ABSTRACT
Extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) bacteremia can have poor clinical outcomes. Thus, determining the predictors of mortality from ESBL-PE bacteremia is very important. The present systematic review and meta-analysis aimed to evaluate studies to determine predictors associated with ESBL-PE bacteremia mortality. We searched PubMed and Cochrane Library databases for all relevant publications from January 2000 to August 2022. The outcome measure was mortality rate. In this systematic review of 22 observational studies, 4607 patients with ESBL-PE bacteremia were evaluated, of whom 976 (21.2%) died. The meta-analysis showed that prior antimicrobial therapy (RR, 2.89; 95% CI, 1.22–6.85), neutropenia (RR, 5.58; 95% CI, 2.03–15.35), nosocomial infection (RR, 2.46; 95% CI, 1.22–4.95), rapidly fatal underlying disease (RR, 4.21; 95% CI, 2.19–8.08), respiratory tract infection (RR, 2.12; 95% CI, 1.33–3.36), Pitt bacteremia score (PBS) (per1) (RR, 1.35; 95% CI, 1.18–1.53), PBS ≥ 4 (RR, 4.02; 95% CI, 2.77–5.85), severe sepsis (RR, 11.74; 95% CI, 4.68–29.43), and severe sepsis or septic shock (RR, 4.19; 95% CI, 2.83–6.18) were found to be mortality predictors. Moreover, urinary tract infection (RR, 0.15; 95% CI, 0.04–0.57) and appropriate empirical therapy (RR, 0.39; 95% CI, 0.18–0.82) were found to be a protective factor against mortality. Patients with ESBL-PE bacteremia who have the aforementioned require prudent management for improved outcomes. This research will lead to better management and improvement of clinical outcomes of patients with bacteremia caused by ESBL-PE.
Introduction
Extended-spectrum beta-lactamases (ESBLs) are enzymes that confer resistance to various types of beta-lactam antibiotics, including oxyimino-cephalosporins (cefotaxime, ceftriaxone, cefuroxime, cefixime, ceftazidime, cefepime, and cefpirome) and monobactams (aztreonam) [Citation1, Citation2]. ESBLs are produced by Enterobacteriaceae bacteria, mainly Escherichia coli and Klebsiella pneumoniae [Citation3]. In recent years, the spread of ESBL-producing Enterobacteriaceae (ESBL-PE) has increased rapidly worldwide [Citation4]. Patients with ESBL-PE bacteremia can have poor clinical outcomes due to delayed appropriate antimicrobial therapy and limited therapeutic options [Citation5]; hence, ESBL-PE has become a clinically critical issue. Recent studies reported mortality rates ranging from approximately 12% to 41% among patients with ESBL-PE bacteremia [Citation6-11]. Therefore, when treating patients with ESBL-PE bacteremia, determining the predictors of mortality from ESBL-PE bacteremia is very important.
Several recent studies have reported different risk factors associated with ESBL-PE bacteremia mortality, including nosocomial infection [Citation10, Citation11], transfer to intensive care unit [Citation12, Citation13], respiratory tract infection [Citation6, Citation12], non-urinary tract infection [Citation13, Citation14], age [Citation10, Citation14], and severe sepsis or septic shock [Citation8, Citation9, Citation11, Citation14]. However, our literature search revealed no study focusing on the meta-analysis of predictors associated with ESBL-PE bacteremia mortality. The present systematic review and meta-analysis aimed to evaluate studies in order to determine predictors associated with ESBL-PE bacteremia mortality.
Materials and methods
Literature review
The systematic review and meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Furthermore, the research model was constructed based on previous studies [Citation15,Citation16], with slight modifications. A methodical search for the reviewed literature was conducted in PubMed and Cochrane Library databases for all publications from January 2000 to August 2022. Our search comprised three keywords or phrases: “extended spectrum beta lactamase or ESBL,” “bacteremia or bloodstream infection,” and “mortality or fatality or lethality or prognosis or predictor.” The search was conducted taking into account all the three keywords and the phrases in combination. Results were restricted to full-text articles available in English.
