Abstract
Drought and salt stress are the most common abiotic stresses resulting in yield reduction or complete loss of agricultural production in recent years. Stress mitigation by external biostimulator molecules has been an active research topic recently. Neurotransmitters (NTs) dopamine and progesterone, found in both animal and plant kingdom were shown to take role in plant abiotic and biotic stress defense in limited number of studies. This study investigated the effects of exogenous dopamine and progesterone application in tomato seedlings under drought and salt stress by examining various morphological and physiological parameters (tissue length and weights, relative water content, ion leakage, malondialdehyde and proline levels), as well as expressions of various genes encoding enzymes; superoxide dismutase (FeSOD), catalase (CAT2), glutathione reductase (GR1), ascorbate peroxidase (APX1), 1-aminocyclopropane-1-carboxylic acid synthase (ACS2) and delta 1-pyrroline-5-carboxylate synthase (P5CS), that play a direct role in the antioxidative defense system or measured as stress indicators. The results showed that dopamine and progesterone alleviated drought stress mainly by increasing superoxide dismutase and catalase antioxidative enzyme gene expressions and decreasing ethylene production in tomato seedlings, thereby improving cell membrane integrity and increasing root dry weight. Although morphological and physiological responses of the seedlings were mostly similar under drought and salinity stresses, antioxidative defense enzyme gene expressions were not upregulated under salinity stress, except for the GR1 expression under progesterone treatment.
REVIEWING EDITOR:
Introduction
Drought and salinity are among the most common abiotic stresses restricting current agricultural production worldwide (Angon et al., Citation2022; Kopecká et al., Citation2023). In recent years, unexpected droughts have become very common throughout the world due to climate change (Yadav et al., Citation2020; Zomer et al., Citation2017). Drought stress results in cellular dehydration, which destabilizes membrane structure and denatures proteins leading to necrosis (Sato et al., Citation2019).
High salt concentrations in the soil also restrict agricultural production due to the intensive usage of synthetic fertilizers and irrigation systems depending on groundwater, which contains high amounts of dissolved salts (Haj-Amor et al., Citation2022). Salinity stress exerts further destructive effects by causing ion toxicity and nutritional imbalances (I. Ahmad et al., Citation2023).
The common denominators of drought and salinity stresses are osmotic and oxidative stress resulting from stomatal closure, decreased turgor and photosynthesis efficiency, and accumulation of reactive oxygen and nitrogen species (Abdelaal et al., Citation2022). Both stresses reduce cell division and expansion thereby impairing agronomical traits and resulting in reduced growth, yield and quality (Muhammad et al., Citation2024).
Various molecules that regulate growth and development in plants are being investigated for their ameliorative potential under various stresses (Zulfiqar & Ashraf, Citation2020). Bioregulators and biostimulants are natural substances that improve crop productivity as well as stress tolerance and their use possesses a great potential for environmentally friendly and sustainable food production (Ma et al., Citation2022). Many bioregulator substances including methyl jasmonate, salicylic acid and melatonin were studied intensively, whereas a promising substance which is a biogenic amine or catecholamine dopamine received limited research attention. It is biosynthesized from tyrosine and is a very common active molecule in both plants and animals (Ahammed & Li, Citation2023).
Although the roles of dopamine in animals as a neurotransmitter regulating metabolism, hormone secretion, and control of movement, reward, and addiction processes are widely studied, its roles in plant tissues are largely unknown (Howe & Dombeck, Citation2016). Dopamine takes role in various plant physiological processes, including indole acetic acid (IAA) metabolism, photosynthesis, flowering, herbivory defense and nitrogen fixation (Roshchina, Citation2022). Recognition of the stress-mitigating effects of dopamine is a promising new field of study. A recent study reported increased levels of dopamine in apple leaves under drought and salt stress (Zhang et al., Citation2022). Exogenous dopamine application alleviated stress symptoms in apple plants under various stresses including drought, salt, nutrient deficiency, alkali, cadmium and waterlogging stress (Cao et al., Citation2023; Du et al., Citation2022; Gao et al., Citation2021; Jiao et al., Citation2019; C. Li et al., Citation2015; Liang et al., Citation2017; Zhang et al., Citation2023). Dopamine treatment was also effective under cadmium and floride stresses in duckweed and rice as well as under chilling and nitrogen deficiency stress in banana and lettuce (Ali et al., Citation2023; Chakraborty et al., Citation2022; Farouk et al., Citation2023; W. Wang et al., Citation2023). Dopamine also alleviated bisphenol A-induced phytotoxicity in cucumber plants and hydrocarbon stress in Brassica oleracea (Ahammed et al., Citation2020; A. Ahmad et al., Citation2021). Commonly observed effects of dopamine under stress conditions include increased photosynthetic rates, higher water use efficiencies, and improved antioxidant capacity (Gao et al., Citation2021; Lan et al., Citation2020; C. Li et al., Citation2015; Liang et al., Citation2017; Yogendra et al., Citation2016). Dopamine and its oxidation product melanin also show strong antioxidant capacities similar to those of ascorbic acid or catechin and play a direct role in the scavenging of reactive oxygen species (Gomes et al., Citation2014). In the presence of reactive oxygen species inside or outside of the cell, dopamine is transformed into red pigment dopachrome to mark the location of stress, which finally oxidized into black pigment melanin (Roshchina, Citation2022).
Another promising bioregulator molecule is progesterone, a well-known animal steroidal hormone essential for ovulation, luteinization, and pregnancy in mammals. It is also regarded as a neurotransmitter because of its effects of rapid change on the activity of neuronal systems by signaling through various receptors (Rudolph et al., Citation2016). Progesterone was detected in plants in the 1960s, and its presence has been demonstrated in a wide variety of plant species and in various plant tissues (Iino et al., Citation2007). Similar to animals, its roles in flowering and generative development have been characterized (Janeczko et al., Citation2003; Janeczko & Filek, Citation2002). Iino et al. (Citation2007) proposed that progesterone acts as a growth hormone in plant tissues and that its role and function are different from those of brassinosteroids, which are another group of plant steroidal hormones.
