Abstract
Salinity is one of the main factors that limit rice production globally. In Sub Saharan Africa, soil salinity has affected many countries. Understanding the available screening techniques and the mechanisms of salinity tolerance in rice is very important for dealing with soil salinity problem. This review summarizes the extent of soil salinity problems in some of the affected African countries and the available salt stress-tolerant rice genotypes. The problems, challenges and opportunities of salt-affected soils in Sub Saharan Africa are thoroughly described in this review, which also looks at breeding techniques which have been used for developing rice cultivars adapted to salt stress. Among the best option to deal with salinity-related problems is through the use of tolerant rice varieties, as many other available management approaches are not economically feasible for small-scale farmers. Also, the review discusses different approaches, both conventional and molecular breeding approaches that have greatly enhanced the current rice breeders’ toolboxes for developing salt-tolerant rice varieties. The review suggests that more efforts are required to leverage conventional breeding with molecular techniques for speedy identification of tolerant cultivars, useful markers and quantitative trait loci (QTLs). It is recommended that it is crucial to reinforce collaborative efforts and continuous investment in research, capacity building, and knowledge sharing for developing improved rice tolerant cultivars in order to fully address salinity problems in African.
Reviewing Editor:
1. Introduction
Agriculture is the backbone of the African economy, which accounts for one-third of the Sub-Saharan African (SSA) countries’ gross domestic product (GDP), employing about 65-70% of the African workforce (Mukasa et al., Citation2017; Diao et al., Citation2010). However, the region lags behind other regions worldwide regarding agricultural productivity. This is due to several reasons which apply at different levels of the food value chain, making Africa a food-insecure continent. A core problem with soils in Africa is degradation caused by escalating salinization, accelerated soil erosion, acidification, plant nutrient depletion, and soil biodiversity loss, (Lal & Stewart, Citation2019). Salinity is one of the main factors that limit rice production globally. An increasing amount of land is becoming unfeasible for crop production each year due to land salinization, and it is predicted that by 2050 about 50% of the land will be unsuitable for crop production (Subudhi et al., Citation2020). It will take significant efforts to reduce the yield gap to keep up with the rising population, especially with the increasing per capita rice consumption extending from 12 kg year−1 in the 1960s to 27.4 kg year−1 recently (van Oort & Zwart, Citation2018; Van Oort et al., Citation2015; Rahman et al., Citation2021).
Rice (Oryza sativa L.) is the main food source for approximately 3.5 billion people worldwide and provides more than 20% of dietary calories (Muthayya et al., Citation2014). Rice is the most important crop in Asia, and about 90% of the crop is produced in the region, providing almost 70% of their dietary calories (Segal & Minh, Citation2019). Rice has also been shown to have an increased consumption rate in the Caribbean and Latin America, with a reported increase in per capita rice consumption from 10 kg to 30 kg in the twentieth century (Calvert et al., Citation2004). In sub-Saharan Africa, rice is becoming an important crop, as shown in its increasing per capita consumption which has doubled since 1970 (Muthayya et al., Citation2014). The reported rice yield in SSA is around 2.8 tons/ha, which is still very low compared to the 4.61 tons/ha reported in Asia (Arouna et al., Citation2021).
The low rice yields in SSA are due to several reasons, such as salinization, diseases, social issues, labour, climate change such as drought incidences, poor soil fertility, and various forms of soil and land degradation, (Lal & Stewart, Citation2019; Bjornlund et al., Citation2020). In addition, flooding also have immensely contributed to low rice yields in SSA. Available quantitative data highlights the immense loss in crop and soil productivity associated with soil and land degradation (Obalum et al., Citation2012), which imposes one of the core problems of crop production in Africa. Furthermore, salinization, soil and land degradation is equally, caused by, accelerated soil erosion, acidification, loss of soil organic matter, plant nutrient depletion, and soil biodiversity loss (Lal & Stewart, Citation2019). However, salinity has been reported to be a major environmental factor promoting soil degradation (Thiam et al., Citation2021) and is known to negatively affect crop yields, vegetation growth, and soil quality (Ma et al., Citation2020; Wang et al., Citation2018).