For the purpose of our review, we included clinical trials, cohort studies, case–control studies, and cross-sectional studies that had determined cases of ESBL-producing Enterobacteriaceae bacteremia. By contrast, reviews, systematic reviews, meta-analyses, guidelines, editorials, letters to the editor, comments, case reports, animal research, in vitro studies, research focused on children, studies involving < 20 patients per group, and studies performing inappropriate multivariate analysis using automatic selection methods such as stepwise regression and wherein only items with a predefined small p-value in the univariate analysis were included in the multivariate regression model were excluded. Both monomicrobial and polymicrobial episodes were considered for inclusion. The primary outcome was mortality. We did not have any limitation in place regarding the cause of death (infection-attributed or not) or the days to death.
Data extraction and quality assessment
Data were extracted from the literature by two independent reviewers and standardized using an established format that included the following study variables: first author, country, study design, year of publication, study duration, number of patients with ESBL-producing Enterobacteriaceae bacteremia, mortality, and statistical analysis method. Discrepancies or disagreements, if any, were resolved by mutual discussion and consensus. We performed quality assessment using the Newcastle–Ottawa Scale.
Statistical analyses
Multivariate model-adjusted measurements were used as main effect estimates. Risk ratio (RR; odds ratio or hazard ratio [HR]) is an appropriate effect estimate for cohort and case–control studies, and only studies that reported or allowed for the calculation of RR and error estimates (confidence intervals [CIs] and standard error) were included in the quantitative data synthesis. We performed a meta-analysis of the parameters for which RR was reported in three or more studies and wherein at least one statistically significant association was identified. Furthermore, we conducted a subgroup analysis to compare the clinical effectiveness of carbapenems and carbapenem-sparing regimens. We estimated the RR and 95% CIs for all-cause mortality based on the number of individuals at risk and number of deaths. We performed a meta-analysis of identical regimens in three or more studies. As the background factors and combinations of confounding factors were different in different articles, a meta-analysis was conducted using a random-effects model. The results of the meta-analysis are presented as forest plots; the Cochran's Q test was used to assess heterogeneity. An I2 value between 50% and 100% was a prerequisite for considering the presence of statistical heterogeneity. Publication bias and small-study effects were investigated by visually assessing the funnel plots. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a modified version of the R Commander that includes the statistical functions that are frequently used in biostatistics.
Results
Study selection and characteristics
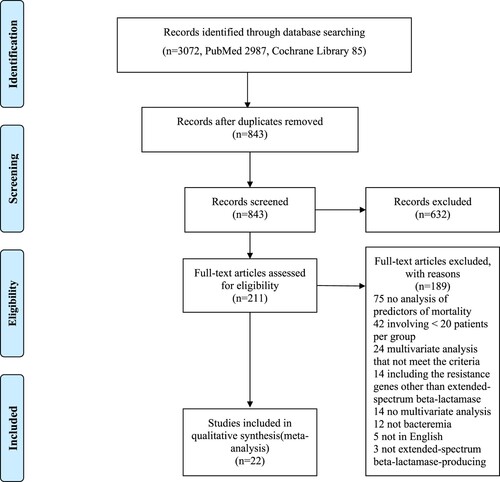
summarizes the study identification process in the form of a PRISMA flow diagram. The search revealed that 3072 existent studies were relevant to the present literature review. After the elimination of 2229 duplicates, 843 records were screened based on the title and abstract. Of these, 632 records were further eliminated after we reviewed the title and/or the abstract. The remaining 211 papers were assessed in a full-text review, and 189 articles were further excluded at this stage for the following reasons: no analysis of the predictors of mortality (n = 75), did not meet the case-load criteria (n = 42), multivariate analysis that did not meet the required criteria (n = 24), inclusion of resistance genes other than ESBL (n = 14), no multivariate analysis (n = 14), no bacteremia (n = 12), not in English (n = 5), and not including ESBL-PE (n = 3) (). After a complete textual appraisal, 22 studies were included in the present review [Citation9-11,Citation13,Citation17-34]. The key attributes of the selected articles are summarized in . These studies were published between 2004 and 2022. The studies were carried out in 16 countries (Korea, Italy, Spain, Taiwan, the United States of America, Israel, Singapore, Germany, Greece, Turkey, South Africa, Canada, Argentina, France, China, and Japan) on five continents (Asia, Europe, North America, Africa, and South America) and were all observational; one was a prospective study, 20 were retrospective studies, and the study type of one was unknown. The sample size ranged from 48 to 622. A total of 4607 patients with ESBL-PE bacteremia were evaluated, of whom 976 (21.2%) died. The assessment of the outcomes was set at 30 days in more than half of the studies.