Progesterone was also shown to decrease necrosis and ion leakage and improve photosystem II efficiency in Arabidopsis under a biotic stress, Pseudomonas syringae infection (Janeczko et al., Citation2013). Progesterone was also shown to play a role in various abiotic stresses. Erdal (Citation2012a) showed that progesterone protects germinating maize seeds under salt stress. The molecule also protected wheat and chickpea seedlings from salt stress by both enzymatic and non-enzymatic antioxidative defense mechanisms and by increasing the anabolism of carbon compounds (Erdal, Citation2012b; Erdal & Dumlupınar, Citation2011). Progesterone mitigates drought, heat, and light stress in wheat by enhancing antioxidant defense system and photosystem II activity, by way of facilitating D1 protein phosphorylation (Janeczko et al., Citation2013; Su et al., Citation2014; Xue et al., Citation2017). Progesterone was also shown to protect chickpea and maize seedlings from chilling damage and to enter the lipid bilayer (Erdal & Genisel, Citation2016; Genisel et al., Citation2013). Similar to other sterols, it plays a role in membrane fluidity and frost tolerance in winter wheat (Filek et al., Citation2017; Janeczko et al., Citation2019).
Plant breeding strategies had limited success on the improvement of agricultural production under stress pressure, mostly due to the complexity of the tolerance traits which are controlled by multigene families, as well as the increase in the intensity and duration of stresses in recent years due to anthropogenic factors (Waqas et al., Citation2019). Application of bioregulator substances on plant tissues or as a soil amendment is a promising agricultural practice. Progesterone, being a sterol is capable of entering lipid bilayer and restore membrane integrity, whereas dopamine possesses strong antioxidative properties, which directly involve in ROS scavenging (Janeczko et al., Citation2019; Roshchina, Citation2022). Although recently the role of both neurotransmitters under abiotic stresses received increased research attention, to our knowledge none of these studies investigated the responses of tomato plants. Tomato (Solanum lycopersicum L.) is a model plant for the study of fruits. It is also an economically important crop exhibiting many health benefits due to its high vitamin and lycopene content. According to the latest FAO agricultural production statistics (2020), tomato is the most widely produced vegetable crop worldwide. The high water requirement of tomato makes it very vulnerable to both drought and soil salinity (Battilani et al., Citation2012).
This study was performed to investigate the stress mitigation potential of two bioregulator substances dopamine and progesterone in tomato plants under drought and salinity stresses. While searching for potential solutions to improve agricultural production in the era of global warming, potential mechanisms of action for both bioregulators were also investigated by observing morphological responses, common physiological stress indicators including membrane damage and osmoprotectant proline levels, as well as gene expressions of the enzymatic antioxidative defense system; superoxide dismutase (FeSOD), catalase (CAT2), glutathione reductase (GR1), ascorbate peroxidase (APX1), non-enzymatic defense system; delta 1-pyrroline-5-carboxylate synthase (P5CS) and the key enzyme in ethylene production; 1-aminocyclopropane-1-carboxylic acid synthase (ACS2), under both drought and salinity stresses.
Materials and methods
The Kayra F1 tomato variety (Anamas Seed Company, Turkey) was used as the plant material.
Plant growth and treatment
Tomato seeds were placed in polypropylene containers with a total volume of 400 ml, containing sterile perlite, as one seed in each container. Germination was achieved in all pots by irrigation with sterile Hoagland solution (Hoagland & Arnon, Citation1950) every other day, to make sure that 2/3 of the container is filled with the solution. Plant growth was continued for 21 days in a 16-h light/8-h dark cycle, at 24 °C in a plant growth cabinet containing 50% humidity. Drought stress was started at the end of the 21st day, 0 water potential was reached on the 3rd day, and at the end of the 5th day, the tissue samples were collected for analysis. Salt stress was initiated with Hoagland solution containing 200 mM NaCl at the end of the 21st day and applied for 5 days as in drought stress.
Dopamine was applied by adding 100 µM dopamine hydrochloride (Dopasel, Haver Farma, Turkey), and progesterone was applied by adding 1 µM progesterone (Progestan, Kocak Farma, Turkey) into Hoagland solution, starting from seed sowing, and the application continued throughout stress applications. The same concentration of dopamine and progesterone were applied regularly in Hoagland solution, starting from day one of culture period until plant harvest, by irrigation every other day. Dopamine and progesterone concentrations used throughout the study were determined by pre-testing with 10 plants that received treatments at three different concentrations of 50, 100 and 200 µM dopamine and of 100 nM, 1 µM and 10 µM progesterone. After evaluation of the root and stem lengths of the plants and ion leakage measurements, the concentration with the best anatomical and physiological response, which was 100 µM for dopamine and 1 µM progesterone, was used throughout the study. Salt stress was applied at a concentration of 200 mM NaCl based on the observation that it induced a severe stress response for the same tomato variety within a week in our previous studies (Akcay & Okudan, Citation2023).
Determination of physiological plant stress indicators
In the presence or absence of dopamine or progesterone application and stress, after 21 days of development and 5 days of stress application, all plants were removed from perlite and washed under tap water, and root/stem lengths were determined as cm. Shoot length was measured as the length from the tip of the youngest leaf to the first root fringe. Root length was determined by measuring the distance from the first root fringe to the tip of the fibrous root system. Root and stem tissues were separated and weighed, and after drying at 60 °C for 48 h, they were weighed again and their dry weight (g) was determined.
Relative water contents (RWC) were determined according to the formula; RWC (%) = (Wet weight-Dry weight)/(Turgid weight-Dry weight) X 100 as specified in Smart and Bingham (Citation1974). Turgid weight was determined by keeping the leaves in distilled water at room temperature for 24 h.
The levels of the osmoprotectant amino acid proline were determined by the method of Bates et al. (Citation1973) without any modifications. Cellular membrane damage was determined by measuring the phospholipid peroxidation product malondialdehyde (MDA) level according to Ohkawa et al. (Citation1979) without any modifications. Another indicator of cell wall and membrane damage, ion leakage levels, were determined according to Nanjo et al. (Citation1999) without any modifications.
