ABSTRACT
Because of the large number of infected individuals, an estimate of the future burdens of the long-term consequences of SARS-CoV-2 infection is needed. This systematic review examined associations between SARS-CoV-2 infection and incidence of categories of and selected chronic conditions, by age and severity of infection (inpatient vs. outpatient/mixed care). MEDLINE and EMBASE were searched (1 January 2020 to 4 October 2022) and reference lists scanned. We included observational studies from high-income OECD countries with a control group adjusting for sex and comorbidities. Identified records underwent a two-stage screening process. Two reviewers screened 50% of titles/abstracts, after which DistillerAI acted as second reviewer. Two reviewers then screened the full texts of stage one selections. One reviewer extracted data and assessed risk of bias; results were verified by another. Random-effects meta-analysis estimated pooled hazard ratios (HR). GRADE assessed certainty of the evidence. Twenty-five studies were included. Among the outpatient/mixed SARS-CoV-2 care group, there is high certainty of a small-to-moderate increase (i.e. HR 1.26–1.99) among adults ≥65 years of any cardiovascular condition, and of little-to-no difference (i.e. HR 0.75–1.25) in anxiety disorders for individuals <18, 18–64, and ≥65 years old. Among 18–64 and ≥65 year-olds receiving outpatient/mixed care there are probably (moderate certainty) large increases (i.e. HR ≥2.0) in encephalopathy, interstitial lung disease, and respiratory failure. After SARS-CoV-2 infection, there is probably an increased risk of diagnoses for some chronic conditions; whether the magnitude of risk will remain stable into the future is uncertain.
Introduction
In addition to disrupting the global economy [Citation1], SARS-CoV-2 has infected millions of people worldwide and more than 4.5 million Canadians [Citation2]. Potential long-term consequences of SARS-CoV-2 infection were raised in the first year of the pandemic [Citation3]. Combined with the large number of infected individuals, it is necessary to derive some estimate of the future burdens of the long-term consequences of SARS-CoV-2 infection so that health policy and other decision makers can make informed decisions and healthcare systems can prepare for a potential increase in need for care and resources.
Many reviews in the literature have examined post-COVID-19 condition (previously called Long COVID) [Citation4–6], and many reviews reporting on other long-term sequalae, such as the development of chronic conditions after SARS-CoV-2 infection, have been limited to a single condition or cluster of conditions [Citation7–9] and/or did not require included studies to have a control group in order to quantify attributable risk [Citation10–12]. In order to understand how SARS-CoV-2 may change the future burden of health outcomes on healthcare resources in the future, it is important to assess whether there is actually an association between SARS-CoV-2 infection and increased risk of long-term sequelae.
Therefore, we set out to conduct a systematic review to answer the question: What are the associations between SARS-CoV-2 infection and the incidence of new diagnoses or exacerbations of chronic conditions in groups based on age and severity of infection?
Methods
This review followed an a priori protocol developed in consultation with disease leads (NC, DZ, LS, HG, JM, and others) at the Public Health Agency of Canada. The protocol was prospectively registered and is available on PROSPERO (CRD42022364883). This review has been reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 reporting guideline (Appendix 1 in the supplement) [Citation13].
Study eligibility
We included prospective or retrospective observational studies carried out in high-income Organisation for Economic Co-operation and Development (OECD) member countries [Citation14] and comparing individuals with suspected or confirmed SARS-CoV-2 infection (exposed) to those without (controls). Pre-prints and other reports not peer-reviewed were eligible. Conference abstracts frequently present preliminary results and rarely report sufficient methods to adequately assess quality and were therefore excluded. We limited inclusion to records published in English or French, as these are the official languages of Canada and limits on non-English language studies has not been shown to bias systematic review conclusions [Citation15]. Table S1 in the Supplement outlines our eligibility criteria in greater detail.
To be eligible, studies had to report on severity of SARS-CoV-2 infection (i.e. hospitalization status), adjust for possible confounding by at least sex and two or more comorbidities (i.e. by matching, propensity scores, or multivariable regression), and report outcomes by age to allow for allocation to the most appropriate age group for analysis and synthesis of findings by key life-course stages: 0–17y, 18–64 y, and ≥65y. Study outcomes reported using differing age groupings were analysed within the most appropriate age group. Where a study reported an age group that spanned two of our categories, we weighted the data based on the number of years contributed to the age category. For example, data reported for 60–69 year-olds contributed to both the 18–64-year-old and ≥65-year-old groups but was given half of the 60–69-year-old age group’s overall weight. We included studies comparing people with confirmed (e.g. by laboratory testing) or suspected (e.g. physician diagnosed, regardless of test status) SARS-CoV-2 infection to those without. To ensure we would have some relevant studies to include, we did not require control groups to test negative for SARS-CoV-2. There was also no requirement for control groups to be healthy individuals (i.e. they could include hospitalized patients or individuals with other respiratory infections such as influenza but without SARS-CoV-2), to control for possible confounding, such as due to hospitalization not specific to SARS-CoV-2 infection.
