?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Maize production is an important enterprise in Ghana, providing livelihood for thousands of people. It is challenged by fall armyworm infestation causing destruction to many cultivated lands in production enclaves and threatening food security. Effective control, however, requires information on the actual pest infestation as well as the effect of climate factors on infestation in these enclaves. The study assessed the incidence, prevalence and severity of fall armyworm infestation on maize farms in two major maize enclaves (Ejura and Ejisu) in Ghana. Data was taken on the presence, infestation levels and damage of fall armyworms as well as the climatic conditions in each district. Data collection was done by sampling 50 maize plants each on 40 maize farms in both districts and assessing them for the incidence, larval prevalence, leaf damage and severity. Results showed varying infestation in both districts (p < 0.0001) with Ejisu having a higher prevalence (0.10 ± 0.04 larvae per plant) than Ejura (0.05 ± 0.03 larvae per plant) in the minor season at seedling stage. At the vegetative stage, Ejisu recorded a higher prevalence in both seasons. A low severity was recorded at the seedling stage in both districts for all seasons which, however, varied among seasons at vegetative stage. Climatic variables including rainfall, temperature, relative humidity and wind were found to significantly impact infestation in both districts. The study thus, showed fall armyworm infestation to be a major challenge to maize production in both districts confirming it as a major constraint to maize production in the region.
Reviewing Editor:
1. Introduction
Maize (Zea mays L.), known for its important benefits is described as the “queen of cereals” (Huma et al., Citation2019). It is believed to have been transformed from a wild grass into a food source by Native Americans, although it originated about 7000 years ago from central Mexico (Sharon et al., Citation2020). It is one of the most cultivated food crops worldwide with 63% of its production from China, Brazil and the USA. In Ghana, maize is the most consumed staple food with a per capita consumption of 45 kg/year. It accounts for over 50–60% of the total cereal production of the country occupying over one million hectares of land (Ragasa et al., Citation2014; Obour et al., Citation2022). Maize grows well in almost every part of the country thriving well in forest, northern savannah, coastal savannah and transitional zones (Wongnaa et al., Citation2019). It is principally grown in the Ashanti, Eastern and Brong Ahafo regions with production from these areas accounting for 70–80% of total maize production in Ghana (Darfour & Rosentrater, Citation2016).
Despite the economic benefits of maize production such as ensuring food security and providing livelihoods for people, Ghana records one of the lowest yields in the world (Ragasa et al., Citation2014). Reports by Wongnaa et al. (Citation2019) and Ragasa et al. (Citation2014) revealed maize yield in Ghana to be growing by only 1.1% per annum with current yield ranging between 1.73 to 1.92 metric ton/ha. This is somewhat attributed to the lack of access of farmers to the required resources needed for increasing productivity as larger proportion of maize farmers are smallholder farmers. Given this, many interventions have been put in place by both governmental and non-governmental agencies in the last decade to help improve maize production and increase yield (Obour et al., Citation2022). Regardless of these efforts, production is faced with pest infestation with an important one being fall armyworm.
The first invasion of the fall armyworm (FAW) in Africa occurred in January 2016 in Nigeria (Jing et al., 2021). This later spread to other countries invading Ghana in April 2016. By the end of 2017, it was reported to have invaded over 38 African countries (Sharon et al., Citation2020). The FAW, scientifically known as Spodoptera frugiperda, is a lepidopteran insect belonging to the family Noctuidae (Regier et al., Citation2017). It originated from the Americas causing devastating impacts on a variety of food crops including rice, sorghum, maize and sugarcane with being its main host (Manjula et al., Citation2019). The invasion and survival of the insect involves a complex interplay of factors such as dispersal patterns, reproductive behaviour and interactions with natural enemies. FAWs are strong migrants having the ability to fly over long distances to food sources before oviposition (Li et al., Citation2023). Female adults lay eggs in clusters on host plants which hatch into larvae that undergoes six instars. These larvae voraciously feed on foliage, leading to substantial crop damage (Deshmukh et al., Citation2021). Their rapid reproductive range is facilitated by favourable climatic conditions and the diversity of their host plants contributing to their invasive and survival success. For instance, a temperature around 30 °C is optimal for its development and while any increase in temperature increases their feeding and foraging habits (Du Plessis et al., Citation2020). This results in its all-year round survival in the African continent as climatic conditions are conducive for their growth as well the availability of host plants (Ahmed-Seid, Citation2022).
The impact of FAW infestations in different African countries was thus, reported by Sagar et al. (Citation2020). In Malawi, infestation has been shown to lead to greater economic losses. Tanzania was reported to experience a yield loss of 3.2 million tonnes, while Uganda and Ethiopia faced staggering losses of 13.91 million and 30.54 million tonnes respectively. In Kenya, FAW affected over 250,000 hectares of agricultural land, accounting for 11% of the total maize cultivation area in the country. Similarly, Zambia suffered 40% production losses respectively. The incidence of FAW infestation in Ghana greatly impacts maize production and it resulted in the destruction of large hectares of cultivated maize lands and loss of about US$177 million worth of maize highlighting the urgent need for its effective management (MoFA, Citation2023). Since then, farmers have used various means in controlling them but efforts have not yielded great results. However, for effective strategies for management to be put in place, information on the actual infestation of the pest in an area is essential. Also, the influence of changes in climatic conditions on the infestation is essential in the management of the pest. In view of this, the study investigated the incidence, prevalence and severity of fall armyworm infestation as well as the effect of climatic variables on infestation in two major maize growing areas; Ejura-Sekyedumase and Ejisu-Juabeng in Ghana to inform decisions towards interventions for sustainable management.
2. Materials and methods
2.1. Study area
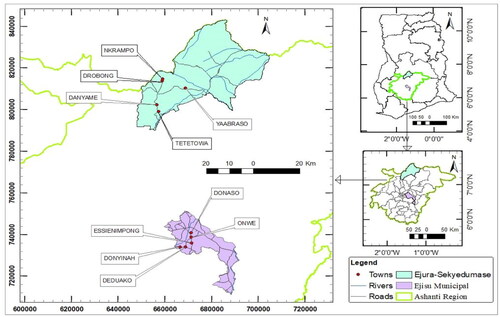
The study was carried out in the Ejisu-Juabeng district and the Ejura-Sekyedumase district in the Ashanti Region of Ghana (). The Ejura-Sekyedumase district is located at the northern part of the Ashanti Region and lies between longitude 1.5° W and 1.39° W and latitudes 7.9° N and 7.3° N (Obour et al., Citation2022). It covers about 7.3% of the estimated land cover of the Ashanti Region with a population of about 121.765 in year 2020 (MoFA, Citation2023). The district lies within the Guinea Savanna and the transitional semi-deciduous forest zones and experiences a mean temperature of 21 °C to 30 °C monthly. It has a bimodal rainfall pattern with the major season between March and August and minor season between September to November. Its flat and undulating topography as well as climatic conditions and soil profile makes is suitable for farming. Soils have a deep profile, are well aerated and have a moderate amount of nutrients (Obour et al., Citation2022). Maize production is the main agricultural activity, accounting for about 41% of the total area cultivated (Cossar et al., Citation2016). The Ejisu-Juabeng district lies in the central part of the region and covers an estimated area of 637.4 km2 (Appiah et al., Citation2015). It, also, has a bimodal rainfall pattern with a wet equatorial climate. Its major rain season spans from March to July while the minor season last from September to November. Its temperature ranges between 20 °C in August and 32 °C in March. The distribution of rainfall allows for agriculture and cultivation of many cash and food crops (MoFA, Citation2023).