Determination of gene expression
Total RNA isolation was performed according to the instructions of the Thermo GeneJET plant mini kit (Thermo, USA), followed by treatment with Thermo RNase-free DNase I (Thermo, USA) in accordance with the instructions for quantitative RT-PCR. The amount of total RNA obtained was determined spectrophotometrically using Nanodrop 2000. The quality of total RNAs was also determined by separation and imaging with 1% agarose gel electrophoresis followed by cDNA synthesis, which was performed according to Thermo RevertAid FirstStrand cDNA synthesis kit instructions (Thermo, USA).
In this study, ACS2, P5CS, FeSOD, CAT2, GR1, and APX1 gene expressions were measured in tomato leaves. ACS2 is the rate-limiting enzyme in the production of ethylene, which is the primary stress hormone in plants with climacteric type fruits, including tomato. P5CS is the key enzyme in proline biosynthesis, which is an important component of non-enzymatic antioxidative defense system in plants. GR1 is responsible for the presence of steady supply of reduced glutathione, another non-enzymatic antioxidant in the cells. FeSOD, CAT2 and APX1 are the direct scavengers of reactive oxygen species; superoxide and hydrogen peroxide. The data were normalized using the elongation factor-1 (EF-1) reference gene, and the relative quantification of genes was compared with the control group using a Biorad CFX Connect Real-Time PCR device and CFX Maestro software (Biorad, USA). All amplification reactions were performed using Biorad iTaq Universal SYBR Green Supermix as specified in the user manual. The primers used in the study were optimized with PrimerPremier 5.0 software (Premier Biosoft International, USA) to provide optimum amplification conditions. PCR conditions included initial denaturation of 30 s at 95 °C, followed by amplification for 5 s at 95 °C and 30 s at 54 °C, repeating 40 cycles. RT-qPCR reactions included two biological and two technical replicates. The relative expression of genes was determined according to the 2−ΔΔCT method. ΔΔCT values were calculated by subtracting the mean ΔCT values of the samples from the mean ΔCT values of the controls, and then these values were used to determine the 2−ΔΔCT differences.
Statistical analysis
The study was conducted in triplicate, and each replicate included 20 plants. The data obtained in this study were evaluated using the SPSS 16.0 program. Differences between the means were determined by the one-way ANOVA and Tukey’s test.
Results
Determination of physiological plant stress indicators
Salt and drought stress significantly reduced the length, dry weight, and water content of shoot tissues, whereas dopamine application did not have any significant effect on the morphological parameters except for slight improvements in dry weights (). Salt and drought stress did not have any significant effect on root length, dry weight, or water content, except for 50% reduction in root dry weight under salt stress. Dopamine application improved the dry weights under both salt and drought stresses. Dopamine application also significantly increased the root length by 26% under salt treatment.
Table 1. Effect of different stress and hormone treatments on plant morphological parameters.
Progesterone treatment also had no significant effect on shoot and root lengths, however reduced shoot and root dry weights (). Although not significant, progesterone treatment increased shoot dry weights under drought and root dry weights under salinity stress. Both stresses slightly reduced the water content, and neither dopamine nor progesterone did not show any significant effect.
All physiological stress indicators were measured using tomato leaf tissues (). Under both abiotic stress conditions, leaf relative water content decreased and dopamine had no improvement effect. Although stress treatments did not significantly change leaf ion leakage levels, the value was at its minimum under drought stress/dopamine co-treatment, which was significantly lower than the control treatment. MDA contents also increased under abiotic stresses; however, dopamine treatment effectively reduced MDA content back to control levels under salt treatment. The proline contents showed 1925% and 628% increments under salt and drought stresses, respectively. However, dopamine treatment slightly reduced proline levels under both stresses.
Table 2. Effect of different stress and hormone treatments on physiological stress indicators.
Progesterone also did not have any significant effect on the relative water content (). The ion leakage value was significantly lower than that of every other treatment, including control, upon progesterone treatment under salt stress. Unlike ion leakage, both stress levels increased MDA contents, whereas progesterone treatment reduced the level back to the control level under salinity stress. The proline content was the most reactive parameter to both drought and salt stresses, with 7- and 20-fold increases, respectively. The presence of progesterone resulted in 9% and 38% decrease in proline content under salinity and drought stresses, respectively.
Determination of gene expression
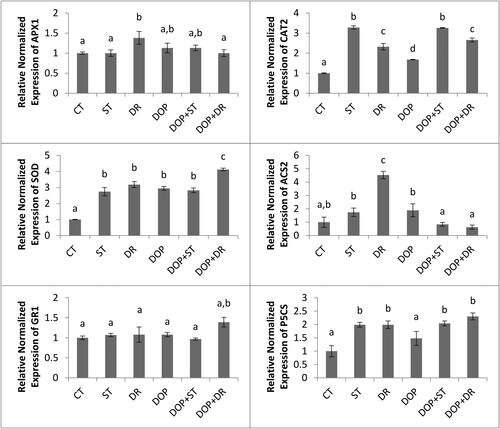
Transcript levels of ACS2, which is the key enzyme in plant stress hormone ethylene synthesis, and P5CS, which is the rate-limiting enzyme in proline synthesis, increased significantly under drought stress and reduced back to control levels upon dopamine application (). Both drought and salt stress treatments, as well as dopamine application alone, significantly increased FeSOD and CAT2 gene expressions compared with control treatment. However, under drought stress, dopamine application significantly increased FeSOD and CAT2 expressions compared with drought treatments alone. The only treatment that significantly changed APX1 gene expression was drought. Unlike FeSOD and CAT2 gene expressions, the expression was reduced back to control levels under dopamine treatment.
Figure 1. Graph of relative normalized gene expressions for different treatment groups. CT, ST, DR, DOP, DOP + ST and DOP + DR indicate control, salt, drought, dopamine, dopamine + salt and dopamine + drought applications, respectively. Different letters indicate significant differences (p ≤ 0.05, n = 60) determined by Tukey test of One Way ANOVA.