Table 1. Characteristics of studies included in the systematic literature review and meta-analysis.
Meta-analysis
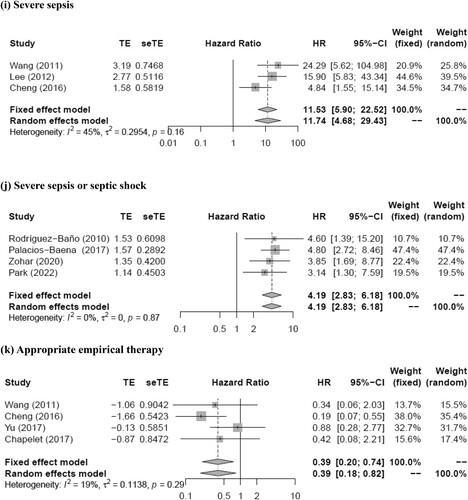
lists the parameters for which the RR for death caused by ESBL-PE bacteremia was reported in the multivariate analysis of the 22 selected studies. The parameters for which the RR was reported in three or more studies and for which at least one statistically significant association was identified were prior antimicrobial therapy (within 30 days before bacteremia), neutropenia, nosocomial infection, Charlson score, rapidly fatal underlying disease, respiratory tract infection (especially pneumonia), urinary tract infection, Pitt bacteremia score (PBS; per1), PBS ≥ 4, severe sepsis, severe sepsis or septic shock, appropriate empirical therapy, and piperacillin/tazobactam.
Table 2. Results of studies performing multivariable analyses regarding mortality in patients with ESBL-PE bacteremia.
The meta-analysis showed that prior antimicrobial therapy (total number of patients, 614; pooled RR, 2.89; 95% CI, 1.22–6.85; p = 0.016), neutropenia (total number of patients, 464; pooled RR, 5.58; 95% CI, 2.03–15.35; p = 0.0009), nosocomial infection (total number of patients, 1420; pooled RR, 2.46; 95% CI, 1.22–4.95; p = 0.012), rapidly fatal underlying disease (total number of patients, 661; pooled RR, 4.21; 95% CI, 2.19–8.08; p < 0.0001), respiratory tract infection (total number of patients, 1817; pooled RR, 2.12; 95% CI, 1.33–3.36; p = 0.0015), urinary tract infection (total number of patients, 611; pooled RR, 0.15; 95% CI, 0.04–0.57; p = 0.0056), PBS (per1) (total number of patients, 688; pooled RR, 1.35; 95% CI, 1.18–1.53; p < 0.0001), PBS ≥ 4 (total number of patients, 1534; pooled RR, 4.02; 95% CI, 2.77–5.85; p < 0.0001), severe sepsis (total number of patients, 475; pooled RR, 11.74; 95% CI, 4.68–29.43; p < 0.0001), severe sepsis or septic shock (total number of patients, 1235; pooled RR, 4.19; 95% CI, 2.83–6.18; p < 0.0001), and appropriate empirical therapy (total number of patients, 412; pooled RR, 0.39; 95% CI, 0.18–0.82; p = 0.013) were mortality predictors (). Moreover, most of these factors had I2 values < 50% in terms of heterogeneity (prior antimicrobial therapy: I2 = 28%, Q = 2.77, p = 0.25; neutropenia: I2 = 0%, Q = 1.07, p = 0.59; nosocomial infection: I2 = 57%, Q = 11.51, p = 0.04; rapidly fatal underlying disease: I2 = 16%, Q = 2.37, p = 0.31; respiratory tract infection: I2 = 43%, Q = 12.32, p = 0.09; urinary tract infection: I2 = 42%, Q = 5.21, p = 0.16; PBS (per1): I2 = 38%, Q = 3.22, p = 0.20; PBS ≥ 4: I2 = 10%, Q = 4.44, p = 0.35; severe sepsis: I2 = 45%, Q = 3.62, p = 0.16; severe sepsis or septic shock: I2 = 0%, Q = 0.70, p = 0.87; appropriate empirical therapy: I2 = 19%, Q = 3.72, p = 0.29). The funnel plot showed no publication bias for any of the predictive factors. Meanwhile, no statistically significant pooled RR for mortality was detected for the Charlson score (pooled RR, 1.07; 95% CI, 0.94–1.21; p = 0.30) and piperacillin/tazobactam (pooled RR, 1.80; 95% CI, 0.81–3.98; p = 0.15). Moreover, both these factors had I2 values >50% in terms of heterogeneity (Charlson score, 51%; piperacillin/tazobactam, 55%).
