanghai.
In the context of high vaccination coverage, a common question of interest could be the effect of vaccination on reducing the severity of disease or probability of death in symptomatic cases or infections. This study aimed to assess the vaccine effects on post-infection outcomes and provide more robust evidence to improve vaccine confidence.
Materials and methods
Study participants
Subjects who had positive SARS-CoV-2 polymerase chain reaction (PCR) results during the local outbreak in Shanghai (between February 17, 2022 and May 15, 2022) and who were admitted to designated or cabin hospitals were invited to participate in this study. The designated hospitals received patients suffering from mild-to-moderate or severe symptoms, and cabin hospitals received patients suffering from slight or mild symptoms of the novel coronavirus. The patients who had two consecutive negative PCR results in hospital were allowed to be discharged. Subjects who were aged 3 years and older and provided informed consent were eligible for this study. We excluded individuals who ever had a positive PCR test before the start of the study period.
Data collection
Participants’ demographic information (age, gender, comorbidity), clinical course (diagnosis date, admission date, symptoms, antiviral treatment (Paxlovid), disease severity) and vaccination information (doses, vaccine product, vaccination date) were extracted from medical records. The participants with incomplete records were interviewed face-to-face or by telephone to supplement relevant data as much as possible. The comorbidity categories were defined using standardized definitions and were obtained through electronic medical record review by trained surveillance personnel, and the data included hypertension, diabetes, cardiovascular disease, haematological and haemodialysis disease, chronic renal disease, chronic lung disease, chronic liver disease, chronic rheumatism, neurological disease, human immunodeficiency virus (HIV) infection, malignant tumour and transplantation. The diagnosis, treatment and disease severity classification (asymptomatic infection, mild-to-moderate cases, severe cases, critical cases and death) were conducted according to the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 8 before March 15, 2022 [Citation4] and Trial Version 9 after March 15, 2022 [Citation5]) issued by the National Health Commission of the People’s Republic of China. Critical cases and deaths were grouped into severe cases in this study.
Vaccination status
Vaccination status was defined as unvaccinated, partial, primary and booster immunization based on the national recommendation of each product-specific COVID-19 vaccine. Participants who received a two-dose regimen of inactivated vaccines, a one-dose regimen of ad-vectored vaccine or a three-dose regimen of protein subunit vaccine were considered to have completed primary immunization. The combined use of vaccines on different platforms was classified as mixed regimens. A 14-day lag after the vaccination series was considered when classifying the vaccination status.
Statistical analyses
The demographics of the eligible participants were summarized for disease severity (asymptomatic infection, mild-to-moderate and severe illness). Vaccine effects on post-infection outcomes were represented as VEp. VEp against progression to high disease severity was estimated using multivariable logistic regression, comparing the odds of vaccination status among asymptomatic infection vs. mild-to-moderate illness and nonsevere illness vs. severe illness separately after adjusting for potential confounders, including age, sex, presence of comorbidity (continuous variable) and antiviral medications. Subjects who received antivirals before progression to severe clinical symptoms were classified as receiving antivirals group. VEp was calculated as (1 – adjusted odds ratio) × 100%. The results were stratified by age group (3–17, 18–59, 60–79, ≥80 years) and vaccine platforms. Subgroup analyses for time since receipt of the vaccine (<180 days and ≥180 days post primary vaccination, <90 days and ≥90 days post booster vaccination) were conducted and were restricted to the inactivated vaccine recipients. The duration of infection was defined as the interval between the diagnosis date (the date of positive PCR retest result) and the discharge date and this analysis was restricted to participants diagnosed after March 15 due to the updated the Diagnosis and Treatment Protocol for COVID-19. The mean and corresponding 95% confidence interval (95% CI) were calculated. Comparisons of the duration of infection between the participants with different vaccination statuses were performed by Group t tests. All analyses were conducted using R 4.0.2.