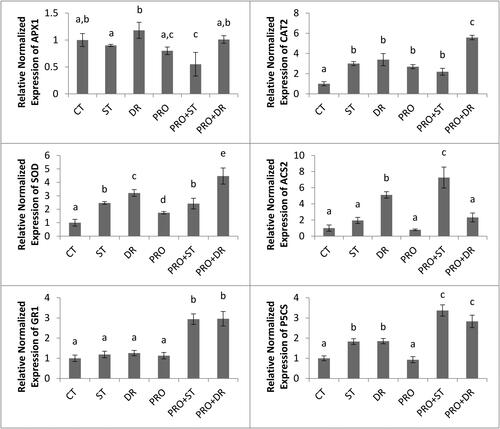
Progesterone treatment alone usually did not change gene expression except for FeSOD and CAT2, which increased with progesterone treatment (). Progesterone treatment under drought stress increased FeSOD, CAT2, GR1, and P5CS gene expression significantly compared with drought stress treatments alone. GR1 and P5CS gene expression also increased with progesterone treatment under salinity stress in addition to ACS2 gene expression, whereas FeSOD, CAT2, and APX1 expression was unaffected.
Figure 2. Graph of relative normalized gene expressions for different treatment groups. CT, ST, DR, PRO, PRO + ST and PRO + DR indicate control, salt, drought, progesterone, progesterone + salt and progesterone + drought applications, respectively. Different letters indicate significant differences (p ≤ 0.05, n = 60) determined by Tukey test of One Way ANOVA.
Discussion
In this study, severe drought and salinity stress were imitated for a short duration of time. Soils are classified as saline when they contain 40 mM NaCl salt (Shelden & Munns, Citation2023). In this study salinity stress was established with 200 mM NaCl and drought stress was applied by exposing plants to 0 water potential. In a very short period of time (5 days) both drought and salt stresses reduced tissue lengths, weights, and water contents, and shoot tissues were more sensitive to stress treatments compared to root tissues as also supported by the literature (Y. Li, Citation2009). However, no significant effect of dopamine was reported on the morphological parameters of tomato plants, which is also supported by the related literature. Two contradictory studies have reported root growth inhibition in soybean seedlings and root growth promotion in Acmella oppositifolia and Nicotiana tabacum by dopamine treatment, indicating dose-dependent plant-specific responses that cannot be generalized (Guidotti et al., Citation2013; Protacio et al., Citation1992). In this study, dopamine application only slightly improved morphological parameters under stress treatments, while it reduced ion leakage under drought stress, MDA levels under salinity stress, and proline levels under both stresses. Limited literature data also supported these observations. For example, Y. Wang et al. (Citation2020) reported that overexpression of the tyrosine decarboxylase gene (MdTyDC), which is responsible for the synthesis of dopamine derivatives, resulted in higher dopamine levels, increased fresh weight, and reduced MDA levels in apple callus under salt stress. Apple MdTyDC overexpression lines also exhibited lower ion leakage levels and water loss rates in addition to enhanced photosynthetic performance under drought stress (Gao et al., Citation2021). Exogenous dopamine treatment in cucumber, rice and banana plants also decreased tissue MDA levels and increased the components of enzymatic antioxidative defense system under very different stresses, including bisphenol-A, floride and chilling, respectively (Ahammed et al., Citation2020; Ali et al., Citation2023; Chakraborty et al., Citation2022).
Progesterone treatment reduced shoot and root dry weights while increasing root length. As dopamine, there are contrasting results in the literature. H. Li et al. (Citation2022) concluded that low concentrations of progesterone have growth promoting effect while high concentrations have growth inhibitory effect, although the situation also differs depending on the plant species and different growth stages, which is also the case with auxin and brassinosteroids. Although limited in number, previous studies have also reported oxidative stress mitigation under exogenous progesterone treatment. Our study showed enhancements in root length and dry weight upon progesterone application under salinity stress. The same observation was performed for maize seedlings under salt stress (Erdal & Dumlupınar, Citation2011). The results of our study also showed that the levels of MDA and ion leakage were reduced by progesterone treatment under salinity stress. Similar effects were also reported by other researchers for wheat, chickpea, and maize under heat, drought, salt, and cold stresses (Erdal & Dumlupınar, Citation2011; Erdal & Genisel, Citation2016; Genisel et al., Citation2013; Su et al., Citation2014; Xue et al., Citation2017). Our results showed that the effects of progesterone were more prominent under salt stress compared to drought stress in terms of morphological and physiological stress parameters.
Intensity and duration of drought and salt stresses used in the present study were not enough to induce ion leakage, mainly defined as potassium ion efflux from plant cells under severe stress conditions, which eventually leads to programmed cell death (Demidchik et al., Citation2014). However, dopamine application under drought stress and progesterone application under salt stress significantly reduced ion leakage levels in tomato tissues, lesser than the control treatment levels, indicating protective roles of both bioregulators. Although not effective under drought stress, both bioregulators also decreased lipid peroxidation significantly under salinity stress, possibly by way of different mechanisms. Being a sterol, progesterone is capable of entering lipid bilayer, restore membrane integrity and directly control electrolyte leakage, whereas dopamine possesses strong antioxidative properties, which directly involve in ROS scavenging (Janeczko et al., Citation2019; Roshchina, Citation2022).
In this study, ACS2 gene expression increased significantly with drought stress and decreased in the presence of dopamine under stress. ACS2 is the rate-limiting enzyme in ethylene synthesis, and its expression results in the production of ethylene hormone, which plays an important role in aging and senescence followed by necrosis. In plants with climacteric type of fruits including apple, peaches, banana and tomato, ethylene is the key regulator of senescence (Iqbal et al., Citation2017; McAtee et al., Citation2013). Ethylene is usually considered as a negative regulator of plant tolerance under abiotic stress conditions (Riyazuddin et al., Citation2020). To the best of our knowledge, there are no reports on dopamine application/ACS2 expression interaction in the literature. The findings showed potential low levels of ethylene, resulted from low levels of ACS2 expression, under drought stress in the presence of dopamine, indicating mitigation of the stress conditions. Another data for the interaction of ethylene and dopamine comes from the observation that dopamine regulates the expression of ERF family transcription factors, which are ethylene responsive proteins, indicating the role of dopamine in ethylene signaling under drought stress (Gao et al., Citation2021).