2.2. Farm selection
The survey was caried out in 10 farming communities (5 from each district) which were extensively known for maize cultivation (). A total of 40 farms were selected from each district with the number of farms per community ranging from 5 to 10 depending on the extent of farming activities in the community. One acre sized farms were selected with farmers’ consent.
Table 1. Number of farms selected per community in districts.
2.3. Data collection
Data were collected on the incidence of FAW infestation, prevalence of FAW larvae leaf damage and severity of FAW infestation on farms. This was done at both the seedling stage (3-4 weeks after germination) and the vegetative stage (6–8 weeks after germination) during both the minor season (September 2020 to December 2020) and major season (April 2021 to August 2021) of maize cultivation. Where farms were close in proximity, the first farm was selected and at least five farm lands were skipped before sampling the next field. On each field, 10 plants each were selected at 5 locations using the W-sampling model as described by Prasanna et al. (Citation2018) and observed for infestation. The incidence of FAW was confirmed by the presence of fecal pellets and frass on the leaves of maize and fall armyworm larvae was identified by a Y-shaped mark on the dorsal surface of the head and four dots arranged in a square pattern on the last abdominal segment (Singh et al., Citation2023). This was conducted between September 2020 and June 2021.
2.3.1. Incidence of FAW infestation and leaf damage
Incidence of FAW infestation was defined in this study as the presence of fall armyworm infestation of farms surveyed. Where there is an incidence of infestation of FAW, the level of leaf damage was assessed. This was estimated by expressing the number of damaged leaves as a percentage of the total number of leaves per plant surveyed. This was graded as: 0 = no damaged leaf on plants, 1-20% = low leaf damage, 21-49% = moderate leaf damage and 50-100% for severe leaf damage. This is determined at the community and district level.
2.3.2. Prevalence of FAW larvae on farms
Prevalence of FAW larvae on farms was determined at the community and district level. It was determined by the number of larvae counted on plants per the number of plants sampled (Baudron et al., Citation2019).
2.3.3. Severity of FAW infestation
The severity of FAW infestation on farms was done by visually rating the damage caused to leaves using the Davis and Williams (Citation1992) scale of 0 – 9 (). This scale represents different levels of damage with 9 representing the highest damage score and 0 representing no damage. The damage score of farms at the community and district level were calculated using the mode of the scores recorded per plant. A score of 0 – 4, 5 – 7 and 8–9 indicated a low, medium and high severity respectively.
Plate 1. Visual rating scales for screening leaf damage by FAW (Prasanna et al., Citation2018).

2.4. Acquisition of climate data
Climate variables namely; surface air temperature, wind speed and direction and relative humidity were obtained on the monthly timescale in 2020 and 2021 from the European Centre for Medium-Range Weather Forecasts Reanalysis Fifth Generation (ERA5; Hersbach et al., Citation2020). These global observations were done by ERA5 with model data to produce a consistent dataset based on physical laws (Copernicus Climate Change Service (C3S), Citation2017), which were quality controlled to produce daily hourly estimates of climate variables globally at a 0.25° ×0.25° horizontal grid resolution. Therefore, for each study area, the nearest neighbour interpolation method was used to extract the variables from the gridded resolution using their coordinates. Additionally, monthly rainfall was extracted from the Climate Hazards Group Infrared Precipitation combined with Station data (CHIRPS; Peterson et al., Citation2013) from 2020 and 2021. CHIRPS combined global climatology, in-situ rainfall observations and satellite estimates were used to produce precipitation with timesteps ranging from daily to seasonal (Funk et al., Citation2015) at a finer spatial resolution of 0.05° ×0.05°.
2.5. Data analyses
Data were analysed using SPSS version 27. The standard error of mean (SEM) was calculated for percentage leaf damage and prevalence of FAW infestation in communities and districts. ANOVA was used to test for the differences in percentage leaf damage, prevalence and severity between communities, seasons and districts at each growing stage while Tukey test was used to separate means at district level. Pearson correlation analysis was done to determine the relationship between prevalence, severity and leaf damage as well as determine their association with the various climate variables. Linear regression analyses were also performed to predict the influence of climatic variables on infestation of fall armyworm.
3. Results
3.1. Incidence of fall armyworm infestation and leaf damage
FAW infestation was recorded across all maize farms surveyed in both Ejisu and Ejura Districts, causing significant damage to maize farms. Percentage leaf damage on plants was consistently higher during the major season compared to the minor season at every growth stage observed.
During the seedling stage, in particular, the contrast was stark between the districts: the minor season showed a significantly (p < 0.001, df = 2) higher percentage leaf damage in Ejisu (66.1 ± 16.3) compared to Ejura (29.8 ± 12.4). Moreover, leaf damage differed significantly across seasons and among various communities (p < 0.001). In the Ejisu District, every community experienced over 20% leaf damage, with each one reporting severe leaf damage (50-100%) (). The Onwe community stood out, registering the highest percentage damage (70.6 ± 14.3) across farms, while Donaso reported the least (59.5 ± 26.0). Interestingly, Donaso had the largest proportion of farms with moderate leaf damage (37.5%), contrasting with Essienimpong, which had no farms exhibiting this level of damage. All farms in Essienimpong uniformly experienced severe leaf damage, with all leaves of plants sampled being damaged. Conversely, in the Ejura District, Yaabraso had the largest mean percentage leaf damage (36.1 ± 25.6), whereas Danyame reported the least (24.5 ± 6.5). All communities, except Yaabraso, recorded less than 50% leaf damage, with Yaabraso reporting severe damage on 37.5% of its farms. Only the Drobong community had all farms experiencing moderate percentage leaf damage ().
Table 2. Level of leaf damage caused by FAW at seedling stage.
During the major season, Ejisu consistently recorded a higher average percentage of leaf damage (70.1 ± 16.0) compared to Ejura (62.6 ± 14.2). Within Ejisu, the Donyinah community rrecorded the most extensive leaf damage (78.6 ± 11.1), while Deduako recorded the least (62.4 ± 11.6) among its farms. All communities in Ejisu had over 20% leaf damage, except for Donaso, where only 12.5% of its farms experienced low leaf damage ranging from 1 – 20%. Conversely, in Ejura, a different trend emerged as all communities recorded either moderate or high levels of leaf damage across their farms. Specifically, Yaabraso stood out with all of its farms reporting high levels of leaf damage. Among these communities, Drobong documented the largest percentage of leaf damage (65.7 ± 11.5), while Yaabraso reported the least (58.5 ± 8.1) ().