Developing stress-tolerant rice varieties has been an effective approach to coping with adverse effects soil salinity. Although conventional breeding methods have significantly improved crop production and yield, biotechnological breeding approaches offer the possibility to obtain improved and genome-edited crops in a short period of time (Anwar & Kim, Citation2020). Additionally, advances in molecular marker technologies, such as marker-assisted selection (MAS), marker-assisted backcrossing (MAB), and genomic selection (GS), have also provided breeders with more tools for quick development of stress-tolerant rice cultivars. The contribution of other biotechnological techniques, such as transgenic, mutation, and genome editing approaches, have been reviewed. These technological advances have been found to reduce breeding time and improved the accuracy of breeding programs when significant environmental effects may have reduced the effectiveness of selection (Bhowmik et al., Citation2009). This review illustrates the extent of soil-related problems, and of particular interest, the salt-affected soils in Africa, challenges, opportunities and the approaches available for developing stress-tolerant rice varieties for improving food security in Africa. Relevant information was gathered, selected, and presented in meaningful ways, using tables and figures. The flow of the review has been arranged in topics and subtopics relevant for themes discussed.
2. Degraded soils and their effect on rice production in Africa
2.1. Soil salinization as a major process of land degradation in Africa
Salinization, is one type of soil and land degradation, that occur through primary rock weathering releasing salts that if they are not adequately washed away particularly in areas where precipitations are lower than evapotranspiration. Also, secondary salinization processes caused by human activities such irrigation with saltwater, poor drainage and irrigation water management resulting to elevated water tables, overuse of fertilizers and over-exploitation of groundwater leads to accumulation of salts to hazardous levels in soils (Hassani et al., Citation2021). Experience reveals that soil salinity, both natural and human-induced, is dynamic and widespread in most countries (Shahid et al., Citation2018).
Additionally, soil and land degradation attributed to excessive sodium accumulation in the soil by the process called sodification, results in sodic soils. The forming processes are the same as salinization but differs due to high sodium concentration (SAR > 13 and ESP >15%) and electrical conductivity above 4 dSm−1, as summarized in (). Both saline and sodic soils are found in equal proportions in Tanzania, affecting rice growth, yield, and overall productivity (FAO, Citation2000). Furthermore, future climate change scenarios are projected to increase the problem of soil salinity due to elevated sea levels, rising temperatures, and increased evaporation (Shahid et al., Citation2018).
Table 1. Classification of salt-affected soils based on chemical properties (Daba & Qureshi, Citation2021).
Soil salinization results in the build-up and movement of salts in the agricultural landscape, which can occur naturally or as a result of poor management practices (Kayode et al., Citation2021). The salinization of soils results in several physical, biological, and chemical problems. More frequently, salinization is linked to waterlogging arising from poor drainage and pollution brought on by improper irrigation practices. There are many more human induced factors causing salinization in agricultural lands including poorly designed and operated irrigation systems, intensive agriculture, and low-quality water for irrigation (Machado & Serralheiro, Citation2017) and of recent the occurrence of climate change.
Salts in soils occur in the ionic form, released due to the weathering of rocks and soil parent materials in the soil. When there is no adequate rainfall to wash away ions from the soil profile, the salt accumulates, resulting into soil salinity and/or sodicity. Plants absorb important nutrients through soluble salts, but oversupply severely inhibits plant growth. Over the centuries, the physical, chemical, and/or biological processes that degrade soils have severely impacted the world’s natural resources (Shrivastava & Kumar, Citation2015) as indicated with different proportions in different countries in Africa ().
Table 2. Extent of salt affected soils in some African countries.
The total area in Africa affected by salinity is around 122.9 million hectares and it may continue to increase annually due to the expansion of irrigation practices in new areas (Shahid et al., Citation2018). It has been established that, soil salinity and sodicity have already affected more than 19 million hectares of sub-Saharan Africa (Tully et al., Citation2015).