Figure 2. Forest plot of risk ratio for mortality of patients with (a) prior antimicrobial therapy, (b) neutropenia, (c) nosocomial infection, (d) rapidly fatal underlying disease, (e) respiratory tract infection, (f) urinary tract infection, (g) Pitt bacteremia score (per1), (h) Pitt bacteremia score ≥4, (i) severe sepsis, (j) severe sepsis or septic shock, and (k) appropriate empirical therapy for extended-spectrum beta-lactamase-producing Enterobacteriaceae bacteremia.
Using subgroup analysis, the mortality associated with ESBL-PE bacteremia based on carbapenem- and carbapenem-sparing regimens is summarized in . Identical regimens were reported in three or more studies and included only carbapenems versus piperacillin-tazobactam (PTZ). Data from three studies, involving 443 patients, were subjected to meta-analysis of mortality rate. Carbapenems and PTZ were administered to 236 and 207 patients, respectively. Mortality occurred in 57 patients (24.2%) receiving carbapenems and 54 patients (26.1%) receiving PTZ. The meta-analysis showed no statistically significant difference in mortality among the two groups (Favours carbapenems: pooled RR, 0.55; 95% CI, 0.25–1.19; p = 0.13) (). Moreover, this factor had I2 values of < 50% in terms of heterogeneity (I2 = 46%, Q = 3.73, p = 0.15).
Figure 3. Forest plot showing the odds ratio of the mortality for carbapenems versus non-carbapenems in patients with extended-spectrum beta-lactamase-producing Enterobacteriaceae bacteremia.
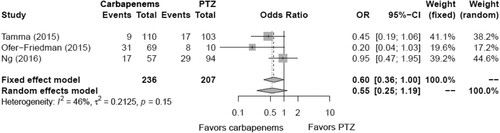
Table 3. Summary of mortality in ESBL-PE bacteremia patients according to antibiotic comparisons.
Discussion
To date, studies investigating the predictors of mortality in patients with ESBL-PE bacteremia have yielded inconsistent results. Therefore, access to a systematic and comprehensive summary of the existing evidence is essential for all clinicians involved in the care of patients with infectious diseases to ensure appropriate diagnosis, treatment, and preventive measures. In this systematic literature review, we assessed 22 observational studies on ESBL-PE bacteremia published between 2004 and 2022, which included 4607 patients, approximately 7.5 times the number of patients included in the largest simplex research. The meta-analysis revealed that prior antimicrobial therapy, neutropenia, nosocomial infection, rapidly fatal underlying disease, respiratory tract infection, urinary tract infection, PBS (per1), PBS ≥ 4, severe sepsis (or septic shock), and appropriate empirical therapy were predictors of mortality caused by ESBL-PE bacteremia. Of the above predictors, nosocomial infection, respiratory tract infection, and appropriate empirical therapy are considered to be of particular clinical importance.