Results
From February 17 to May 15, 2022, a total of 51,775 patients with laboratory-confirmed infections were admitted to the designated or cabin hospitals, and 48,243 participants were included in this study. Participants with mild-to-moderate illness (67.9%) and adults aged 18–59 years old (82.0%) accounted for the majority ( and ). The proportions of the participants who received at least one dose of COVID-19 vaccines were 87.8%, 90.0% and 23.8% in the asymptomatic, mild-to-moderate and severe groups, respectively, and most of the vaccines were inactivated vaccines. The median days between the last vaccination dose and positive PCR test result were approximately 280 days post primary immunization and approximately 100 days post booster immunization (). More than 80% of the patients with asymptomatic infection and mild-to-moderate illness reported no comorbidity, and 86.9% of the patients with severe disease had at least one comorbidity ().
Figure 1. Flowchart of the included participants. Severe outcomes included the clinical outcomes of the severe, critical and fatal cases.
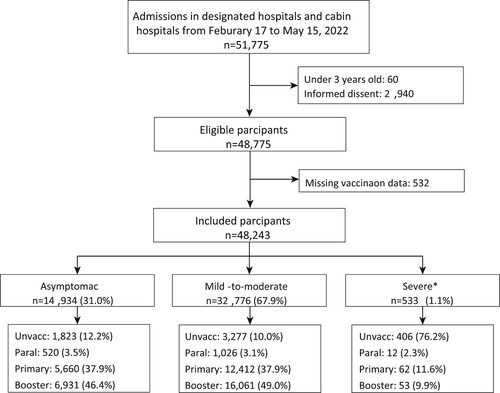
Table 1. Demographic characteristics (n, %).
All the lower limits of the 95% CI for the vaccine effect estimations in preventing asymptomatic infection from developing into mild-to-moderate cases were smaller than 0, except that of ad-vectored vaccines among the elderly aged 60–79 years (). In contrast, the lower limits of the 95% CI were greater than 0 for all thresholds when assessing the vaccine effects in preventing nonsevere illness progressing to severe illness among the patients who completed primary or booster immunization (). Homologous booster immunization with inactivated vaccines prevented more than 70% of disease progression to severe outcomes in all age groups above 18 years (VEp 79.5, 95% CI 58.0-90.3 for the 18–59 age group; VEp 71.1, 95% CI 57.4-80.9 for the 60–79 age group; VEp 71.1, 95% CI 23.8-91.5 for the 80 years and above age group; ). COVID-19 vaccines provided a relatively lower level of protection in preventing the cases of nonsevere illness from progressing to severe disease for the elderly aged 80 years and older than that for the 18–59 age group (p = 0.02) and the 60–79 age group (p = 0.03). The protection provided by three doses of inactivated vaccines against severe disease progression was durable in the adults of three age group (18–59 age group: VEp 74.1, 95% CI 33.6–91.6 within 90 days, VEp 81.6, 95% CI 57.7–92.9 above 90 days, p = 0.56; 60–79 age group: VEp 71.4, 95% CI 48.4–85.7 within 90 days, VEp 66.6, 95% CI 46.1–80.3 above 90 days, p = 0.71; 80 years and above age group: VEp 70.1, 95% CI −61.9–98.4 within 90 days, VEp 56.8, 95% CI −43.3–90.6 above 90 days, p = 0.71; ). Meanwhile, there were no significant differences in the effects between the three age groups after booster immunization.
Table 2. Vaccine effects against disease progression by the vaccination status within the age categories (VEp, 95% CI).
Table 3. Time-varying vaccine effects against disease progression from nonsevere to severe outcomes within the age categories among the inactivated vaccine recipients (VEp, 95% CI).
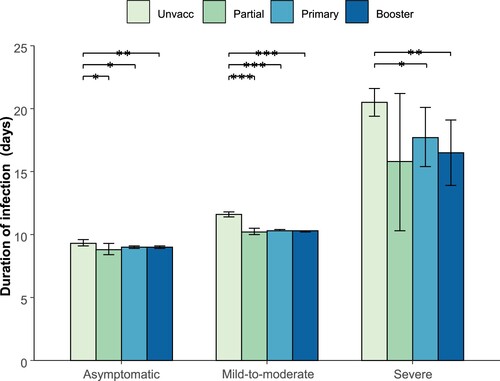
Among the unvaccinated participants, the mean infection durations of the asymptomatic infections, mild-to-moderate cases and severe cases were 9.3 days (95% CI: 9.1–9.6), 11.6 days (95% CI: 11.4–11.8), and 20.5 days (95% CI: 19.4–21.6), respectively, which were significantly longer than those among the vaccinated participants (). The duration of infection was similar among the participants with different vaccination statuses, regardless of disease severity ().