At the vegetative stage, Ejisu still recorded a larger percentage of leaf damage in both the minor (58.9 ± 22.8) and major seasons (63.5 ± 18.8) compared to Ejura (16.1 ± 10.3 and 46.5 ± 12.1). Statistical analysis still showed a significant variation in percentage leaf damage between the two districts and among communities (p < 0.001). In the minor season, Onwe in the EJisu District recorded the highest percentage leaf damage (78.0 ± 8.9), while Deduako had the least (50.2 ± 21.4). On the other hand, within Ejura, Yaabraso had the highest percentage damage (31.8 ± 13.2), while Tetetowa recorded the least (11.4 ± 3.4). Remarkably, while Ejisu had only two of its communities (Donyinah and Essienimpong) recording 1 – 20% leaf damage, Ejura had all its communities recording 1 – 20% leaf damage with only Yaabraso having farms with both moderate and severe leaf damage on farms ().
Table 3. Level of leaf damage caused by FAW at vegetative stage.
In the major season, all communities in both districts recorded more than 20% leaf damage. In Ejisu, Onwe had the worst damage (78.5 ± 5.5), where every farm suffered a high level of damage. On the other hand, Donyinah reported the least with Ejisu (48.1 ± 18.9). In Ejura, Drobong recorded the largest percentage leaf damage (56.4 ± 12.5), while Tetetowa reported the least (36.8 ± 11.0) ().
3.2. Prevalence of FAW larvae on farms
Generally, prevalence of FAW larvae in both districts was higher in the major season compared to the minor season across all growth stages. A significant difference in larval prevalence was observed between growth stages in both districts during both the minor (p = 0.030 for Ejisu, p = 0.001 for Ejura) and major seasons (p = 0.726 for Ejisu, p = 0.000 for Ejura). During the minor season, Ejisu reported a higher mean prevalence of FAW at the seedling stage (0.1 ± 0.04 larvae per plant sampled) compared to the vegetative stage (0.09 ± 0.02 larvae per plant sampled). In Ejura however, the prevalence during the seedling stage (0.05 ± 0.03) was almost the same as that recorded at the vegetative stage (0.05 ± 0.04). On the other hand, both districts exhibited high prevalence during the vegetative stage in the major season signifying a consistent pattern across the districts ().
Table 4. Prevalence of FAW larvae.
At the seedling stage, Ejisu recorded a higher mean prevalence (0.10 ± 0.04) in the minor season compared to Ejura (0.05 ± 0.03). This varied within districts, ranging from 0.04 ± 0.08 in Deduako to 0.15 ± 0.10 in Onwe in Ejisu, and from 0.02 ± 0.02 in Tetetowa to 0.10 ± 0.11 in Drobong in Ejura. In the major season, however, the mean prevalence was contrary, being higher in Ejura (0.23 ± 0.02) than in Ejisu (0.17 ± 0.02). Tetetowa recorded the highest prevalence (0.27 ± 0.08) in Ejura, while Yaabraso reported the least (0.21 ± 0.06). In Ejisu, Donyinah (0.19 ± 0.11) recorded the highest prevalence, whereas Onwe recorded the least (0.2 ± 0.07). An analysis of variance, showed prevalence to vary significantly between seasons (p < 0.001). However, there was no significant difference in prevalence concerning districts (p = 0.781) and communities (p = 0.274) ().
At the vegetative stage, Ejisu consistently showed a higher prevalence of larvae compared to Ejura in both seasons. In the minor season, Donyinah, the Ejisu District reported the lowest prevalence (0.06 ± 0.08), while Deduako had the highest (0.1 ± 0.09). In the Ejura district, Tetetowa recorded the least prevalence (0.01 ± 0.02), while both Drobong and Yaabraso recorded the highest (0.10 ± 0.08). In the major season, Essienimpong had the highest FAW prevalence in the Ejisu district, whereas Onwe had the least (0.25 ± 0.07). On the other hand, Tetetowa maintained the lowest prevalence (0.26 ± 0.07), while Drobong exhibited the highest (0.35 ± 0.09) in Ejura (). Prevalence of FAW at the vegetative stage significantly differed between seasons (p < 0.001). However, there was no significant variance in prevalence regarding districts (p = 0.347) or communities (p = 0.740).
3.3. Severity of FAW damage infestation
Severity of FAW infestation in the districts was higher during the major season compared to the minor season, which was observed across both seedling and vegetative stages. Notably, this difference in severity was statistically significant solely among growth stages during the minor and major seasons at Ejisu (p < 0.001). In Ejura, however, there was no significant variation in severity between growth stages for both minor (p = 0.094) and major (p = 0.268) seasons.
At the seedling stage, both districts recorded a low severity of infestation, though the individual scores differed between the minor and major seasons (1 and 3 respectively) (). In the minor season, all communities in Ejisu reported the same severity (score 1) on their farms. Conversely, severity varied among communities in Ejura, with Danyame and Yaabraso recording the highest scores (3), while the rest recorded the least (1). During the major season, communities within both districts reported varying scores, yet an overall, low severity (score 3) was recorded at district level. Donaso reported the highest score (4), whereas Deduako and Onwe recorded the least (2) in Ejisu. In Ejura, Tetetowa reported the highest score (4), while Drobong recorded the least (2). Upon analysis, a significant difference in severity was observed among seasons (p < 0.001). However, no significant variations were found among districts (p = 0.41) and communities (p = 0.208).
Table 5. Severity of FAW.
During the vegetative stage, both districts exhibited a lower severity during the minor season compared to the major season (). This trend persisted consistently across all communities in both districts. In Ejisu, Deduako and Donyinah communities recorded the highest severity score (4), while Onwe and Donaso had the lowest (1) in the minor season. In Ejura, all communities, except Danyame, reported a severity score of 3. Severity during the major season increased across all communities and in both districts. In Ejisu, Donyinah had the highest score (6), while Essienimpong had the least (3). Similarly, in Ejura, all communities had a severity score of 5, except Tetetowa and Nkrampo, which recorded a score of 3. There was no significant variance in severity between districts (p = 0.155). However, severity among seasons (p < 0.001) and among communities different significantly (p = 0.012).
3.4. Association between climatic factors and FAW infestation
A correlational analysis showed a significant relationship among infestation parameters; leaf damage, prevalence and severity (). Prevalence positively and significantly correlated with leaf damage (r=.539, p<.01) and severity (r=.358, p<.01). Thus, an increase in prevalence resulted in significantly increased leaf damage and severity of infestation on the farms. It was, also, shown that leaf damage was significantly associated with severity (r=.148, p<.01) suggesting an increase in the leaf damage of FAW infestation on maize farms to result in an increase in the severity of infestation.
Table 6. Association between climatic factors and infestation of fall armyworms.
With regards to the association between infestation and climatic factors, results showed varying effects for infestation parameters (). Prevalence exhibited associations with various climatic elements such as rainfall, temperature, wind direction, wind speed, and relative humidity. Specifically, prevalence showed a significantly positive correlation with rainfall during both the month of data collection (r = .269, p < .01) and the preceding month (r = .194, p < .01). This suggests that as rainfall increased, the prevalence of larvae on farms also increased. Remarkably, while rainfall was shown to have a positive influence on prevalence, only rainfall from the month before data collection had a significant association with prevalence (r = .504, p < .01).