In addition to the articles included in this review, a few studies reported that nosocomial infection was a predictor of mortality from ESBL-PE bacteremia [Citation35,Citation36]. A recent study also showed that nosocomial ESBL-PE bacteremia are associated with higher mortality compared with community-onset ESBL-PE bacteremia [Citation18]. We believe that two factors play a major role in the high mortality associated with nosocomial ESBL-PE bacteremia: differences in pathogenicity and differences in antimicrobial susceptibility. Regarding differences in pathogenicity, some studies reported that the frequency of highly pathogenic sequence type 131 C1/H30-R and/or C2/H30-Rx in nosocomial ESBL E. coli was relatively high [Citation37,Citation38]. Furthermore, Liu et al. showed that the incidence of hypervirulent strains in nosocomial ESBL K. pneumoniae has increased [Citation39]. Meanwhile, regarding differences in antimicrobial susceptibility, previous studies indicated that nosocomial ESBL-producing isolates were more resistant than community-acquired isolates [Citation40,Citation41]. Therefore, patients with nosocomial infection may receive inappropriate antimicrobial therapy more frequently than community-acquired patients. A few studies have reported that the length of hospital stay from admission to onset is longer in non-survivors of ESBL-PE bacteremia than in the survivors [Citation19,Citation42]. A prolonged hospital stay can lead to adverse events, including nosocomial infections and a decline in functional status [Citation43]. Furthermore, Marfil-Garza et al. demonstrated that a prolonged hospital stay is associated with increased mortality and other poor outcomes [Citation44]. The abovementioned factors support the finding that nosocomial infection is associated with an increased risk mortality in patients with ESBL-PE bacteremia.
In addition to the articles examined in our review, a few studies have cited respiratory tract infection as a predictor of mortality caused by ESBL-PE bacteremia [Citation6,Citation45]. Cheng et al. reported that both severity and mortality among patients with bacteremic pneumonia caused by ESBL-producing E. coli or K. pneumoniae were very high (PBS ≥4, 42.3%; severe sepsis, 52.3%; crude mortality, 55.9%) [Citation20]. Furthermore, Harada et al. showed that higher amounts of exposed bacteria in pulmonary infections caused by ESBL K. pneumoniae increased the minimal inhibitory concentration of antibiotics due to the inoculum effect [Citation46]. These findings support that respiratory tract infection is associated with an increased risk of mortality in patients with ESBL-PE bacteremia. Therefore, we need to be particularly vigilant for respiratory tract infections among various infection sources in patients with ESBL-PE bacteremia.
In addition to the articles included in this review, a few studies have reported that appropriate empirical therapy was a protective factor against mortality from ESBL-PE bacteremia [Citation47]. Biehl et al. indicated that inadequate treatment for patients with ESBL-PE infection led to worse outcomes and survival [Citation48]. Furthermore, Gutiérrez-Gutiérrez and Rodríguez-Baño reported that delay in initiating active antibiotic therapy may be associated with a high mortality rate for ESBL infections [Citation49]. These findings suggest that appropriate empirical therapy is associated with a decreased mortality risk in patients with ESBL-PE bacteremia. In our study, PTZ did not account for a statistically significant increase in mortality compared with carbapenems. However, the number of studies and cases may be too low for definitive conclusions. Current treatment options for ESBL-PE infections include ceftolozane/tazobactam, ceftazidime/avibactam, aminoglycosides, and fosfomycin, as well as carbapenems and PTZ [Citation50]. Selecting appropriate antimicrobial agents for ESBL-PE bacteremia according to infection source and severity is very important. Therefore, to establish the optimal treatment for patients with ESBL-PE bacteremia, we need to collect and analyze data from more patients and compare the clinical effectiveness of carbapenems and carbapenem-sparing regimens.
In addition to the articles included in this review, a few studies have reported that prior antimicrobial therapy was a predictor of mortality from ESBL-PE bacteremia [Citation51,Citation52]. However, in general, prior antimicrobial therapy leads to the acquisition of or infection by multi-drug resistant organisms, instead of mortality [Citation53]. Ku et al. reported that the association between prior antimicrobial therapy and increased mortality might be due to a high degree of antimicrobial resistance of causative organisms isolated from patients with a history of antimicrobial therapy [Citation21]. Therefore, such patients may develop more complicated infections with a poor prognosis. These findings suggest that whether prior antimicrobial therapy is a predictor of mortality from ESBL-PE bacteremia is debatable. Future studies are required to collect and review further data related to prior antimicrobial therapy and increased mortality.