Primary outcomes of interest were incidence and exacerbations of chronic conditions after SARS-CoV-2 infection compared to controls. Conditions of interest fell into the following categories: cardiovascular diseases, neurological conditions, cancer, chronic kidney disease, diabetes (excluding gestational diabetes), musculoskeletal disorders (e.g. osteoarthritis, gout, etc.), respiratory diseases, mental disorders, and stroke. Individual conditions within each category were also evaluated. Because of the limited clinical and epidemiologic relevance [Citation16], we did not look at dementia/cognitive impairment outcomes in individuals <18 years. Outcomes could be ascertained at any time after the acute phase of infection (i.e. immediately after discharge in hospitalized patients and ≥4 weeks in outpatients) and no minimum follow-up time was required. We attempted to only include studies reporting on diagnoses of chronic conditions, defined as those that were at a minimum documented by a healthcare provider in medical records; however, there may not have been standard diagnostic testing performed in all cases. Variables of interest for subgroup analyses were time since infection, vaccination status, and different SARS-CoV-2 variants of concern.
Search strategy
An information specialist (MT) developed a search strategy combining concepts for SARS-CoV-2 infection, post-acute/follow-up, outcomes (e.g. incidence), and chronic conditions of interest using vocabulary and syntax specific to each database searched. The search strategy was peer-reviewed by a second research librarian using the PRESS 2015 checklist [Citation17]. Searches were carried out on 4 October 2022 in Ovid MEDLINE® ALL 1946 to 3 October, and EMBASE 1974 to 3 October 2022. Search results were limited to those on or after 1 Jan 2020, and filters were applied to remove case reports, commentaries, and conference abstracts. The full searches for MEDLINE and EMBASE are available in Appendix 2 in the Supplement. In addition to database searches, a review lead (LG or JP) screened the reference lists of included studies and pertinent systematic reviews identified during screening for potentially relevant studies. Screening of reference lists and systematic reviews was completed on 7 November 2022.
Study selection
Search results were uploaded to an EndNote library (v. 20.3, Clarivate Analytics, Philadelphia, PA) and deduplicated before screening. Unique records were then uploaded to DistillerSR (Evidence Partners, Ottawa, Canada) and screened in a two-stage process, first by title and abstract (screening) and then by full-text (selection). Using standardized forms, all reviewers involved in screening and selection (LG, JP, SS) piloted the screening form with a random sample of 200 records and piloted the selection form with 16 full-text records from the database searches. Screening and selection proceeded once sufficient agreement between reviewers was reached.
During screening, DistillerSR’s machine learning feature (DistillerAI) was enabled. DistillerAI learns from human reviewers’ inclusion decisions to assign a likelihood score (0–1, with values closer to 1 indicating higher likelihood of inclusion) for each unscreened record and prioritizes the most relevant records for screening by the human reviewers (i.e. the most relevant records are screened first) [Citation18]. Further, when threshold likelihood score for inclusion is applied, DistillerAI can act as a second reviewer with high specificity and sensitivity [Citation19]. Thus, two reviewers independently screened the first 50% of titles and abstracts, after which DistillerAI acted as a second reviewer with likelihood threshold of 0.7. All remaining records with a DistillerAI-assigned likelihood >0.7 proceeded to selection and the rest were manually screened by one human reviewer for final exclusion. After screening, attempts were made to retrieve the full texts of all potentially relevant records. Two reviewers independently reviewed all retrieved full-texts and came to consensus on inclusion, with adjudication by a review lead or other reviewer (e.g. statistician) when necessary.
Data extraction and management
We developed standardized data extraction forms in Microsoft Office Excel (v. 2019, Microsoft Corporation, Redmond, WA) which were independently piloted by all reviewers involved in extraction (LG, JP, SS). Thereafter, one reviewer extracted data from the included studies, and a second reviewer independently verified results data for accuracy and completeness. Disagreements were resolved by discussion. When relevant findings were reported in figures, data was extracted using Web Plot Digitizer (https://automeris.io/WebPlotDigitizer/). We only recorded zero events of a condition when it was explicitly reported.