In contrast, a significantly negative correlation was observed between prevalence and wind direction (r = -.329 and r = -.258, p < .01 for the month of and month preceding data collection respectively). Moreover, wind speed in both the month of data collection and the previous month displayed a significant and positive correlation with prevalence. Essentially, a unit increase in wind speed during the month of data collection led to a 0.423 increase in prevalence, while the same increase in wind speed from the previous month resulted in a 0.422 increase in prevalence. Lastly, relative humidity showcased a negative yet significant association with prevalence, suggesting that an escalation in relative humidity led to a decrease in prevalence and vice versa (r = -.388 and r = -.451, p < .01 for the month of data collection and the month prior to data collection respectively) ().
Leaf damage exhibited significant and positive relationships with several climatic factors. Specifically, it showed positive and significant associations with average rainfall during the month of data collection (r = .257, p < .01) and the previous month (r = .379, p < .01), as well as with temperature during the month of data collection (r = .110, p < .05). Additionally, there was a positive and significant correlation with wind speed during both the month of data collection (r = .361, p < .01) and the preceding month (r = .189, p < .01). These findings imply that an increase in these climatic factors leads to a corresponding increase in leaf damage caused by fall armyworms (). On the other hand, there existed significant negative relationships between leaf damage and certain wind-related factors. Precisely, there were negative relationships with wind direction during the month of data collection (r = -.159), wind speed of data collection month (r = -.067), and wind speed of month prior (r = -.095) ().
The severity of infestation showed clear correlations with various climatic factors. Notably, it displayed positive and significant correlations with temperature (r = .148 and r = .527 at p < .01 for data collection and preceding month respectively) as well as with wind speed (r = .359 and r = .355, p < .01 for data collection and preceding month). On the contrary, severity exhibited negative correlations with rainfall, wind direction, and relative humidity for both data collection and preceding months, although the correlation between severity and rainfall was not deemed significant. These findings imply that an increase in these parameters leads to a decrease in the severity of infestation caused by fall armyworm ().
3.5. Climatic determinants of prevalence of FAW on farms
A linear regression analysis was done to predict the impact of climate variables on larval prevalence on farms ( and ). Results indicated that all climate variables; rainfall, temperature, wind direction, wind speed, and relative humidity, recorded during both the month of data collection and the preceding month significantly influenced (p < 0.001) prevalence (). Specifically, the findings revealed that rainfall (OR: 0.001; 95% CI: 0.001, 0.002), temperature (OR: 0.09; 95% CI: 0.07, 0.1), wind direction (OR: 0.01; 95% CI: 0.007, 0.011), and wind speed (OR: 0.01; 95% CI: 0.005, 0.009) recorded in the month of data collection were more likely to increase prevalence. On the other hand, for climate variable recorded in the month preceding data collection (temperature, wind direction, wind speed, and relative humidity) were more likely found to increase prevalence. However, rainfall in the month before sampling was found to having a decreasing effect on prevalence.
Table 7. Climate determinants of prevalence of FAW armyworm on farms.
Table 8. Summarized results for multiple linear regression for prevalence of fall armyworm infestation.
The analysis revealed that 46% of the fluctuations in prevalence across districts, seasons, and growth stages could be explained by variations in rainfall, temperature, wind direction, wind speed, and relative humidity (). Examining individual districts, these climate factors accounted for 44% of the prevalence variation in Ejisu, while a higher proportion of variations (64%), was attributed to these climate factors in Ejura. Similarly, when considering the seasonal and growth stage variations, these variables accounted for 11% of the variation in prevalence during the minor season, 40% at the seedling stage, and 64% at the vegetative stage. In the major season, wind speed was the only variable that exhibited no influence on prevalence, while all the other factors collectively accounted for 27% of the variations observed.
3.6. Climate determinants of leaf damage by fall armyworms on farms
Regression analysis showed that all climatic factors significantly predicted leaf damage caused by fall armyworms on farms with the exceptions of rainfall during and prior to the month of data collection and wind direction of month prior to data collection (). The analysis revealed a 1 °C unit increase in temperature in data collection and preceding month to increase leaf damage by 20.82 times and 20.37 times respectively. Additionally, a unit increase in windspeed (1 ms−1) in month of data collection and preceding month increased leaf damage by 1.49 and 0.99 times respectively. Furthermore, 1% increase in relative humidity recorded during and prior to the month of data collection increased leaf damage by 2.67 and 3.08 times respectively. These findings emphasized the influential role of these climatic factors in escalating leaf damage by fall armyworms.
Table 9. Climate determinants of leaf damage on farms.
A multiple regression analysis attributed 53% of the variations in leaf damage across districts, seasons, and growth stages to rainfall, temperature, wind direction, wind speed, and relative humidity (). However, considering individual districts, these climatic factors accounted for only 5% of the variations in leaf damage in Ejisu and 69% in Ejura. Furthermore, these climatic factors were responsible for 54% and 56% of the variations in leaf damage during the seedling and vegetative stages respectively.
Table 10. Summarized results for multiple linear regression for leaf damage by fall armyworms.
3.7. Climate determinants of severity of FAW infestation on farms
The severity of fall armyworm infestation was significantly influenced by all climate factors analyzed, except rainfall (). Temperature (OR: 0.70; 95% CI: 0.429, 0.961), wind direction (OR: 0.086; 95% CI: 0.429, 0.961), and wind speed (OR: 0.07; 95% CI: 0.046, 0.096) during the month of data collection were identified as factors more likely to intensify severity of infestation. Conversely, relative humidity during the same period (OR: 0.04; 95% CI: -0.081, 0.006) appear to less likely increase severity. While temperature, wind speed, and relative humidity in the month of data collection were found to be more likely to increase severity of infestation, wind direction from the preceding month was less likely to increase severity levels.
Table 11. Climate determinants of severity of fall armyworm infestation on farms.
Results presented in revealed that all climatic variables collectively explained 28% of the variations in infestation severity across districts, seasons, and growth stages. However, regarding individual districts, these factors accounted for 46% of the variations in Ejisu, while it explained a slightly lower proportion of variations in severity (31%) in Ejura. Further insights emerged regarding specific growth stages: all climatic factors were responsible for 29% of the variations in the minor season, 42% at the seedling stage, and 32% at the vegetative stage. Notably, in the major season, rainfall, temperature, wind direction, and relative humidity jointly contributed to 16% of the variations in severity.
Table 12. Summarized results for multiple linear regression for severity of fall armyworm infestation.
4. Discussion
4.1. Incidence and leaf damage by fall armyworm infestation
There was 100% incidence of FAW infestation on farms in both districts. This could be attributed to the constant food supply in the communities surveyed as these areas are major farming areas and maize production enclaves and could host abundant food supplies for fall armyworm to thrive. According to Paudel Timilsena et al. (Citation2022), all-year round supply of food enhances the survival of FAW explaining its incidence in the two districts surveyed in this study. In addition, FAW feeds on a wide range of crops and as such, an absence of maize when the growing season is over does not break their cycle of development as they feed on other food crops and come back to maize plants when they become available (Acharya et al., Citation2020). The higher percentage of damaged leaves in the major season compared to the minor season could be attributed to the different weather conditions in each season (Skendžić et al., Citation2021). Weather conditions have an effect on the growth of maize, with optimal climatic factors enhancing growth and serving the needs of FAW (Wang et al., Citation2021). As such, maize grown in the major season might have been much fresher and more nutritious to FAW than those in the minor season. This assertion that FAW prefers fresher leaves is in line with work done by Manjula et al. (Citation2019) which reported FAW to prefer leaves in the whorl which are much fresher than the outer leaves. The differences in percentage leaf damage in both districts could also be attributed to the differences in weather conditions in these districts. From Obour et al. (Citation2022) and MoFA (Citation2023), Ejisu and Ejura Districts are located in different ecological zones with differences in climatic factors. These climatic conditions could thus, affect the growth of maize as well as the extent of infestation in both districts. As such, there is a need for sustainable measures which are location and season-specific to be put in place to control FAW.