The present study has several limitations. First, we used only two databases (PubMed and Cochrane Library) for the literature search. Second, we limited our selection to only articles written in English, which restricts the scope of our analysis. Third, although we attempted to minimize the effects of confounding factors as much as possible by excluding studies with inappropriate multivariate analysis and by performing a meta-analysis using a random-effect model, we were unable to completely eliminate their influence. Fourth, most studies used a retrospective study design and may have been susceptible to selection bias. Fifth, the outcome of mortality has not been assessed at the same point of time in all studies; nevertheless, it was assessed at 30 days in approximately half of the studies. Sixth, obtaining a clear inference from the current evidence may be difficult because some studies may have included participants with polymicrobial bacteremia or resistance genes other than ESBL. Seventh, concerns remain about the sample size for each predictor as only three to five studies were included in most meta-analyses. Finally, we performed the analysis without unifying the bacterial species or considering the genotypes. Therefore, future studies are required to analyze the bacterial species or genotypes in ESBL-PE causing bacteremia during data collection and to further establish the predictive factors against mortality.
In conclusion, the present study demonstrated that prior antimicrobial therapy, neutropenia, nosocomial infection, rapidly fatal underlying disease, respiratory tract infection, urinary tract infection, PBS (per1), PBS ≥ 4, severe sepsis (or septic shock), and appropriate empirical therapy are predictors of mortality caused by ESBL-PE bacteremia. Therefore, patients with ESBL-PE bacteremia who have the aforementioned factors require prudent management for improved outcomes. Based on the above findings, we believe that this research will lead to better treatment and improvement of the clinical outcomes of patients with ESBL-PE bacteremia.
Authors’ contributions
NH designed the study, performed literature search, reviewed the literature, and wrote the first draft. IW performed literature search and reviewed the literature. YK and KH designed the study and critically revised the draft. TY, KY, and ST critically revised the draft. All authors contributed to the final version of the manuscript and approved its submission.
Acknowledgements
We would like to thank Honyaku Center Inc. for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
- Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166.
- Peirano G, Pitout JDD. Extended-spectrum β-lactamase-producing Enterobacteriaceae: update on molecular epidemiology and treatment options. Drugs. 2019;79:1529–1541.
- Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951.
- Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in U.S. Hospitalized patients, 2012-2017. N Engl J Med. 2020;382:1309–1319.
- McDanel J, Schweizer M, Crabb V, et al. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiellainfections in the United States: a systematic literature review. Infect Control Hosp Epidemiol. 2017;38:1209–1215.
- Xiao T, Wu Z, Shi Q, et al. A retrospective analysis of risk factors and outcomes in patients with extended-spectrum beta-lactamase-producing Escherichia coli bloodstream infections. J Glob Antimicrob Resist. 2019;17:147–156.
- Hung WT, Cheng MF, Tseng FC, et al. Bloodstream infection with extended-spectrum beta-lactamase-producing Escherichia coli: the role of virulence genes. J Microbiol Immunol Infect. 2019;52:947–955.
- Namikawa H, Yamada K, Yamairi K, et al. Mortality caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae bacteremia; a case control study: alert to Enterobacteriaceae strains with high minimum inhibitory concentrations of piperacillin/tazobactam. Diagn Microbiol Infect Dis. 2019;94:287–292.
- Zohar I, Schwartz O, Yossepowitch O, et al. Aminoglycoside versus carbapenem or piperacillin/tazobactam treatment for bloodstream infections of urinary source caused by Gram-negative ESBL-producing Enterobacteriaceae. J Antimicrob Chemother. 2020;75:458–465.
- Benetazzo L, Delannoy PY, Houard M, et al. Combination therapy with aminoglycoside in Bacteremiasdue to ESBL-producing Enterobacteriaceae in ICU. Antibiotics (Basel). 2020;9:777.
- Park JJ, Jung EJ, Kim JY, et al. Thirty-day mortality rates in patients with extended-spectrum β-lactamase-producing Enterobacterales bacteremia receiving ertapenem versus other carbapenems. Antimicrob Agents Chemother. 2022;66:e0028722.