We extracted the following information from each study: study characteristics (i.e. author, year, country, funding source, location of registration/protocol, design), population characteristics (i.e. inclusion and exclusion criteria, sample size, population demographics (age, sex, ethnicity, relevant comorbidities), SARS-CoV-2 infection confirmation method and timing), care setting during acute phase (outpatient, inpatient, mixed out- and inpatients), comparator(s), length of follow-up, analysis details (i.e. variables considered in analysis), outcome details (i.e. methods of ascertainment), and findings. For each condition category and/or individual condition of interest we extracted both relative (i.e. incidence rate ratios [IRRs] or hazard ratios [HRs]) and per-group incidence rates or cumulative incidence, when available. If an adjusted incidence rate or cumulative incidence was not reported (but participants were matched by at least sex and comorbidities), we extracted the crude number of events and estimated the cumulative incidence based on the denominator for each group. When results were reported for multiple time points, we took the longest follow-up. We extracted outcome data even when it was not able to be meta-analysed, for example if only a p-value between groups was reported, to help interpret data and document possible reporting biases. Adjusted findings (i.e. from the most adjusted model) were prioritized in all cases. We extracted any within-study analyses by time since infection, SARS-CoV-2 vaccination status, and different SARS-CoV-2 variants of concern and synthesized these narratively. Data extracted for this review are available on reasonable request from the authors.
Risk of bias assessment
To assess risk of bias of included studies we used the JBI critical appraisal checklist for cohort studies [Citation20]. After piloting, a review lead (LG) assessed the risk of bias for each study and brought any questions or concerns about included studies to the review team for discussion and consensus. We specifically considered in our assessment the validity of SARS-CoV-2 infection confirmation, with laboratory confirmed (using RT–PCR or antigen test) based on medical records being low risk and all others having some concerns. We also had concerns when a prospective study did not censor control participants who contracted COVID-19 during the follow-up period. We assigned an overall risk of bias rating (low, moderate or high) based on the number of questions answered “No” for each study (0 for low, 1 for moderate, ≥ 2 for high). Final assessments were incorporated into our certainty of evidence assessments guided by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach (see below) [Citation21].
Data synthesis
We conducted random-effects meta-analysis using inverse variance weighting in Review Manager (RevMan; v5.4, The Cochrane Collaboration, 2020) to estimate a pooled hazard ratio when two or more studies reported on a condition category, or individual condition, by age category and COVID-19 care setting (inpatient vs. outpatient/mixed). Because our analysis was based on planned sub-groups, we did not investigate further into potential sources of heterogeneity. Forest plots were generated in RevMan to visually display results of the meta-analyses. Data not appropriate for meta-analysis were synthesized narratively. For all meta-analyses, a relative effect of 0.75–1.25 was considered little-to-no association; 0.51–0.74 and 1.26–1.99 small-to-moderate association (decrease or increase, respectively), and ≤0.50 or ≥2.00 large association. All studies with useable data were included in the meta-analyses for each condition category or individual condition they reported on. Since we identified no eligible studies with data on exacerbations of pre-existing conditions and new diagnosis of a condition can only occur once, we considered reported hazard ratios and incidence rate ratios to be interchangeable. When only cumulative incidence or crude events were reported, we estimated the incidence rates for each group by dividing the number of events by the average follow-up period (in years) multiplied by the number of participants.
We conducted separate analyses for each of the following categories of chronic conditions: cardiovascular disease, neurological conditions, chronic kidney disease, diabetes, musculoskeletal disorders (e.g. osteoarthritis, gout, etc.), respiratory diseases, mental disorders, and stroke. Although cancer was also among our chronic conditions of interest, we did not identify any eligible studies reporting on this outcome. The disease leads helped to ensure conditions reported by each included study were appropriately categorized. We also analysed individual conditions within each condition category (e.g. dementia/mild cognitive disorder within the category of neurological conditions) when there was condition-specific data and a sample size of >2000 in the SARS-CoV-2 infection group.
For studies that reported data for multiple diagnoses falling within the label of an individual condition (e.g. tachycardia and ventricular arrhythmia, which would both contribute to the condition labelled “arrhythmias”), we calculated an estimated average for the condition weighted by the inverse of the variance to give more weight to results with more reliable estimates. This process was also used when a study reported multiple individual conditions within a condition category, if the study did not report a suitable composite outcome for the condition category.
For all categories and individual conditions with low, moderate or high certainty of some direction of effect (i.e. small-to-moderate or large increase/decrease), we estimated the excess incidence in the SARs-CoV-2 group per 1000 people over 6 months. We used a hierarchy to identify the most relevant data to use for the control (non-SARS-CoV-2) event rate. If at least one study reported a composite outcome (e.g. any cardiovascular event) within a condition category, we used the study’s reported incidence for that composite outcome. When a condition category had no directly reported composite incidence, we looked at the individual conditions in that condition category. Where we considered conditions within a condition category to be mutually exclusive (broadly speaking), we took the sum of their incidence in the control group as an estimate of the control event rate. Where conditions within a condition category were not mutually exclusive, we used the individual condition with the highest incidence as a conservative estimate. When multiple studies reported a control event rate for a condition category or individual condition, we took an average weighted by sample size. We converted all control event rates to a standard 6-month period, which was most representative of follow-up duration in the included studies. For example, a 1-year incidence rate was divided by 2 to estimate the incidence over 6 months. We estimated excess incidence by subtracting the control event rate from the product of the control event rate and the relative effect.