2.2. Effect of salt-affected soils on rice production
Plants are classified as glycophytes or halophytes based on their ability to thrive in salty environments, and each group responds differently to salt stress. They differ in terms of uptake of toxic ions, compartmentation and/or exclusion of ions, photosynthetic electron transport, osmotic regulation, CO2 assimilation, chlorophyll content, fluorescence, antioxidant defences, and generation of reactive oxygen species (ROS) (Stepien & Johnson, Citation2009; Munns & Munns, Citation2005; Tang et al., Citation2014). Plant adaption to salinity is associated with specific morphological and anatomical changes (Moatabarniya et al., Citation2022).
Salt-affected soils (SAS) limit plant development from germination, vegetative growth, and reproductive growth stages due to high osmotic pressure which suck out water from the growing plant/shoot hence creating harsh environmental conditions for survival (Machado & Serralheiro, Citation2017). This means, excess salts in the root zone prevent plant roots from drawing water from the soil. For all major crops, average yields in salt-affected areas are only a fraction, between 0%, 20%, and 50% of the total yields (Shrivastava & Kumar, Citation2015), instead water move out of the plants into the salty soils. It is therefore, evident that salt-affected soils (SAS) negatively affects agricultural productivity and quality, biodiversity, water quality, the availability of water for essential human needs and industry, the durability and quality of infrastructures, and human livelihoods (Shrivastava & Kumar, Citation2015; Kumar & Sharma, Citation2020).
Furthermore, salt stress also negatively affect plant establishment, tillers, panicles, time to heading, leaf area index, and flowering (Singh et al., Citation2021). It has been established that seedlings under salinity and sodicity stress develop smaller, thinner, and fewer roots with less root mass compared to plants not exposed to salt stress (Dasgupta et al., Citation2018; Grattan et al., Citation2002).
However, research results indicate that there are genetic differences in salt tolerance, and the level of tolerance can differ within plant species and subspecies. Barley (Hordeum vulgare) is among the most important crops that exhibit a higher level of salt tolerance than wheat (Triticum aestivum) and rice (Oryza sativa). shows threshold levels of rice compared to other field crops. Glycophytes, which include most crop plants, cannot grow well in the presence of high salt concentrations. At the same time, halophytes can thrive relatively well in the presence of high concentrations of salts (400 mM NaCl) due to their improved salt tolerance mechanism (Acosta-Motos et al., Citation2017; Mishra & Tanna, Citation2017; Hoang et al., Citation2016).
Table 3. Salinity threshold levels for rice compared with other field crops.
2.3. Nutrient-depleted soils in Africa
Nutrient-depleted soils exist worldwide and seriously impede agricultural production by affecting plant roots’ ability to absorb water and mineral nutrients from the soil (Gomiero, Citation2016; Ismail & Thomson, Citation2011). In some areas, nutrients have been depleted, causing previously fertile lands to no longer be suitable for agriculture, and some land areas have been abandoned from farming (Mosier et al., Citation2021). These soils possess serious physical and chemical limitations leading to soil degradation, which requires special management strategies to address different crop requirements (Osman, Citation2013).
Soil degradation is a decrease in the ability to provide ecosystem functions that are useful to humans and nature, such as agriculture, construction, recreation, and transport (Jie et al., Citation2002). It is one of the main factors for low agricultural productivity in sub-Saharan Africa (SSA), which affects the environment and people’s livelihoods (Obalum et al., Citation2012). About 65% of agricultural land in Africa is degraded, reducing annual agricultural GDP by about 3% and making land productivity the lowest compared to other regions worldwide (Agegnehu et al., Citation2021).
Furthermore, the effects of soil and land degradation caused by salinity can encompass a variety of negative impacts on ecosystems, agriculture, and the broader environment (Stavi et al., Citation2021). Raised soil salinity levels can hinder the absorption of vital plant nutrients. It is widely documented that salinity significantly affects the availability and uptake of essential nutrients by plants (Ehtaiwesh, Citation2022). Excessive salt in the soil can disturb the accessibility of crucial elements, such as nitrogen, phosphorus, potassium, iron, and zinc, all of which are essential for the growth and development of plants (Shrivastava & Kumar, Citation2015).