- Joo EJ, Park DA, Lee NR, et al. Impact of appropriateness of empiric therapy on outcomes in community-onset bacteremia by extended-spectrum-β-lactamase producing Escherichia coli and Klebisella pneumoniae definitively treated with carbapenems. Eur J Clin Microbiol Infect Dis. 2017;36:2093–2100.
- Ko JH, Lee NR, Joo EJ, et al. Appropriate non-carbapenems are not inferior to carbapenems as initial empirical therapy for bacteremia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: a propensity score weighted multicenter cohort study. Eur J Clin Microbiol Infect Dis. 2018;37:305–311.
- Scheuerman O, Schechner V, Carmeli Y, et al. Comparison of predictors and mortality between bloodstream infections caused by ESBL-producing Escherichia coli and ESBL-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2018;39:660–667.
- Namikawa H, Oinuma KI, Yamada K, et al. Predictors of hypervirulent Klebsiella pneumoniae infections: a systematic review and meta-analysis. J Hosp Infect. 2023;134:153–160.
- Namikawa H, Oinuma KI, Yamada K, et al. Differences in severity of bacteraemia caused by hypermucoviscous and non-hypermucoviscous Klebsiella pneumoniae. Int J Antimicrob Agents. 2023;61:106767.
- Kang CI, Kim SH, Park WB, et al. Bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother. 2004;48:4574–4581.
- Palacios-Baena ZR, Gutiérrez-Gutiérrez B, De Cueto M, et al. Development and validation of the INCREMENT-ESBL predictive score for mortality in patients with bloodstream infections due to extended-spectrum-β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2017;72:906–913.
- Xiao T, Yang K, Zhou Y, et al. Risk factors and outcomes in non-transplant patients with extended-spectrum beta-lactamase-producing Escherichia coli bacteremia: a retrospective study from 2013 to 2016. Antimicrob Resist Infect Control. 2019;8:144.
- Cheng WL, Hsueh PR, Lee CC, et al. Bacteremic pneumonia caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: appropriateness of empirical treatment matters. J Microbiol Immunol Infect. 2016;49:208–215.
- Ku NS, Kim YC, Kim MH, et al. Risk factors for 28-day mortality in elderly patients with extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae bacteremia. Arch Gerontol Geriatr. 2014;58:105–109.
- Tumbarello M, Sali M, Trecarichi EM, et al. Bloodstream infections caused by extended-spectrum-β-lactamase- producing Escherichia coli: risk factors for inadequate initial antimicrobial therapy. Antimicrob Agents Chemother. 2008;52:3244–3252.
- Rodríguez-Baño J, Picón E, Gijón P, et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-β-lactamase-producing Escherichia coli. J Clin Microbiol. 2010;48:1726–1731.
- Wang SS, Lee NY, Hsueh PR, et al. Clinical manifestations and prognostic factors in cancer patients with bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. J Microbiol Immunol Infect. 2011;44:282–288.
- Chung HC, Lai CH, Lin JN, et al. Bacteremia caused by extended-spectrum-β-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob Agents Chemother. 2012;56:618–622.
- Lee NY, Lee CC, Huang WH, et al. Carbapenem therapy for bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli or Klebsiella pneumoniae: implications of ertapenem susceptibility. Antimicrob Agents Chemother. 2012;56:2888–2893.
- Tamma PD, Han JH, Rock C, et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis. 2015;60:1319–1325.
- Ofer-Friedman H, Shefler C, Sharma S, et al. Carbapenems versus piperacillin-tazobactam for bloodstream infections of nonurinary source caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Infect Control Hosp Epidemiol. 2015;36:981–985.
- Lee CH, Su LH, Chen FJ, et al.. Comparative effectiveness of flomoxef versus carbapenems in the treatment of bacteraemia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae with emphasis on minimum inhibitory concentration of flomoxef: a retrospective study. Int J Antimicrob Agents. 2015;46:610–615.
- Ng TM, Khong WX, Harris PN, et al. Empiric piperacillin-tazobactam versus carbapenems in the treatment of bacteraemia due to extended-spectrum beta-lactamase-producing Enterobacteriaceae. PLOS ONE. 2016;11:e0153696.