Discussion
Our study showed that primary and booster vaccination provided limited protection to prevent asymptomatic infection progressing to mild-to-moderate disease and durable protection, the vaccine prevented nonsevere illness into progressing into severe illness. Partial vaccination fails to provide effective protection in any situation. The level of vaccine effects on disease progression for the elderly over 80 years old was relatively lower compared with the other age groups. The duration of infection among the participants who received at least one dose of COVID-19 vaccine was significantly shorter than that among the unvaccinated participants regardless of disease severity.
The participants in the vaccinated group who become infected would be those with weaker immune systems, whereas the infected participants in the unvaccinated group would be those with a weaker immune system as well as those with a stronger immune system. In this situation, if we compare a post-infection outcome in the vaccinated group with that in the unvaccinated group, it could appear that the vaccine makes things worse, even if vaccination has absolutely no effect on anything after infection [Citation6]. However, results associated with lower limits of the 95% CI that were greater than 0 for all thresholds when assessing the vaccine effects on nonsevere illness progressing to severe illness indicated that primary and booster vaccination substantially decreased the severity of breakthrough disease in adults who received two or three doses of vaccines compared with that in unvaccinated adults.
Studies in Hong Kong and Shanghai have revealed the strong protection provided by two-dose schedules of inactivated vaccines against severe disease and death in those under 80 years of age and the limited protection against Omicron infection [Citation2, Citation6, Citation7], which was consistent with our results. Both McMenamin et al. [Citation2] and Huang et al. [Citation7] obtained data from aggregated electronic databases that lacked the biological factors and therapeutic options that are significantly associated with severe outcomes.
The predominant variant Omicron BA.4/5 has been indicated to be more likely to escape immunity than the Omicron BA.2 sub-lineage [Citation8, Citation9]. Several studies in the real world indicated that the protection provided against these two sublineages by first-generation COVID-19 vaccines was similar, and the more important factor associated with loss of protection was the waned vaccine effects over time [Citation10, Citation11]. Most of the participants in our study received vaccines more than 6 months before infection, suggesting that vaccination with booster doses in time rebounded the immune responses for stronger protection. As of March 2022 (at the beginning of the outbreak), Shanghai residents were vaccinated with domestically developed inactivated vaccines, and the vaccination coverage was relatively low in the elderly (only approximately 62% of individuals aged 60 years or older had completed the primary schedule) [Citation3] and has been at a standstill since then (approximately 70.1% as of July 24, 2022) [Citation12]. Everyone needs to be individually protected by vaccination when the herd immunity from any of the current generation vaccines is hard to build and when facing the emerging variants, and this is especially true within clinically vulnerable populations. Older people, those with multimorbidity, and those with specific underlying health conditions remain at increased risk of severe COVID-19 and death after the initial vaccine booster [Citation13]. Every person vaccinated and every person boosted receives clinically meaningful direct protection from serious/critical/fatal COVID-19 [Citation14]. Inducing mucosal immunity by first-generation COVID-19 vaccines [Citation15, Citation16] and accelerating the next generation of COVID-19 vaccines [Citation17] would be other ways to establish a higher level of population immunity. To mitigate the viral pandemic in progress in China, the second booster doses are urgent to be provided as the additional protection against severe outcomes has been demonstrated [Citation18, Citation19], especially for the healthcare workers and clinically vulnerable population. Inhaled and heterologous doses could induce higher levels of neutralization antibody titres, which should be recommended preferentially. As the first-generation COVID-19 vaccines couldn’t reduce the spread of SARS-CoV-2 virus, more researches are appealed on mucosal vaccines and broad-spectrum vaccines.