Other than age and COVID-19 care setting, we did not conduct any quantitative subgroup or sensitivity analyses. However, we planned to narratively summarize any time-varying effects and any within-study sub-group analyses for different SARS-CoV-2 variants of concern or by SARS-CoV-2 vaccination status.
Certainty of evidence
Two reviewers reached consensus through discussion on the certainty about conclusions in relation to our thresholds of effect of the relative effects for each outcome, guided by GRADE [Citation21,Citation22]. We started the evidence at high certainty [Citation23] and down rated to lower levels (i.e. moderate, low, and very low certainty) based on study quality in five domains (i.e. risk of bias, indirectness, inconsistency, imprecision, reporting biases). For each domain, we rated down by 0, 1, or 2 levels depending on the seriousness of the concerns, i.e. how much the domain appeared to impact the conclusions. We used thresholds as the targets of our certainty: a relative effect of 0.75–1.25 was considered little-to-no association, 0.51–0.74 and 1.26–1.99 was considered small-to-moderate, and ≤0.50 or ≥2.00 large. For example, we did not rate down for risk of bias when both high and low risk of bias studies had estimates surpassing the threshold for magnitude of association. Similarly, we did not rate down if some of our concerns in one domain likely stemmed from another domain, for example, we did not rate down for inconsistency if differences in estimates across studies were judged to be primarily related to risk of bias. If only one or two conditions contributed to a condition category estimate, we considered this an indirectness concern. To assess reporting biases, we compared outcomes specified in each report’s Methods section (or protocol if available) with the outcomes reported in the Results section. Outcomes in the results section that were not specified in the methods or specified in the methods but not reported in the results were considered concerns. We rated down for inconsistency/lack of consistency when there was a single study in an analysis, when there was concerning variation not accounted for in other domains in study estimates (in relation to our thresholds), or when a single study contributed >80% weight to an estimate. Finally, we rated down once or twice for imprecision when one or both of the ends, respectively, of the confidence interval extended across an effect threshold (i.e. from effect into little-to-no difference or vice versa). When considering imprecision, we made conclusions about the results for which we had the highest certainty; for example when a point estimate surpassed our threshold for a large association but with imprecision, we instead made conclusions about a small-to-moderate association without imprecision concerns.
Results
The flow of records through the selection process is depicted in , and Appendix 3 in the Supplement lists relevant studies that did not meet key eligibility criteria, with reasons for exclusion. After screening 4,648 unique database records and 24 records identified from other sources, we included 25 studies from six countries: United States (15), Germany (4), United Kingdom (3), Denmark (1), Korea (1), and Sweden (1). The included studies (median sample size [IQR] N = 488,552 [226,380–2,568,874]) are summarized in . Eight (32%) of the included studies confirmed that the control group was negative for SARS-CoV-2 using laboratory testing. Only 2 (8%) eligible studies reported on chronic conditions after hospitalization with SARS-CoV-2. We did not identify any eligible studies reporting on cancer, osteoarthritis, or gout after SARS-CoV-2 infection, nor did we identify any eligible studies reporting exacerbations of pre-existing chronic conditions.
Figure 1. PRISMA flow diagram for a systematic review of the associations between SARS-CoV-2 infection and incidence of new chronic condition diagnoses. Template From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting.
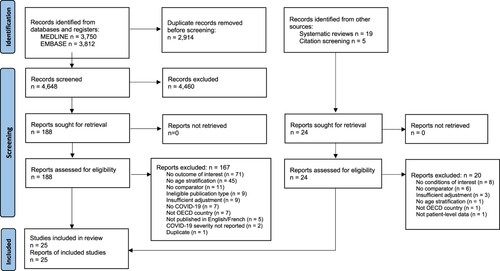
Table 1. Study characteristics of included studies for a systematic review of new diagnoses of chronic conditions after SARS-CoV-2 infection.
Risk of bias assessments are presented in . The majority of studies (18/25, 72%) were considered moderate risk of bias with only three having low risk. The most frequent concern for risk of bias was the potential for misclassification, largely due to differential exposure ascertainment methods between groups mostly from not confirming the absence of exposure with negative tests in the control group. Our assessment of potential reporting biases did not identify evidence of missing outcome data in any of the studies included in the meta-analysis.
Table 2. Risk of bias assessment according to JBI’s Cohort Studies tool.