- Yu WL, Lee MF, Chen CC, et al. Impacts of hypervirulence determinants on clinical features and outcomes of bacteremia caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Microb Drug Resist. 2017;23:376–383.
- Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50:355–361.
- Chapelet G, Boureau AS, Dylis A, et al. Association between dementia and reduced walking ability and 30-day mortality in patients with extended-spectrum beta-lactamase-producing Escherichia coli bacteremia. Eur J Clin Microbiol Infect Dis. 2017;36:2417–2422.
- Mitsuboshi S, Tsuruma N, Watanabe K, et al. Advanced age is not a risk factor for mortality in patients with bacteremia caused by extended-spectrum β-lactamase-producing organisms: a multicenter cohort study. Jpn J Infect Dis. 2020;73:288–292.
- Peralta G, Lamelo M, Alvarez-García P, et al. Impact of empirical treatment in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. bacteremia. A multicentric cohort study. BMC Infect Dis. 2012;12:245.
- Cho SY, Kang CI, Cha MK, et al. Clinical features and treatment outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli sequence type 131. Microb Drug Resist. 2015;21:463–469.
- Price LB, Johnson JR, Aziz M, et al. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 2013; 4:e00377–e00313.
- Merino I, Hernández-García M, Turrientes MC, et al. Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J Antimicrob Chemother. 2018;73:3039–3043.
- Liu C, Du P, Xiao N, et al. Hypervirulent Klebsiella pneumoniae is emerging as an increasingly prevalent K. pneumoniae pathotype responsible for nosocomial and healthcare-associated infections in Beijing, China. Virulence. 2020;11:1215–1224.
- Latifpour M, Gholipour A, Damavandi MS. Prevalence of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in nosocomial and community-acquired urinary tract infections. Jundishapur J Microbiol. 2016;9:e31179.
- Jobayer M, Afroz Z, Nahar SS, et al. Antimicrobial susceptibility pattern of extended-spectrum beta-lactamases producing organisms isolated in a tertiary Care Hospital, Bangladesh. Int J Appl Basic Med Res. 2017;7:189–192.
- Sakellariou C, Gürntke S, Steinmetz I, et al. Sepsis caused by extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: comparison of severity of sepsis, delay of anti-infective therapy and ESBL genotype. PLoS One. 2016;11:e0158039.
- Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002;39:238–247.
- Marfil-Garza BA, Belaunzarán-Zamudio PF, Gulias-Herrero A, et al. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS One. 2018;13:e0207203.
- Meini S, Laureano R, Tascini C, et al. Clinical outcomes of elderly patients with bloodstream infections due to extended-spectrum β-lactamase-producing Enterobacteriaceae in an Italian Internal Medicine ward. Eur J Intern Med. 2018;48:50–56.
- Harada Y, Morinaga Y, Kaku N, et al. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2014;20:831–838.
- Rodríguez-Baño J, Mingorance J, Fernández-Romero N, et al. Outcome of bacteraemia due to extended-spectrum β-lactamase-producing Escherichia coli: impact of microbiological determinants. J Infect. 2013;67:27–34.
- Biehl LM, Schmidt-Hieber M, Liss B, et al. Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients – review of the literature from a clinical perspective. Crit Rev Microbiol. 2016;42:1–16.
- Gutiérrez-Gutiérrez B, Rodríguez-Baño J. Current options for the treatment of infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae in different groups of patients. Clin Microbiol Infect. 2019;25:932–942.
- Karaiskos I, Giamarellou H. Carbapenem-sparing strategies for ESBL producers: when and how. Antibiotics (Basel). 2020;9:61.
- Du B, Long Y, Liu H, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae bloodstream infection: risk factors and clinical outcome. Intensive Care Med. 2002;28:1718–1723.
- Perianes-Díaz ME, Novo-Veleiro I, Solís-Díaz K, et al. Bacteriemia por Escherichia coli y Klebsiella pneumoniae productoras de betalactamasas de espectro extendido: factores asociados a mortalidad y reingreso hospitalario. Med Clin (Barc). 2014;142:381–386.
- Reuland EA, Naiemi A, Kaiser N, et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J Antimicrob Chemother. 2016;71:1076–1082.