P5CS, which is responsible for proline production, also exhibited a similar expression pattern to ACS2 gene expression, increasing significantly with stress and being insensitive to the presence of dopamine under stress. The transcript levels were also in agreement with tissue proline levels. Proline levels and P5SC expression results of this study contradict those of Y. Wang et al. (Citation2020, Citation2021), who reported increased levels with dopamine presence, which possibly shows plant, tissue, and/or dose-dependent effects of dopamine.
The results of the present study also showed that the transcript levels of FeSOD and CAT2 increased in the presence of dopamine, whereas APX1 gene expression decreased in the presence of dopamine under drought stress. SOD and CAT are the two main antioxidative defense enzymes that detoxify superoxide radicals and hydrogen peroxide, respectively. Catalase enzymes function independently from reducing equivalents, whereas APX1, which is also an H2O2 scavenging enzyme, requires a steady supply of NADPH. This requirement may result in sensitivity to severe stress and decreased transcript levels. Similar to our study, Guidotti et al. (Citation2013) and Gomes et al. (Citation2014) also reported increased SOD and decreased or unaffected POD activity upon dopamine application. Salinity stress upregulated FeSOD and CAT2 gene expressions, however, the expression levels were insensitive to dopamine presence under salinity stress. Gao et al. (Citation2021) investigated transcriptomic responses of apple under drought stress upon dopamine application and identified 1052 differentially expressed genes. Dopamine activated Ca2+ signaling pathways and strongly regulated WRKY, ERF and NAC transcription factors, as well as the genes related to amino acid, nitrogen and secondary plant metabolism. These observations showed that in addition to its direct ROS scavenging capacity, dopamine is also a very early regulator of stress. Its role in amino acid and secondary metabolism may have resulted in the regulation of proline levels and its role in nitrogen metabolism may explain increased expression levels of enzymatic antioxidative defense system in tomato plants, observed in the present study.
Erdal and Dumlupınar (Citation2011) showed that, progesterone application alone increased SOD and CAT enzyme activities and decreased lipid peroxidation levels in chickpea plants under normal growth conditions, which is also in agreement with our findings. In this study, P5CS, FeSOD, CAT2, and GR1 expressions were significantly higher in progesterone-treated plants under drought stress, whereas only P5CS and GR1 expressions were higher under salt-stressed plants. Unlike drought, salt stress also has an ion toxicity component in addition to osmotic stress, which may be destructive to some antioxidative defense enzymes. However, under salinity stress tomato roots are longer and have more biomass with progesterone treatment. Also, the ion leakage and MDA levels were lower and proline levels were higher with progesterone treatment under salinity stress. Decreased levels of ion leakage and MDA, in addition to increased levels of reduced glutathione and proline under postharvest chilling stress in banana and mango upon progesterone treatment were also reported (Chen et al., Citation2022; Hao et al., Citation2019; Khedr & Khedr, Citation2023). Non-enzymatic defense system including high levels of reduced glutathione, produced by GR1 expression and high levels of proline seems to provide protection under salinity stress upon progesterone treatment. Also being a sterol, progesterone seems to be very effective in terms of cell membrane protection.
Conclusion
External dopamine and progesterone applications improved the morphological and physiological responses of tomato plants to drought and salt stress. Dopamine and progesterone also boosted FeSOD and CAT2 expression under drought stress. Reduced tissue ethylene levels and decreased levels of reactive oxygen species, superoxide and H2O2 in turn resulted in cell membrane integrity, improved tissue lengths and weights in tomato plants. Further studies, including transcriptomics technologies, are necessary to decipher the exact mechanism of action of dopamine and progesterone under oxidative stress conditions. However, the results of the study showed that practices such as external application or seed treatment with dopamine or progesterone might be promising in abiotic stress mitigation for sustainable agricultural production.
Authors’ contributions
Ufuk Celikkol Akcay, Ph.D., designed the experiment, performed all the analyses and prepared the manuscript. Reyhan Gencay and Fatma Zehra Koc were M.S. students in the Department of Agricultural Biotechnology. They performed the analyses under the supervision of Ufuk Celikkol Akcay and revised the manuscript for intellectual content.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the raw data used in the preparation of the manuscript is available upon request.
References
- Abdelaal, K., Alsubeie, M. S., Hafez, Y., Emeran, A., Moghanm, F., Okasha, S., Omara, R., Basahi, M. A., Darwish, D. B. E., Ibrahim, M. F. M., El-Yazied, A. A., Rashwan, E. A., Elkelish, A., Mady, M. A., & Ibraheem, F. (2022). Physiological and biochemical changes in vegetable and field crops under drought, salinity and weeds stresses: Control strategies and management. Agriculture, 12(12), 1. https://doi.org/10.3390/agriculture12122084
- Ahammed, G. J., & Li, X. (2023). Dopamine-induced abiotic stress tolerance in horticultural plants. Scientia Horticulturae, 307, 111506. https://doi.org/10.1016/j.scienta.2022.111506
- Ahammed, G. J., Wang, Y., Mao, Q., Wu, M., Yan, Y., Ren, J., Wang, X., Liu, A., & Chen, S. (2020). Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environmental Pollution, 259, 113957. https://doi.org/10.1016/j.envpol.2020.113957
- Ahmad, A., Khan, W. U., Shah, A. A., Yasin, N. A., Ali, A., Rizwan, M., & Ali, S. (2021). Dopamine alleviates hydrocarbon stress in Brassica oleracea through modulation of physio-biochemical attributes and antioxidant defense systems. Chemosphere, 270, 128633. https://doi.org/10.1016/j.chemosphere.2020.128633
- Ahmad, I., Zhu, G., Zhou, G., Younas, M. U., Suliman, M. S. E., Liu, J., Zhu, Y. M., & Salih, E. G. I. (2023). Integrated approaches for increasing plant yield under salt stress. Frontiers in Plant Science, 14, 1215343. https://doi.org/10.3389/fpls.2023.1215343
- Akcay, U. C., & Okudan, N. (2023). Exogenous serotonin improves drought and salt tolerance in tomato seedlings. Plant Growth Regulation, 101(1), 239–12. https://doi.org/10.1007/s10725-023-01016-x
- Ali, A. F., Hatamnia, A. A., Malekzadeh, P., Sayyari, M., & Aghdam, M. S. (2023). Exogenous dopamine ameliorates chilling injury in banana fruits by enhancing endogenous dopamine and glycine betaine accumulation and promoting ROS scavenging system activity. Postharvest Biology and Technology, 205, 112521. https://doi.org/10.1016/j.postharvbio.2023.112521
- Angon, P. B., Tahjib-Ul-Arif, M., Samin, S. I., Habiba, U., Hossain, M. A., & Brestic, M. (2022). How do plants respond to combined drought and salinity stress? A systematic review. Plants, 11(21), 2884. https://doi.org/10.3390/plants11212884
- Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. https://doi.org/10.1007/BF00018060
- Battilani, A., Prieto, M. H., Argerich, C., Campillo, C., & Cantore, V. (2012). Tomato. In P. Steduto, T. C. Hsiao, E. Fereres, & D. Raes (Eds.), Crop yield response to water, ırrigation and drainage paper no. 66 (pp. 192–198). FAO.