Appendix 4 in the Supplement contains all forest plots. presents the summary of findings, including the certainty of evidence for the relative effects and estimates of the excess cumulative incidence in 1000 people over 6 months (for outcomes with low, moderate or high certainty of a direction of effect). The GRADE domain(s) that led to rating down our certainty are documented in the table footnotes. One study included in our review reported on 31 conditions in 7 categories, but only provided non-stratified numeric results for adults (≥18y; ∼15% of sample was ≥65 y); [Citation35] this study reported results for children (<18 y) as a broad statement of no difference without effect estimates or variance, and thus was unable to be included in the meta-analysis, although it still met the inclusion criteria detailed in our protocol. We do not report directions of effect for outcomes in which we had very low certainty. The condition categories and individual conditions for which we had moderate or high certainty were limited to the outpatient/mixed care group and are outlined below.
Table 3. Summary of Findings for new diagnoses of chronic conditions after SARS-CoV-2 infection.
For ≥65 year-olds, we have high certainty that SARS-CoV-2 infection is associated with a small-to-moderate increase of any cardiovascular disorder, acute coronary disease, arrhythmias/dysrhythmias, and heart failure. For 18–64 year-olds, we have high certainty of a large increase in cardiomyopathy and of a small-to-moderate increase of heart failure. For all age groups (<18 y, 18–64 y, ≥ 65 y), we have high certainty of little-to-no difference in anxiety/anxiety disorders.
For individuals <18 years old, we have moderate certainty of a small-to-moderate increase in trauma and stress disorders, chronic fatigue syndrome, and stroke; and moderate certainty of little-to-no difference in arrhythmias/dysrhythmias, chronic kidney disease, type 2 diabetes, and asthma.
For 18–64 year-olds, we have moderate certainty of a large increase in encephalopathy, interstitial lung disease, and respiratory failure; moderate certainty of a small-to-moderate increase of any cardiovascular disorder, acute coronary disease, arrhythmias/dysrhythmias, hypertension, chronic kidney disease, myoneural junction/muscle disease, dementia/mild cognitive disorder, and haemorrhagic stroke; and moderate certainty of little-to-no difference for depression/mood disorders.
Among individuals ≥65 years old, we have moderate certainty of a large increase of encephalopathy, interstitial lung disease, and respiratory failure; moderate certainty of a small-to-moderate increase of cardiomyopathy, hypertension, any diabetes, type 2 diabetes, any mental disorder, psychosis/psychotic disorders, myoneural junction/muscle disease, communication and motor disorders, dementia/mild cognitive disorder, epilepsy, haemorrhagic stroke, and transient ischaemic attack; and, moderate certainty of little-to-no difference for depression/mood disorders.
Two studies reported on how associations varied across time since infection or by variant of concern (). Change in risk over time since SARS-CoV-2 infection likely differs between conditions; however, there is not enough evidence to draw condition-specific conclusions at this time. One study reported on differing risks across variants of concern which suggested that risks may differ across variants, but these differences may also be confounded by average severity of acute disease and mortality of each variant. We did not identify any studies eligible for our review that looked at differences in risk between vaccinated vs. unvaccinated groups.
Table 4. Summary of time-varying effects and subgroup analyses by variant in a systematic review of new diagnoses of chronic conditions after SARS-CoV-2 infection.
Discussion
We conducted a systematic review to identify associations between SARS-CoV-2 infection and exacerbations of pre-existing or new diagnoses of chronic conditions. We stratified analyses by age category and severity of SARS-CoV-2 infection (using hospitalization during acute phase of infection as a proxy) to enable meaningful interpretation of the findings and because these are strong predictors of severity of outcomes both in the acute [Citation24] and recovery stages of COVID-19 [Citation25]. After SARS-CoV-2 infection, there is probably an increased risk of new diagnoses for some, but not all chronic conditions. In general, we had the most certainty in associations between SARS-CoV-2 infection and new diagnoses of chronic conditions, especially cardiac conditions, in outpatient/mixed care samples aged ≥65 years. People in this age category are already at increased risk of many chronic conditions and are more susceptible to poor outcomes after SARS-CoV-2 infection [Citation24,Citation26]. We also had moderate to high certainty in associations between SARS-CoV-2 infection and at least a small increase of new diagnoses of several chronic conditions in individuals 18–64 years old and a few chronic conditions in individuals <18 years (i.e. trauma and stress disorders, chronic fatigue syndrome, and stroke). We identified only two eligible studies reporting on associations between hospitalization with SARS-CoV-2 infection and new diagnosis of chronic conditions. While it is widely recognized that severity of initial SARS-CoV-2 infection leads to poorer long-term outcomes [Citation25], we were not able to draw conclusions in any age group regarding an association between SARS-CoV-2 infection and subsequent new diagnoses of chronic conditions among individuals hospitalized during the acute infection phase. Finally, although we did not identify any eligible studies reporting on exacerbations of pre-existing chronic conditions after SARS-CoV-2 infection, this does not preclude the existence of this relationship for some conditions.