Our study has several limitations. Conditioning on an event that occurs subsequent to receipt of vaccine or control could result in selection bias, and comparability of the infected vaccinated and infected unvaccinated groups would lead to biased estimates of the effect of vaccination on post-infection outcomes. However, people of all ages are susceptible to SARS-CoV-2, and we included biological factors (age and presence of comorbidity), which have been indicated to be high-risk factors associated with severe outcomes. Second, due to the pressure on medical systems, the diagnosis date, which was defined as the date of a positive PCR retest result, may lag for two or three days after the first positive PCR test result, which may have led to an underestimated duration of infection in this study. Third, the absence of obvious symptoms may lead to the misclassification of disease severity, but the possibility of misclassification of severe outcomes was very small. Last, we failed to evaluate the effects of protein subunit vaccines and mixed regimens, and the effects against fatal outcomes due to the small sample size of the study. Meanwhile, the number of subjects received primary immunization within 180 days was small which leading to biased results.
Conclusions
Our study results showed that first-generation COVID-19 vaccines could provide durable protection to prevent disease progression from nonsevere to severe outcomes. It is difficult to block infection and transmission of the virus in the context of the highly transmissible and immune escape SARS-CoV-2 variant Omicron and its sublineages. Medical interventions are an important weapon to overcome the pandemic in the next stage, and COVID-19 vaccines play an irreplaceable role.
Data availability statement
The data analyzed are not publicly available as they contain personal information.
Disclosure statement
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, Shanghai Roche Pharmaceutical Company, and SINOVAC Biotech Ltd. None of those research funding is related to this work. The remaining authors declare no competing interests.
Additional information
Funding
References
- Chen Z, Deng X, Fang L, et al. Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: a descriptive study. Lancet Reg Health West Pac. 2022;29:100592.
- McMenamin ME, Nealon J, Lin Y, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–1443.
- Zhang X, Zhang W, Chen S. Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. 2022;399(10340):2011–2012.
- Commission CNH. Diagnosis and treatment protocol for COVID-19 (trial version 8). 2021. [cited Oct 25 2022]. http://www.gov.cn/zhengce/zhengceku/2021-04/15/content_5599795.htm.
- Commission CNH. Diagnosis and treatment protocol for COVID-19 (Trial Version 9). 2022. [cited Oct 25 2022]. http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm.
- Yang B, Wong IOL, Xiao J, et al. Effectiveness of CoronaVac and BNT162b2 Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 Omicron BA.2 Infections in Hong Kong. J Infect Dis. 2022;226(8):1382–1384.
- Huang Z, Xu S, Liu J, et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022;20(1):400.
- Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–608.
- Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593–602.
- Tseng HF, Ackerson B, Bruxvoort K, et al. Effectiveness of mRNA-1273 against infection and COVID-19 hospitalization with SARS-CoV-2 Omicron subvariants: BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. medRxiv. 2022. https://doi.org/10.1101/2022.09.30.22280573
- Surie D, Bonnell L, Adams K, et al. Effectiveness of monovalent mRNA vaccines against COVID-19-associated hospitalization among immunocompetent adults during BA.1/BA.2 and BA.4/BA.5 predominant periods of SARS-CoV-2 Omicron variant in the United States – IVY network, 18 states, December 26, 2021–August 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(42):1327–1334.
- Shanghai Municipal Peoples Government. COVID-19 vaccine coverage of the elderly reached 70.14% as of July 24. 2022. [cited Oct 25 2022]. http://service.shanghai.gov.cn/SHVideo/newvideoshow.aspx?id=F91582A2225BAD75.
- Agrawal U, Bedston S, McCowan C, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet. 2022;400(10360):1305–1320.
- Lance R, Dan W, Zundong Y, et al. Vaccinate with confidence and finish strong. China CDC Weekly. 2022;4(37):828–831.
- Mao T, Israelow B, Peña-Hernández MA, et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science (New York, N.Y.). 2022;78(6622):eabo2523.
- Havervall S, Marking U, Svensson J, et al. Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. N Engl J Med. 2022;387(14):1333–1336.
- Wang Q, Bowen A, Valdez R, et al. Antibody responses to omicron BA.1 4/BA.5 bivalent mRNA vaccine booster shot. bioRxiv. 2022. https://doi.org/10.1101/2022.10.22.513349
- Grewal R, Kitchen SA, Nguyen L, et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. Br Med J. 2022;378:e071502.
- Gazit S, Saciuk Y, Perez G, et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. Br Med J. 2022;377:e071113.