- Cao, Y., Du, P., Yin, B., Zhou, S., Li, Z., Zhang, X., Xu, J., & Liang, B. (2023). Melatonin and dopamine enhance waterlogging tolerance by modulating ROS scavenging, nitrogen uptake, and the rhizosphere microbial community in Malus hupehensis. Plant and Soil, 483(1–2), 475–493. https://doi.org/10.1007/s11104-022-05759-w
- Chakraborty, S., Singh, A., & Roychoudhury, A. (2022). Extensive cross-talk among stress-regulated protective metabolites, biogenic-amines and phytohormone-signalling, co-ordinated by dopamine-mediated seed-priming, governs tolerance against fluoride stress in rice. Plant Cell Reports, 41(12), 2261–2278. https://doi.org/10.1007/s00299-022-02919-1
- Chen, H., Zhou, S., Li, X., & Yang, H. (2022). Exogenous progesterone alleviates chilling injury by upregulating IbAOX1 to mediate redox homeostasis and proline accumulation in postharvest sweetpotato tuberous root. Postharvest Biology and Technology, 183, 111738. https://doi.org/10.1016/j.postharvbio.2021.111738
- Demidchik, V., Straltsova, D., Medvedev, S. S., Pozhvanov, G. A., Sokolik, A., & Yurin, V. (2014). Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. Journal of Experimental Botany, 65(5), 1259–1270. https://doi.org/10.1093/jxb/eru004
- Du, P., Yin, B., Zhou, S., Li, Z., Zhang, X., Cao, Y., Han, R., Shi, C., Liang, B., & Xu, J. (2022). Melatonin and dopamine mediate the regulation of nitrogen uptake and metabolism at low ammonium levels in Malus hupehensis. Plant Physiology and Biochemistry, 171, 182–190. https://doi.org/10.1016/j.plaphy.2022.01.004
- Erdal, S. (2012a). Exogenous mammalian sex hormones mitigate inhibition in growth by enhancing antioxidant activity and synthesis reactions in germinating maize seeds under salt stress. Journal of the Science of Food and Agriculture, 92(4), 839–843. https://doi.org/10.1002/jsfa.4655
- Erdal, S. (2012b). Alleviation of salt stress in wheat seedlings by mammalian sex hormones. Journal of the Science of Food and Agriculture, 92(7), 1411–1416. https://doi.org/10.1002/jsfa.4716
- Erdal, S., & Dumlupinar, R. (2011). Mammalian sex hormones stimulate antioxidant system and enhance growth of chickpea plants. Acta Physiologiae Plantarum, 33(3), 1011–1017. https://doi.org/10.1007/s11738-010-0634-3
- Erdal, S., & Genisel, M. (2016). The property of progesterone to mitigate cold stress in maize is linked to a modulation of the mitochondrial respiratory pathway. Theoretical and Experimental Plant Physiology, 28(4), 385–393. https://doi.org/10.1007/s40626-016-0076-4
- Farouk, S., El-Hady, M. A. M. A., El-Sherpiny, M. A., Hassan, M. M., Alamer, K. H., Al-Robai, S. A., Ali, E. F., & El-Bauome, H. A. (2023). Effect of dopamine on growth, some biochemical attributes, and the yield of crisphead lettuce under nitrogen deficiency. Horticulturae, 9(8), 945. https://doi.org/10.3390/horticulturae9080945
- Filek, M., Rudolphi-Skórska, E., Sieprawska, A., Kvasnica, M., & Janeczko, A. (2017). Regulation of the membrane structure by brassinosteroids and progesterone in winter wheat seedlings exposed to low temperature. Steroids, 128, 37–45. https://doi.org/10.1016/j.steroids.2017.10.002
- Gao, T., Wang, Y., Liu, Y., Ma, M., Li, X., Zhang, D., Ding, K., Li, C., Zou, Y., & Ma, F. (2021). Overexpression of tyrosine decarboxylase (MdTYDC) enhances drought tolerance in Malus domestica. Scientia Horticulturae, 289, 110425. https://doi.org/10.1016/j.scienta.2021.110425
- Genisel, M., Turk, H., & Erdal, S. (2013). Exogenous progesterone application protects chickpea seedlings against chilling-induced oxidative stress. Acta Physiologiae Plantarum, 35(1), 241–251. https://doi.org/10.1007/s11738-012-1070-3
- Gomes, B. R., Soares, R. C. S., Santos, W. D., Marchiosi, R., Soares, A. R., & Filho, O. F. (2014). The effects of dopamine on antioxidant enzymes activities and reactive oxygen species levels in soybean roots. Plant Signaling & Behavior, 9(12), e977704. https://doi.org/10.4161/15592324.2014.977704
- Guidotti, B. B., Gomes, B. R., Siqueira-Soares, R. d C., Soares, A. R., & Ferrarese-Filho, O. (2013). The effects of dopamine on root growth and enzyme activity in soybean seedlings. Plant Signaling & Behavior. 8(9), e25477. https://doi.org/10.4161/psb.25477
- Haj-Amor, Z., Araya, T., Kim, D. G., Bouri, S., Lee, J., Ghiloufi, W., Yang, Y., Kang, H., Jhariya, M. K., Banerjee, A., & Lal, R. (2022). Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. The Science of the Total Environment, 843, 156946. https://doi.org/10.1016/j.scitotenv.2022.156946
- Hao, J., Li, X., Xu, G., Huo, Y., & Yang, H. (2019). Exogenous progesterone treatment alleviates chilling injury in postharvest banana fruit associated with induction of alternative oxidase and antioxidant defense. Food Chemistry, 286, 329–337. https://doi.org/10.1016/j.foodchem.2019.02.027
- Hoagland, D. R., & Arnon, D. I. (1950). The water-culture method for growing plants without soil. Circular - California Agricultural Experiment Station, 347, 1–32.