While previous systematic reviews have reported on the incidence of newly diagnosed chronic conditions after SARS-CoV-2 infection or reported on associations with specific conditions [Citation7–12], this is the first systematic review we are aware of that reports on associations between SARS-CoV-2 infection and new diagnoses of a wide range of chronic conditions specifically by age group. Our strict eligibility criteria, including the requirement to account for sex and relevant comorbidities, also likely reduced the number of eligible studies at high risk of bias. Overall, our findings suggest that there is probably an increased risk of diagnoses for some – but not all – chronic conditions after SARS-CoV-2 infection. In general, cardiovascular diseases and respiratory conditions showed the most consistent effects across adult age categories and disease severities. These associations have implications for decision makers in both policy and healthcare systems at a time when healthcare systems are already under considerable strain As the number of individuals infected by SARS-CoV-2 increases, so too will the number of new diagnoses for chronic conditions, leading to increased health care utilization in the form of specialty care, follow-up with primary care providers and increasing medication and treatment costs at either the patient or system level.
One notable gap highlighted by our review is the lack of evidence around cancer diagnoses after SARS-CoV-2 infection. This is not surprising, as there has likely been insufficient time since the start of the pandemic for disease processes and diagnosis, and longitudinal studies of this association have already been proposed [Citation27]. However, such studies will have to be conducted with careful considerations of the impacts of the pandemic apart from SARS-CoV-2 infection. Public health restrictions during the early waves of the pandemic created access barriers to cancer screening and diagnosis, creating a potential backlog of missed screenings [Citation28]. This may have resulted in delayed diagnoses and therefore will need to be controlled for in the study design of any longitudinal studies examining cancer incidence after SARS-CoV-2 infection.
We also found very few eligible studies examining how the potential risk of being diagnosed with a new chronic condition changes over time since SARS-CoV-2 infection, as well as across different variants of concern. Based on human tissue cultures and animal models, SARS-CoV-2 variants may preferentially infect or replicate in different organ systems or tissues [Citation29–31], and thus may result in a changing constellation of new chronic disease diagnoses after SARS-CoV-2 infection.
Limitations
As with any systematic review, our synthesis comes with some limitations. First, while we made attempts to limit study eligibility to only those reporting on conditions documented or diagnosed by a medical provider, for some conditions it was not always possible to differentiate between chronic disorders versus persistent post-COVID symptoms. Additionally, while most studies included in this review used International Classification of Diseases (ICD)-10 codes (or similar administrative coding systems) to define outcomes of interest, there was substantial variation across studies in which codes were used to define each condition. This likely contributed to the substantial heterogeneity in estimates for some conditions. Second, some of the chronic conditions of interest are much simpler to diagnose than others. For example, diagnosis of type 2 diabetes relies on empirical signs and biological markers that can be objectively measured and diagnosed by primary care providers, whereas it may take a longer time after initially seeking care to be diagnosed with conditions such as chronic fatigue syndrome or mood disorders because they are typically diagnosed by specialists that patients may or may not have access to. Third, we included studies using control groups that did not explicitly require a negative SARS-CoV-2 test; thus it is possible in these studies that some individuals may have had a SARS-CoV-2 infection, especially during later stages of the pandemic when testing patterns shifted towards at-home testing [Citation56]. This potential contamination in some control groups may result in underestimated associations between SARS-CoV-2 infection and new chronic conditions diagnoses, including confounding the true existence of associations for which we have reported little-to-no association. Fourth, although some of the included studies attempted to control for differences in care-seeking behaviour between control and SARS-CoV-2 infected groups (e.g. by matching on index date and only including control participants with at least one health care contact after their determined index visit), we did not evaluate this potential confounder as part of our synthesis. Although we would not expect the ability to obtain a diagnosis to differ between infected and non-infected people who have sought care, there are likely differences in the number of health care contacts between the groups. Thus, some of the associations identified in our review may be the result of surveillance biases. In other words, an undiagnosed chronic condition may have been present in some individuals prior to SARS-CoV-2 infection, but seeking care for the infection (and subsequent health care contacts for follow-up) resulted in the undiagnosed condition being diagnosed when it otherwise may not have been until further on in the disease progression. Lastly, we used the event rates in non-SARS-CoV-2 infected control groups to estimate the excess incidence for conditions in which we had at least low certainty of some direction of effect, standardized to rates over six months; however, event rates were not always reported as 6-month rates. Our estimate of excess incidence assumes that the incidence of new diagnoses in the SARS-CoV-2 group is constant, e.g. that the rate is the same 2 months after infection as it is 6 months after infection, and there is no evidence that this assumption holds true.