4.2. Prevalence of FAW larvae
FAW larvae were prevalent in all communities at all stages and seasons. This may have resulted from a lack of appropriate management strategies for FAW allowing them to thrive in both growing seasons. This in in line with work done by Thiefilder et al. (2018) which attributed the prevailing FAW problem in regions of the world to ineffective management strategies. Tham-Agyekum et al. (Citation2023) and Safo et al. (Citation2023) revealed chemical control to be the dominant control methods in both districts. However, a study by Sisay (Citation2018) reported incidence of FAW resistance to chemical insecticides used against them, rendering the insecticides ineffective. Additionally, differences in prevalence in the two districts could be attributed to the differences in chemical insecticides used in communities under these districts. The prevalence of the insect larvae on farms could result from untreated or unburnt maize stubbles or residues on field from previous growing season leading to no breakage of the life cycle of FAW. This serves as places for diapause of FAW until conditions are favourable to development. This is supported by Lungaju (Citation2021) who asserts that larvae of FAW may diapause in stubbles, stems and other plant remains during unfavourable conditions such as dry or cold periods for months before pupating in appropriate conditions. This is, also, supported by Prasanna et al. (Citation2018) and thus suggests a need for effective control measures to be put in place.
4.3. Severity of FAW infestation
The low to moderate severity of infestation observed in both districts could be due to the chemical insecticides used, the frequency of application and the time of application as shown by Kansiime et al. (Citation2019) and Yang et al. (Citation2021). Generally, farmers spray chemical insecticides at time intervals and S. frugiperda tends to feed more when there is no application. As a result, the time of application is an important influencer of severity. This is because data collected within a period of no insecticide application may record high severity as there is a higher feeding activity within that period and vice versa. Also, the stage of maize growth could also influence severity. According to Huber et al. (Citation2012) growth stages of plants influence their susceptibility to insect pest attack. Thus, young and rapidly growing plants are more susceptible to insect attack compared to older ones. This explains the differences in severity at the seedling and vegetative stages. Furthermore, a higher severity may suggest that the wrong type of insecticides is used for FAW control as well as its concentration and rate. It could also be attributed to applying the right insecticide at the wrong age of plant leading to inefficacy of insecticide (Midega et al., Citation2018) pointing out to the need for control measures that is line farmers’ need and can be easily adopted into agronomical practices on farms
4.4. Climatic determinants of fall armyworm infestation
Literatures have revealed the influence of climatic factors on insect activity and behaviour (Sentis et al., Citation2015; Skendžić et al. Citation2021; Zhang et al., Citation2020). As a result, a significant association between these climatic factors and infestation parameters in the present study suggests an influence of these factors on the activity and feeding habits of FAW. The positive association between temperature and infestation parameters; prevalence, leaf damage and severity are in line with the work of Deutsh et al. (2018), which reports an increase in temperature to have the tendency of increasing feeding rates of agricultural pests reducing crop yield and threatening food supplies. This result, also, supports work by Skendžić et al. (Citation2021) who indicated a rise in the temperature to increase consumption and herbivory in insects. Thus, an increase in temperature increases feeding rates of fall armyworms increasing the prevalence, damage caused and severity on maize farms.
Rainfall, also, impacts insect populations and activities (Skendžić et al., Citation2021). Unlike temperature, the overall impact of rainfall on insect pest species and their interactions with host plants is difficult to assess (Schneider et al., Citation2022). This is because while dry climate provides a suitable environmental condition for development of herbivorous insects, it can limit the availability of food for these insects (Schneider et al., Citation2022; Skendžić et al., Citation2021). Thus, even though heavy rainfall can threaten the survival of insects, it can be optimal for plant growth. Heavy rainfall has been observed to limit the migration of some insects (Chen et al., Citation2019). This could explain the negative correlation of rainfall and severity in this study as movement of fall armyworms to maize farms may be hindered. The negative correlation could also, be explained by the decreasing effects of rainfall on temperature, reducing the feeding activities of FAW. Additionally, very dry seasons and drought can indirectly affect herbivorous insects by limiting availability of food during their development (Schneider et al., Citation2022). On the other hand, an increase in rainfall provides optimal rain needed by plants to grow thereby providing food for insect to feed on. This explains the positive association between rainfall and insect infestation. A study by Niassy et al. (Citation2021) showed FAW infestation to increase at the peak of rainfall in Kenya, Rwanda, Ethiopia and Uganda while it decreased when rainfall subsided.
Wind is an important climate variable which plays a significant role in the movement patterns of insects influencing their infestation in a particular area. It modifies the behaviour of insects by limiting or increasing their access to food (Leonard et al., Citation2016). Wind increases their susceptibility to cuticle desiccation and the same time alters the suitability of plants to insects by increasing thickness of cuticles in plants and secretion phenolics to decrease feeding by larval lepidopterans (Mann et al., Citation2008; McArthur et al., Citation2010, Leonard et al., Citation2016). Reports from Khaliq et al. (Citation2014) and Ludwig et al. (Citation2018) indicate wind to affect insect dispersal by transporting odours of specific plants downwind, which serve as attractive stimuli for herbivorous insects. As indicated by Beyaert and Hilker (Citation2014), moth basically depend on olfaction to track odour plumes and locate their host. However, the transportation of these odour plumes is influenced by both wind direction and wind speed suggesting the impact of wind on FAW infestation.
FAW as strong migratory insects are carried by wind currents over long distances. Thus, favourable winds with optimal direction and speed can facilitate their migration into new areas and areas with abundant food supply and vice versa (Westbrook et al., Citation2019). Wu et al. (Citation2021) reports long range-migratory flights of FAW to be affected by air streams such as direction and wind at high altitudes where they fly. The positive association between wind direction and infestation variables could, thus, be explained as the transport of insects towards food sources when direction is towards these food sources while a negative association suggests the opposite. Prevailing winds, also, may blow insects from infested fields to uninfected crops leading to invasion of and prevalence in these regions. Additionally, wind direction can influence the local dispersal of FAW within a field. For instance, if wind consistently blows from one side of the field to the other, it may affect the distribution of FAW, causing higher prevalence and leaf damage on plants located in the path of the prevailing wind.