- Howe, M. W., & Dombeck, D. A. (2016). Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature, 535(7613), 505–510. https://doi.org/10.1038/nature18942
- Iino, M., Nomura, T., Tamaki, Y., Yamada, Y., Yoneyama, K., Takeuchi, Y., Mori, M., Asami, T., Nakano, T., & Yokota, T. (2007). Progesterone: Its occurrence in plants and involvement in plant growth. Phytochemistry, 68(12), 1664–1673. https://doi.org/10.1016/j.phytochem.2007.04.002
- Iqbal, N., Khan, N. A., Ferrante, A., Trivellini, A., Francini, A., & Khan, M. I. R. (2017). Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Frontiers in Plant Science, 8, 475. https://doi.org/10.3389/fpls.2017.00475
- Janeczko, A., & Filek, W. (2002). Stimulation of generative development in partly vernalized winter wheat by animal sex hormones. Acta Physiologiae Plantarum, 24(3), 291–295. https://doi.org/10.1007/s11738-002-0054-0
- Janeczko, A., Filek, W., Biesaga-Kościelniak, J., Marcińska, I., & Janeczko, Z. (2003). The influence of animal sex hormones on the induction of flowering in Arabidopsis thaliana: Comparison with the effect of 24-epibrassinolide. Plant Cell, Tissue and Organ Culture, 72(2), 147–151. https://doi.org/10.1023/A:1022291718398
- Janeczko, A., Oklešťková, J., Siwek, A., Dziurka, M., Pociecha, E., Kocurek, M., & Novák, O. (2013). Endogenous progesterone and its cellular binding sites in wheat exposed to drought stress. The Journal of Steroid Biochemistry and Molecular Biology, 138, 384–394. https://doi.org/10.1016/j.jsbmb.2013.07.014
- Janeczko, A., Pociecha, E., Dziurka, M., Jurczyk, B., Libik-Konieczny, M., Oklestkova, J., Novák, O., Pilarska, M., Filek, M., Rudolphi-Skórska, E., Sadura, I., & Siwek, A. (2019). Changes in content of steroid regulators during cold hardening of winter wheat - Steroid physiological/biochemical activity and impact on frost tolerance. Plant Physiology and Biochemistry: PPB, 139, 215–228. https://doi.org/10.1016/j.plaphy.2019.03.020
- Jiao, X., Li, Y., Zhang, X., Liu, C., Liang, W., Li, C., Ma, F., & Li, C. (2019). Exogenous dopamine application promotes alkali tolerance of apple seedlings. Plants, 8(12), 580. https://doi.org/10.3390/plants8120580
- Khedr, E. H., & Khedr, N. (2023). Optimization of postharvest progesterone treatment to alleviate chilling injury in mango fruit, maintaining intracellular energy, cell wall stability, and antioxidant activity. Postharvest Biology and Technology, 206, 112572. https://doi.org/10.1016/j.postharvbio.2023.112572
- Kopecká, R., Kameniarová, M., Černý, M., Brzobohatý, B., & Novák, J. (2023). Abiotic stress in crop production. International Journal of Molecular Sciences, 24(7), 6603. https://doi.org/10.3390/ijms24076603
- Lan, G. P., Jiao, C. J., Wang, G. Q., Sun, Y. H., & Yan, S. (2020). Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Scientia Horticulturae. 260, 108790. https://doi.org/10.1016/j.scienta.2019.108790
- Li, Y. (2009). Physiological responses of tomato seedlings (Lycopersicon esculentum) to salt stress. Mod Appl Sci, 3(3), 171–176.
- Li, H., Chen, L., Chen, H., Xue, R., Wang, Y., & Song, J. (2022). The role of plant progesterone in regulating growth, development, and biotic/abiotic stress responses. International Journal of Molecular Sciences, 23(18), 10945. https://doi.org/10.3390/ijms231810945
- Li, C., Sun, X., Chang, C., Jia, D., Wei, Z., Li, C., & Ma, F. (2015). Dopamine alleviates salt-induced stress in Malus hupehensis. Physiologia Plantarum, 153(4), 584–602. https://doi.org/10.1111/ppl.12264
- Liang, B.,Li, C.,Ma, C.,Wei, Z.,Wang, Q.,Huang, D.,Chen, Qi.,Li, C.,Ma, F. (2017). Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiology and Biochemistry, 119, 346–359. 10.1016/j.plaphy.2017.09.012
- Ma, Y., Freitas, H., & Dias, M. C. (2022). Strategies and prospects for biostimulants to alleviate abiotic stress in plants. Frontiers in Plant Science, 13, 1024243. https://doi.org/10.3389/fpls.2022.1024243
- McAtee, P., Karim, S., Schaffer, R. J., & David, K. (2013). A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Frontiers in Plant Science, 4, 79. https://doi.org/10.3389/fpls.2013.00079
- Muhammad, M., Waheed, A., Wahab, A., Majeed, M., Nazim, M., Liu, Y. H., Li, L., & Li, W. J. (2024). Soil salinity and drought tolerance: An evaluation of plant growth, productivity, microbial diversity, and amelioration strategies. Plant Stress, 11, 100319. https://doi.org/10.1016/j.stress.2023.100319
- Nanjo, T., Kobayashi, M., Yoshiba, Y., Kakubari, Y., Yamaguchi-Shinozaki, K., & Shinozaki, K. (1999). Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Letters, 461(3), 205–210. https://doi.org/10.1016/s0014-5793(99)01451-9
- Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95(2), 351–358. https://doi.org/10.1016/0003-2697(79)90738-3
- Protacio, C. M., Dai, Y. R., Lewis, E. F., & Flores, H. E. (1992). Growth stimulation by catecholamines in plant tissue/organ cultures. Plant Physiology, 98(1), 89–96. https://doi.org/10.1104/pp.98.1.89
- Riyazuddin, R., Verma, R., Singh, K., Nisha, N., Keisham, M., Bhati, K. K., Kim, S. T., & Gupta, R. (2020). Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules, 10(6), 959. https://doi.org/10.3390/biom10060959
- Roshchina, V. V. (2022). Biogenic amines in plant cell at norma and stress: probes for dopamine and histamine. In T. Aftab & M. Naeem (Eds.), Emerging plant growth regulators in agriculture (pp. 357–376). Academic Press.