Conclusion
After SARS-CoV-2 infection, there is probably an increased risk of diagnoses for some, but not all, chronic conditions. However, the extent of increased risk that is directly caused by SARS-CoV-2 is uncertain due to other factors, such as increased health care contacts or monitoring in infected individuals, which are difficult to fully account for in observational study designs. Although the findings of this review likely apply well to the pandemic period, reflecting the pandemic’s current impact on healthcare availability and people infected by the virus, it is uncertain whether the impact will remain stable into future years. Finally, how this risk changes over time since infection or by variant of concern is uncertain.
Supplemental Material
Download MS Word (773.4 KB)Acknowledgements
Thank you to Becky Skidmore for peer-reviewing the database search strategies. We also thank Jingxuan Zhang, Murray Weeks, and Cynthia Robitaille for their guidance and insightful comments on this manuscript. The Public Health Agency of Canada funded this work under Contract no. 4500429095. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors. No endorsement by the Public Health Agency of Canada is intended or inferred.
Data availability statement
Data are available from the authors on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Haleem A, Javaid M, Vaishya R. Effects of COVID-19 pandemic in daily life. Curr Med Res Pract. 2020;10(2):78–79.
- Government of Canada. COVID-19 epidemiology update: Key updates Ottawa, Canada. Ottawa: Government of Canada; 2023; [updated 2023-01-16;2023-01-18]. Available from: https://health-infobase.canada.ca/covid-19/#a2.
- Higgins V, Sohaei D, Diamandis EP, et al. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58(5):297–310.
- Zimmermann P, Pittet LF, Curtis N. The challenge of studying long COVID: an updated review. Pediatr Infect Dis J. 2022;41(5):424–426.
- Silva Andrade B, Siqueira S, de A, et al. Hematological profile of pregnant women with suspected Zika virus infection followed up at a referral service in manaus, Brazil. Viruses. 2021;13(4):710.
- Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob. Health. 2021;6(9):e005427.
- Banerjee M, Pal R, Dutta S. Risk of incident diabetes post-COVID-19: a systematic review and meta-analysis. Prim Care Diabetes. 2022;16(4):591–593.
- Veazie S, Lafavor B, Vela K, et al. Mental health outcomes of adults hospitalized for COVID-19: a systematic review. J Affect Disord Rep. 2022 Apr;8:100312.
- Renaud-Charest O, Lui LMW, Eskander S, et al. Onset and frequency of depression in post-COVID-19 syndrome: a systematic review. J Psychiatr Res. 2021;144:129–137.
- Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection. JAMA Netw Open. 2021;4(10):e2128568.
- Yang T, Yan MZ, Li X, et al. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: a systematic review and meta-analysis. Infection. 2022;50(5):1067–1109.
- Zeng N, Zhao YM, Yan W, et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: call for research priority and action. Mol Psychiatry. 2023;28(1):423–433.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89.
- Barua B, Jacques D. Comparing performance of universal health care countries, 2018. Vancouver: Fraser Institute; 2018.
- Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012 Apr;28(2):138–144.
- Tartof SY, Malden DE, Liu IA, et al. Health care utilization in the 6 months following SARS-CoV-2 infection. JAMA Netw Open. 2022;5(8):e2225657.
- McGowan J, Sampson M, Salzwedel DM, et al. Press peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46.
- DistillerSR Inc. Ai review Ottawa, Canada: Evidence Partners; 2022 [cited 2023 Jan 19, 2023]. Available from: http://v2dis-help.evidencepartners.com/1/en/topic/ai-preview-and-rank.
- Burns JK, Etherington C, Cheng-Boivin O, et al. Using an artificial intelligence tool can be as accurate as human assessors in level one screening for a systematic review. Health Info Libr J. 2021;00:1–13.
- Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs institute reviewer's manual. Adelaide: The Joanna Briggs Institute; 2017:219–271.
- Schünemann H, Brożek J, Guyatt G, et al. GRADE Handbook 2013 [cited 2023 Jan 13, 2023]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html.
- Zeng L, Brignardello-Petersen R, Hultcrantz M, et al. GRADE guidance 34: update on rating imprecision using a minimally contextualized approach. J Clin Epidemiol. 2022;150:216–224.
- Foroutan F, Guyatt G, Zuk V, et al. GRADE guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol. 2020 May;121:62–70.
- Gates M, Pillay J, Wingert A, et al. Risk factors associated with severe outcomes of COVID-19: a systematic rapid review to inform national guidance on vaccine prioritization in Canada [pre-print]. MedRxiv. 2021 Nov:2021.04.23.21256014.