Wind speed is important in the infestation of insects as it aids in their dispersal over longer distances. Strong winds may carry the moths or young larvae across fields or even between different regions, contributing to the rapid spread of infestation and can lead to a concentration of feeding in areas with food sources. This explains the positive association between wind speed and infestation parameters. Qi et al. (Citation2021) reports wind speed above 10 m/s to be suitable carrier airflow which enabled the FAW to complete a long-distance migration across the seas in Australia. The effect of windspeed on the infestation of FAW in farms is similar to findings of Shao et al. (Citation2020) which reported wind speed to have a major impact on the population density and incidence rates of Culcula panterinaria. This is, also, corroborated by Ali et al. (Citation2020) who revealed wind speed to favour the increase in population of insect pests of cotton. Additionally, wind speed was shown in the study to have an effect on egg count. Thus, an optimal wind speed might positively impact the hatching of fall armyworm eggs resulting in high prevalence, leaf damage and severity on farms.
Relative humidity, also, plays significant role in the infestation of FAW on farms. It is reported to influence the life cycle and reproductive behavior of insect pests of maize such as FAW (Zulfiqar et al., Citation2010). Higher humidity levels (above 70-80%) create a favorable environment for the survival and reproduction of S. frugiperda (He et al., Citation2021). Warm and humid conditions are conducive to the rapid development and spread of FAW populations. Thus, the positive association between relative humidity and FAW prevalence suggests an increase in relative humidity to optimal levels to potentially lead to higher prevalence FAW in maize farms. On the other hand, the negative association between relative humidity and prevalence could be explained as, a decrease in the relative humidity below optimal levels resulting in a decreasing effect of the population of FAW on farms. Relative humidity also affects the feeding behavior of insects. A study by Contreras et al. (Citation2013) revealed the survival of the hawkmoth, Manduca sexta to increase with higher humidities while foraging maximized at lower humidities. Thus, the activity and voracity of FAW larvae could be influenced by relative humidity. An increased humidity level may promote faster larval growth, leading to more extensive leaf damage within a shorter period. The severity of FAW infestation in maize farms can therefore escalate under favorable humidity conditions.
Thus, climatic factors potentially impact FAW infestation in the two districts. As such, there is a need for climate smart technologies which would help relay information stakeholders and farmers potential changes in the climate prompting them on the necessary actions to take to reduce FAW infestation.
Conclusion
The study investigated the incidence, prevalence and severity of fall armyworm infestation in two districts in the Ashanti Region of Ghana as well as the influence of climatic factors on infestation. The study concludes that there is an incidence of fall armyworm infestation in both districts. Prevalence and severity of fall armyworm varied among districts and growth stages and was higher in the major season than the minor season. Climatic factors including rainfall, temperature, relative humidity, wind speed and wind direction had a significant influence on fall armyworm infestation at district, growth and seasonal levels. Given this infestation across district and the influence of climatic conditions on infestation, effective management strategies that is tailored towards the needs and climatic factors of both districts have to be put in place to reduce infestation.
Authors’ contribution
Author Veronica Frempomaa Siaw conceived the research idea, designed the research, conducted field work, analysed and interpreted data, drafted manuscript and approved the final version to be published. Author Michael Osae conceived research idea, designed research, supervised research, critically reviewed manuscript and approved the final version to be published. Author John Asiedu Larbi shaped research idea, designed and supervised research, critically reviewed manuscript and approved the final version to be published. Author Philip Kweku Baidoo designed research, supervised research, reviewed manuscript and approved of the final version to be published. Author Marian Amoakowaah Osei designed the research, retrieved and curated data for climate variables, reviewed manuscript and approved of final version to be published. All authors take full responsibility for the entirety of the work and commit to address and resolve any queries regarding its accuracy or integrity, ensuring thorough investigation and resolution.
Acknowledgements
The authors are grateful to the Directors and Extension Officers of the Ministry of Food and Agriculture Organization at Ejura and Ejisu in the Ashanti Region of Ghana for serving us as a link between us and the farmers and helping in the administration of questionnaires in all communities. We want to thank David Manu-Bonsu for the enormous help in the organization of the data for statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
We hereby declare that data will be readily made available upon request.
Additional information
Funding
References
- Acharya, S., Kaphle, S., Upadhayay, J., Pokhrel, A., & Paudel, S. (2020). Damaging nature of fall armyworm and its management practices in maize: A review. Tropical Agrobiodiversity, 1(2), 1–17. https://doi.org/10.26480/trab.02.2020.82.85
- Ahmed Seid, S. (2022). Damage level survey, screening of management options and integrated management of fall armyworm (Spodoptera frugiperda) (J.E Smith) (Lepidoptera: Noctuidae) in maize at asossa zone, western Ethiopia (Doctoral dissertation, Asossa University). Retrieved from: http://publication.eiar.gov.et:8080/xmlui/bitstream/handle/123456789/3569/seid%20final%20thesis%20%28MSc%29.pdf?sequence=1andisAllowed=y. Accessed: 14.12.2023
- Ali, A., Akhtar, M. N., Hayat, S., Farooq, A., & Ali, Q. (2020). Effects of weather parameters on incidence of sucking pests and their predators on cotton (Gossypium hirsutum L.). Plant Cell Biotechnology and Molecular Biology, 21(49 & 50), 112–119.
- Appiah, S. T., Otoo, H. and Nabubie, I. B. (2015). Times series analysis of malaria cases in Ejisu-Juaben Municipality. International Journal of Scientific and Technology Research, 4(06), 220–226.
- Baudron, F., Zaman-Allah, M. A., Chaipa, I., Chari, N., & Chinwada, P. (2019). Understanding the factors influencing fall armyworm (Spodoptera frugiperda JE Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Protection, 120, 141–150. https://doi.org/10.1016/j.cropro.2019.01.028
- Beyaert, I., & Hilker, M. (2014). Plant odour plumes as mediators of plant–insect interactions. Biological Reviews of the Cambridge Philosophical Society, 89(1), 68–81. https://doi.org/10.1111/brv.12043
- Chen, H., Chang, X. L., Wang, Y. P., Lu, M. H., Liu, W. C., Zhai, B. P., & Hu, G. (2019). The early northward migration of the white-backed planthopper (Sogatella furcifera) is often hindered by heavy precipitation in southern China during the preflood season in May and June. Insects, 10(6), 158. https://doi.org/10.3390/insects10060158
- Contreras, H. L., Goyret, J., von Arx, M., Pierce, C. T., Bronstein, J. L., Raguso, R. A., & Davidowitz, G. (2013). The effect of ambient humidity on the foraging behavior of the hawkmoth Manduca sexta. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 199(11), 1053–1063. https://doi.org/10.1007/s00359-013-0829-3
- Copernicus Climate Change Service (C3S. (2017).). ERA5: Fifth generation of ECMWF.
- Cossar, F., Houssou., & N., Asante-Addo. (2016). Development of agricultural mechanization in Ghana: Network, actors, and institutions: A case study of Ejura-Sekyedumase District (No. 43) (pp. C. 2–12). International Food Policy Research Institute (IFPRI).
- Darfour, B., & Rosentrater, K. A. (2016, July). Maize in Ghana: An overview of cultivation to processing [Paper presentation]. ASABE Annual International Meeting, Iowa State University, Orland - Florida. https://doi.org/10.13031/aim.20162460492
- Davis, F. M., & Williams, W. P. (1992). Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Technical Bulletin (Mississippi Agricultural and Forestry Experiment Station), (186), 1–9.