- Rudolph, L. M., Cornil, C. A., Mittelman-Smith, M. A., Rainville, J. R., Remage-Healey, L., Sinchak, K., & Micevych, P. E. (2016). Actions of steroids: New neurotransmitters. The Journal of Neuroscience, 36(45), 11449–11458. https://doi.org/10.1523/JNEUROSCI.2473-16.2016
- Sato, H.,Suzuki, T.,Takahashi, F.,Shinozaki, K., &Yamaguchi-Shinozaki, K. (2019). NF-YB2 and NF-YB3 Have Functionally Diverged and Differentially Induce Drought and Heat Stress-Specific Genes. Plant Physiology, 180(3), 1677–1690. 10.1104/pp.19.00391
- Shelden, M. C., & Munns, R. (2023). Crop root system plasticity for improved yields in saline soils. Frontiers in Plant Science, 14, 1120583. https://doi.org/10.3389/fpls.2023.1120583
- Smart, R. E., & Bingham, G. E. (1974). Rapid estimates of relative water content. Plant Physiology, 53(2), 258–260. https://doi.org/10.1104/pp.53.2.258
- Su, X., Wu, S., Yang, L., Xue, R., Li, H., Wang, Y., & Zhao, H. (2014). Exogenous progesterone alleviates heat and high light stress induced inactivation of photosystem II in wheat by enhancing antioxidant defense and D1 protein stability. Plant Growth Regulation, 74(3), 311–318. https://doi.org/10.1007/s10725-014-9920-1
- Wang, Y., Chen, Q., Zheng, J., Zhang, Z., Gao, T., Li, C., & Ma, F. (2021). Overexpression of the tyrosine decarboxylase gene MdTyDC in apple enhances long-term moderate drought tolerance and WUE. Plant Science, 313, 111064. https://doi.org/10.1016/j.plantsci.2021.111064
- Wang, Y., Gao, T., Zhang, Z., Yuan, X., Chen, Q., Zheng, J., Chen, S., Ma, F., & Li, C. (2020). Overexpression of the tyrosine decarboxylase gene MdTyDC confers salt tolerance in apple. Environmental and Experimental Botany, 180, 104244. https://doi.org/10.1016/j.envexpbot.2020.104244
- Wang, W., Yang, Y., Ma, X., He, Y., Ren, Q., Huang, Y., Wang, J., Xue, Y., Yang, R., Guo, Y., Sun, J., Yang, L., & Sun, Z. (2023). New insight into the function of dopamine (DA) during Cd stress in duckweed (Lemna turionifera 5511). Plants, 12(10), 1996. https://doi.org/10.3390/plants12101996
- Waqas, M. A., Kaya, C., Riaz, A., Farooq, M., Nawaz, I., Wilkes, A., & Li, Y. (2019). Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Frontiers in Plant Science, 10, 1336. https://doi.org/10.3389/fpls.2019.01336
- Xue, R. L., Wang, S. Q., Xu, H. L., Zhang, P. J., Li, H., & Zhao, H. J. (2017). Progesterone increases photochemic efficiency of photosystem II in wheat under heat stress by facilitating D1 protein phosphorylation. Photosynthetica, 55(4), 664–670. https://doi.org/10.1007/s11099-016-0681-0
- Yadav, S., Modi, P. D. A., Vijapura, A., Patel, D., & Patel, M. (2020). Effect of abiotic stress on crops. Sustainable Crop Production, 3, 3–24.
- Yogendra, K. N., Dhokane, D., Kushalappa, A. C., Sarmiento, F., Rodriguez, E., & Mosquera, T. (2016). StWRKY8 transcription factor regulates benzylisoquinoline alkaloid pathway in potato conferring resistance to late blight. Plant Science, 256, 208–216. https://doi.org/10.1016/j.plantsci.2016.12.014
- Zhang, Z., Tang, Z., Jing, G., Gao, S., Liu, C., Ai, S., Liu, Y., Liu, Q., Li, C., & Ma, F. (2023). Dopamine confers cadmium tolerance in apples by improving growth, reducing reactive oxygen species, and changing secondary metabolite levels. Environmental and Experimental Botany. 208, 105264. https://doi.org/10.1016/j.envexpbot.2023.105264
- Zhang, Z., Zhang, J., Tang, Z., Wang, Y., Gao, T., Liu, X., Feng, M. A., & Chao, L. (2022). Tissue distribution and changes in dopamine during development and stress responses in Malus germplasm. Journal of Integrative Agriculture, 21(3), 710–724. https://doi.org/10.1016/S2095-3119(20)63590-0
- Zomer, R. T., Bossio, D. A., Sommer, R., & Verchot, L. V. (2017). Global sequestration potential of increased organic carbon in cropland soils. Scientific Reports, 7(1), 15554. https://doi.org/10.1038/s41598-017-15794-8
- Zulfiqar, F., & Ashraf, M. (2020). Bioregulators: unlocking their potential role in regulation of the plant oxidative defense system. Plant Molecular Biology, 105(1–2), 11–41. https://doi.org/10.1007/s11103-020-01077-w