- Pillay J, Rahman S, Guitard S, et al. Risk factors and preventive interventions for post COVID-19 condition: systematic review. Emerg Microbes Infect. 2022;11(1):2762–2780.
- Yek C, Warner S, Wiltz JL, et al. Risk factors for severe COVID-19 outcomes among persons aged >/ = 18 years who completed a primary COVID-19 vaccination series - 465 health care facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep. 2022 Jan 7;71(1):19–25.
- Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. Bioessays. 2021;43(6):2000331.
- Decker KM, Feely A, Bucher O, et al. Evaluating the impact of the COVID-19 pandemic on cancer screening in a central Canadian province. Prev Med. 2022;155:106961.
- Halfmann PJ, Iida S, Iwatsuki-Horimoto K, et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603:687–692.
- Hui KPY, Ho JCW, Cheung MC, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–720.
- Natekar JP, Pathak H, Stone S, et al. Differential pathogenesis of SARS-CoV-2 variants of concern in human ACE2-expressing mice. Viruses. 2022;14(6):1139.
- Abel KM, Carr MJ, Ashcroft DM, et al. Association of SARS-CoV-2 infection with psychological distress, psychotropic prescribing, fatigue, and sleep problems among UK primary care patients. JAMA Network Open. 2021;4(11):e2134803.
- Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-COVID syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ (Clinical Research ed). 2021;372:n693.
- Bohlken J, Weber K, Riedel Heller S, et al. Mild cognitive disorder in post-COVID-19 syndrome: a retrospective cohort study of 67,000 primary care post-COVID patients. Journal of Alzheimer's Disease Reports. 2022;6(1):297–305.
- Chevinsky JR, Tao G, Lavery AM, et al. Late conditions diagnosed 1-4 months following an initial coronavirus disease 2019 (COVID-19) encounter: a matched-cohort study using inpatient and outpatient administrative data-United States, 1 March-30 June 2020. Clin Infect Dis. 2021 Jul 15;73(Suppl 1):S5–S16.
- Cohen K, Ren S, Heath K, et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ (Clinical Research ed). 2022;376:e068414.
- Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ (Clinical Research ed). 2021;373:n1098.
- Donnachie E, Hapfelmeier A, Linde K, et al. Incidence of post-COVID syndrome and associated symptoms in outpatient care in Bavaria, Germany: a retrospective cohort study using routinely collected claims data. BMJ Open. 2022;12(9):e064979.
- Jacob L, Koyanagi A, Smith L, et al. No significant association between COVID-19 diagnosis and the incidence of depression and anxiety disorder? A retrospective cohort study conducted in Germany. J Psychiatr Res. 2022;147:79–84.
- Kompaniyets L, Bull-Otterson L, Boehmer TK, et al. Post-COVID-19 symptoms and conditions Among children and adolescents - United States, march 1, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(31):993–999.
- Park HY, Song I-A, Lee SH, et al. Prevalence of mental illness among COVID-19 survivors in South Korea: nationwide cohort. BJPsych Open. 2021;7(6):e183.
- Pietropaolo M, Hotez P, Giannoukakis N. Incidence of an insulin-requiring hyperglycemic syndrome in SARS CoV-2-infected young individuals: Is it type 1 diabetes? Diabetes. 2022;71(12):2656–2663.
- Qureshi AI, Baskett WI, Huang W, et al. New-Onset dementia among survivors of pneumonia associated with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2022;9(4):ofac115.
- Rao S, Lee GM, Razzaghi H, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. 2022;176(10):1000–1009.
- Rezel-Potts E, Douiri A, Sun X, et al. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med 2022;19(7):e1004052.
- Roessler M, Tesch F, Batram M, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: a matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med. 2022;19(11):e1004122.
- Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815–827.
- Taquet M, Luciano S, Geddes JR, et al. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140.
- Wang L, Davis PB, Volkow ND, et al. Association of COVID-19 with New-onset Alzheimer's disease. Journal of Alzheimer's Disease: Jad. 2022;89(2):411–414.
- Wang W, Wang C-Y, Wang S-I, et al. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. 2022;53:101619.
- Westman G, Zelano J. Epilepsy diagnosis after COVID-19: A population-wide study. Seizure. 2022;101:11–14.
- Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321.
- Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28(3):583–590.
- Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med. 2022;28:2406–2415.
- Zarifkar P, Peinkhofer C, Benros ME, et al. Frequency of neurological diseases after COVID-19, influenza A/B and bacterial pneumonia. Front Neurol. 2022;13:904796.
- Rader B, Gertz A, Iuliano AD, et al. Use of At-home COVID-19 tests - United States, August 23, 2021-march 12, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):489–494.