- Deshmukh, S. S., Prasanna, B. M., Kalleshwaraswamy, C. M., Jaba, J., & Choudhary. B. (2021). Fall armyworm (Spodoptera frugiperda). In Polyphagous pests of crops (pp. 349–372). Springer Nature.
- Deutsch, C. A., Tewksbury, J. J., Tigchelaar, M., Battisti, D. S., Merrill, S. C., Huey, R. B., & Naylor, R. L. (2018). Increase in crop losses to insect pests in a warming climate. Science , 361(6405), 916–919. https://doi.org/10.1126/science.aat3466
- Du Plessis, H., Schlemmer, M. L., & Van den Berg, J. (2020). The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects, 11(4), 228. https://doi.org/10.3390/insects11040228
- Funk, C., Peterson, P., Landsfeld, M., Pedreros, D., Verdin, J., Shukla, S., Husak, G., Rowland, J., Harrison, L., Hoell, A., & Michaelsen, J. (2015). The climate hazards infrared precipitation with stations—a new environmental record for monitoring extremes. Scientific Data, 2(1), 150066. https://doi.org/10.1038/sdata.2015.66
- He, L., Zhao, S., Ali, A., Ge, S., & Wu, K. (2021). Ambient humidity affects development, survival, and reproduction of the invasive fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in China. Journal of Economic Entomology, 114(3), 1145–1158. https://doi.org/10.1093/jee/toab056
- Hersbach, H., Bell, B., Berrisford, P., Hirahara, S., Horányi, A., Muñoz-Sabater, J., Nicolas, J., Peubey, C., Radu, R., Schepers, D., Simmons, A., Soci, C., Abdalla, S., Abellan, X., Balsamo, G., Bechtold, P., Biavati, G., Bidlot, J., Bonavita, M., … Thépaut, J. (2020). The era5 global reanalysis. Quarterly Journal of the Royal Meteorological Society, 146(730), 1999–2049. https://doi.org/10.1002/qj.3803
- Huber, D., Römheld, V., & Weinmann, M. (2012). Relationship between nutrition, plant diseases and pests. In Marschner’s mineral nutrition of higher plants. (pp. 283–298). Academic Press.
- Huma, B., Hussain, M., Ning, C., & Yuesuo, Y. (2019). Human benefits from maize. Sch. J. Appl. Sci. Res, 2(2), 4–7.
- Kansiime, M. K., Mugambi, I., Rwomushana, I., Nunda, W., Lamontagne-Godwin, J., Rware, H., Phiri, N. A., Chipabika, G., Ndlovu, M., & Day, R. (2019). Farmer perception of fall armyworm (Spodoptera frugiderda JE Smith) and farm-level management practices in Zambia. Pest Management Science, 75(10), 2840–2850. https://doi.org/10.1002/ps.5504
- Khaliq, A. M., Javed, M., Sohail, M., & Sagheer, M. (2014). Environmental effects on insects and their population dynamics. Journal of Entomology and Zoology Studies, 2(2), 1–7.
- Leonard, R. J., McArthur, C., & Hochuli, D. F. (2016). Exposure to wind alters insect herbivore behaviour in larvae of U raba lugens (L epidoptera: N olidae). Austral Entomology, 55(3), 242–246. https://doi.org/10.1111/aen.12175
- Li, Y. P., Yao, S. Y., Feng, D., Haack, R. A., Yang, Y., Hou, J. L., & Ye, H. (2023). Dispersal behavior characters of Spodoptera frugiperda larvae. Insects, 14(6), 488. https://doi.org/10.3390/insects14060488
- Ludwig, M., Schlinkert, H., & Meyhöfer, R. (2018). Wind-modulated landscape effects on colonization of B russels sprouts by insect pests and their syrphid antagonists. Agricultural and Forest Entomology, 20(2), 141–149. https://doi.org/10.1111/afe.12237
- Lungaju, S. E. (2021). [Temporal Dynamics of Fall Armyworm, Spodoptera Frugiperda (Je Smith), Stemborer Pests and Associated Natural Enemies in Maize Fields in Semi-arid Zone, Machakos, Kenya ]. [Doctoral dissertation]. Uon.).
- Manjula, K., Saheb, Y. P., Sudheer, M. J., & Rao, A. R. (2019). Studies on biology, feeding habits and natural enemies of fall armyworm, Spodoptera frugiperda, a new invasive pest in India. Journal of Entomology and Zoology Studies, 7(6), 1245–1250.
- Mann, J. A., Tatchell, G. M., Dupuch, M. J., Harrington, R., Clark, S. J., & Mccartney, H. A. (2008). Movement of apterous Sitobion avenae (Homoptera: Aphididae) in response to leaf disturbances caused by wind and rain. Annals of Applied Biology, 126(3), 417–427. https://doi.org/10.1111/j.1744-7348.1995.tb05376.x
- McArthur, C., Bradshaw, O. S., Jordan, G. J., Clissold, F. J., & Pile, A. J. (2010). Wind affects morphology, function, and chemistry of eucalypt tree seedlings. International Journal of Plant Sciences, 171(1), 73–80. https://doi.org/10.1086/647917
- Midega, C. A., Pittchar, J. O., Pickett, J. A., Hailu, G. W., & Khan, Z. R. (2018). A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (JE Smith), in maize in East Africa. Crop Protection, 105, 10–15. https://doi.org/10.1016/j.cropro.2017.11.003
- MoFA. (2023). Ejisu-Juabeng District. Retrieved from: https://mofa.gov.gh/site/sports/district-directorates/ashanti-region/162-ejisu-juaben. Accessed: 24.01.2023.
- Niassy, S., Agbodzavu, M. K., Kimathi, E., Mutune, B., Abdel-Rahman, E. F. M., Salifu, D., Hailu, G., Belayneh, Y. T., Felege, E., Tonnang, H. E. Z., Ekesi, S., & Subramanian, S. (2021). Bioecology of fall armyworm Spodoptera frugiperda (JE Smith), its management and potential patterns of seasonal spread in Africa. PLOS One, 16(6), e0249042. https://doi.org/10.1371/journal.pone.0249042
- Obour, P. B., Arthur, I. K., & Owusu, K. (2022). The 2020 maize production failure in Ghana: A case study of Ejura-Sekyedumase municipality. Sustainability, 14(6), 3514. https://doi.org/10.3390/su14063514
- Paudel Timilsena, B., Niassy, S., Kimathi, E., Abdel-Rahman, E. M., Seidl-Adams, I., Wamalwa, M., Tonnang, H. E. Z., Ekesi, S., Hughes, D. P., Rajotte, E. G., & Subramanian, S. (2022). Potential distribution of fall armyworm in Africa and beyond, considering climate change and irrigation patterns. Scientific Reports, 12(1), 539. https://doi.org/10.1038/s41598-021-04369-3
- Peterson, P., Funk, C., & Husak, G. (2013 The Climate Hazards Group Infrared Precipitation with Sations (CHIRPS): Development and validation [Paper presentation]. AGU Fall Meeting Abstracts, pp In: H33E–1417
- Prasanna, B. M., Huesing, J. E., Eddy, R., & Peschke, V. M. (2018). Fall armyworm in Africa: a guide for integrated pest management. Retrieved from: http://resilience-exchange.s3.amazonaws.com/attachments/uploads/4906/original/Fall_Armyworm_in_Africa__A_Guide_for_Integrated_Pest_Management.pdf. Accessed: 01.07.2023.
- Qi, G. J., Ma, J., Wan, J., Ren, Y. L., McKirdy, S., Hu, G., & Zhang, Z. F. (2021). Source regions of the first immigration of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) invading Australia. Insects, 12(12), 1104. https://doi.org/10.3390/insects12121104
- Ragasa, C., Chapoto, A., & Kolavalli, S. (2014). Maize productivity in Ghana. (Vol. 5). Intl Food Policy Res Inst. Retrieved from: file:///C:/Users/maame/Downloads/p15738coll2_128263.pdf. Accessed: 02.07.2023.
- Regier, J. C., Mitter, C., Mitter, K. I. M., Cummings, M. P., Bazinet, A. L., Hallwachs, W., Janzen, D. H., & Zwick, A. (2017). Further progress on the phylogeny of Noctuoidea (Insecta: L epidoptera) using an expanded gene sample. Systematic Entomology, 42(1), 82–93. https://doi.org/10.1111/syen.12199
- Safo, A., Avicor, S. W., Baidoo, P. K., Addo-Fordjour, P., Ainooson, M. K., Osae, M., Gyan, S. E., & Nboyine, J. A. (2023). Farmers’ knowledge, experience and management of fall armyworm in a major maize producing municipality in Ghana. Cogent Food & Agriculture, 9(1), 2184006. https://doi.org/10.1080/23311932.2023.2184006
- Sagar, G. C., Aastha, B., & Laxman, K. (2020). An introduction of fall armyworm (Spodoptera frugiperda) with management strategies: A review paper. Nippon Journal of Environmental Science, 1(4), 1010. https://doi.org/10.46266/njes.1010
- Schneider, L., Rebetez, M., & Rasmann, S. (2022). The effect of climate change on invasive crop pests across biomes. Current Opinion in Insect Science, 50, 100895. https://doi.org/10.1016/j.cois.2022.100895
- Sentis, A., Morisson, J., & Boukal, D. S. (2015). Thermal acclimation modulates the impacts of temperature and enrichment on trophic interaction strengths and population dynamics. Global Change Biology, 21(9), 3290–3298. https://doi.org/10.1111/gcb.12931
- Shao, X., Zhang, Q., Liu, Y., & Yang, X. (2020). Effects of wind speed on background herbivory of an insect herbivore. Écoscience, 27(1), 71–76. https://doi.org/10.1080/11956860.2019.1666549
- Sharon, B., Michael, M., & Bwayo, M. F. (2020). Severity and prevalence of the destructive fall armyworm on maize in Uganda: A case of Bulambuli District. African Journal of Agricultural Research, 16(6), 777–784.
- Singh, S., Raghuraman, M., Keerthi, M. C., Das, A., Kar, S. K., Das, B., Devi, H. L., Sunani, S. K., Sahoo, M. R., Casini, R., Elansary, H. O., & Acharya, G. C. (2023). Occurrence, Distribution, Damage Potential, and Farmers’ Perception on Fall Armyworm, Spodoptera frugiperda (JE Smith): Evidence from the Eastern Himalayan Region. Sustainability, 15(7), 5681. https://doi.org/10.3390/su15075681
- Sisay, B. (2018). [Evaluation of different management options of fall armyworm (JE Smith) (Lepidoptera: Noctuidae) and assessment of its parasitoids in some parts of Ethiopia ]. [Doctoral dissertation]. Haramaya University.). Retrieved from: http://34.250.91.188:8080/xmlui/bitstream/handle/123456789/1061/Thesis%20%28Birhanu%20Sisay%29%20final%20submitted.pdf?sequence=1&isAllowed=y. Accessed: 18.11.2023.
- Skendžić, S., Zovko, M., Živković, I. P., Lešić, V., & Lemić, D. (2021). The impact of climate change on agricultural insect pests. Insects, 12(5), 440. https://doi.org/10.3390/insects12050440
- Tham-Agyekum, E. K., Appiah, S. K., Ankuyi, F., Andivi Bakang, J. E., Frimpong-Manso, J., Edeafour, P. P., & Asiamah, M. T. (2023). Maize farmers’ response to crisis: The case of fall armyworm infestation in Ejisu Municipality, Ghana. Cogent Social Sciences, 9(2), 2275862. https://doi.org/10.1080/23311886.2023.2275862
- Thierfelder, C., Niassy, S., Midega, C., Subramanian, S., Van den Berg, J., Prasanna, B. M., … Harrison, R. (2018). low-cost agronomic practices and landscape management approaches to control FAW. CIMMYT.
- Wan, J., Huang, C., Li, C-y., Zhou, H-x., Ren, Y-l., Li, Z-y., Xing, L-s., Zhang, B., Qiao, X., Liu, B., Liu, C-h., Xi, Y., Liu, W-x., Wang, W-k., Qian, W-q., Mckirdy, S., & Wan, F-h (2021). Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Journal of Integrative Agriculture, 20(3), 646–663. https://doi.org/10.1016/S2095-3119(20)63367-6
- Wang, Y., Wang, C., & Zhang, Q. (2021). Synergistic effects of climatic factors and drought on maize yield in the east of Northwest China against the background of climate change. Theoretical and Applied Climatology, 143(3–4), 1017–1033. https://doi.org/10.1007/s00704-020-03457-0
- Westbrook, J., Fleischer, S., Jairam, S., Meagher, R., & Nagoshi, R. (2019). Multigenerational migration of fall armyworm, a pest insect. Ecosphere, 10(11), e02919. https://doi.org/10.1002/ecs2.2919
- Wongnaa, C. A., Awunyo-Vitor, D., Mensah, A., & Adams, F. (2019). Profit efficiency among maize farmers and implications for poverty alleviation and food security in Ghana. Scientific African, 6, e00206. https://doi.org/10.1016/j.sciaf.2019.e00206
- Wu, Q. L., Jiang, Y. Y., Liu, J., Hu, G., & Wu, K. M. (2021). Trajectory modeling revealed a southwest-northeast migration corridor for fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Science, 28(3), 649–661. https://doi.org/10.1111/1744-7917.12852
- Yang, X., Wyckhuys, K. A., Jia, X., Nie, F., & Wu, K. (2021). Fall armyworm invasion heightens pesticide expenditure among Chinese smallholder farmers. Journal of Environmental Management, 282, 111949. https://doi.org/10.1016/j.jenvman.2021.111949
- Zhang, P., van Leeuwen, C. H., Bogers, D., Poelman, M., Xu, J., & Bakker, E. S. (2020). Ectothermic omnivores increase herbivory in response to rising temperature. Oikos, 129(7), 1028–1039. https://doi.org/10.1111/oik.07082
- Zulfiqar, M. A., Sabri, M. A., Raza, M. A., Hamza, A., Hayat, A., & Khan, A. (2010). Effect of temperature and relative humidity on the population dynamics of some insect pests of maize. Pakistan Journal of Life and Social Science, 8, 16–18.