Additionally, soil salinity disturbs the equilibrium of ions in the soil, thereby impacting the cation exchange capacity of the soil (Hussain et al., Citation2019). This disruption can diminish the soil’s capacity to both retain and release crucial nutrients needed for plant growth. Apart from diminishing nutrient availability, salt can also give rise to nutrient toxicity. An excess accumulation of salts in the soil can create imbalances in nutrients, resulting in toxic levels of specific ions that can be detrimental to plants, hindering their growth (Fageria et al., Citation2011). Soils depleted of nutrients due to salinity frequently lead to diminished crop yields, a formidable challenge in Africa, where agriculture serves as a primary source of livelihood for numerous individuals.
3. Development of rice varieties for salt affected soils
3.1. Salinity tolerance screening methods
Breeding rice for salinity tolerance needs reliable evaluation methods which can accommodate large number of genotypes produced (Gregorio et al., Citation1997). Salinity tolerance screening techniques plays a vital role in identifying and selecting salt-tolerant rice genotypes for use in breeding programs. The most commonly used methods to evaluate for salinity tolerance in rice are hydroponic screening, pot and field screenings. Due to lack of uniform growth conditions, consistency and reproducibility of results amongst laboratories globally, it remain a challenge despite the available hydroponic screening protocol (Gregorio et al., Citation1997) for salinity stress tolerance evaluation. Screening for salt tolerance requires the use of several approaches at different growth stages. Rad et al. (Citation2012) reported the potential salt concentration for screening rice genotypes for salinity tolerance, with salinity level of up to 6 dSm−1, reduced potential rice productivity by 50% and salinity level of 12 dSm−1 or higher induces crop failure. The normal soil electrical conductivity suitable for rice is below 4 dSm−1 (FAO, Citation2005).
3.1.1. Hydroponic screening
Hydroponic screening is one of the most extensively used technique to evaluate salinity tolerance in rice especially at seedling stage (Ali et al., Citation2014). Through this technique, rice seedlings are raised in hydroponic solution such as IRRI’s hydroponic screening protocol (Gregorio et al., Citation1997) with varying levels of salt concentration. Seedling growth and physiological responses are then monitored and measured to assess their level of salt tolerance. Growth parameters, such a biomass accumulation, shoot and root length, leaf chlorophyll content, and physiological responses including ion accumulation and antioxidant enzyme activity, are measured (Zeng & Shannon, Citation2005; Baker, Citation2008; Kakar et al., Citation2019). This method allows for precise control of salt levels and is widely used for initial screening of large numbers of rice genotypes (Munns & Tester, Citation2008).
3.1.2. Pot screening
Pot screening for salinity tolerance in rice involves growing rice plants in pots filled with saline soil or soil mixed with varying salt concentrations. This technique helps researchers to simulate real field conditions to assess salinity tolerance at various growth stages, from seedling to maturity (Kakar et al., Citation2019). Plant growth parameters such as tiller number, grain yield, plant height, leaf senescence, shoot and root biomass, and grain quality are assessed. Also, this method provides a comprehensive assessment of salinity tolerance in rice, bearing in mind the long-term effects of salt stress on growth and yield (Issue et al., Citation2006; Ismail & Horie, Citation2017).
3.1.3. Field screening
Field screening technique involves growing rice genotypes in salt-affected fields or areas with high salt content. The technique allows evaluation for agronomic traits, yield components, grain quality, and overall performance under natural salt stress conditions. This method provides more realistic results than that under-laboratory conditions, as plants under field conditions experiences not only the effect of soil salinity but also, soil heterogeneity and the effect of other biotic and abiotic factors (Krishnamurthy et al., Citation2016). Under field condition genotypes’ response would be due to the effect of mixture of salts and the biotic and abiotic factors prevailing in the soil that contributes to biochemistry and physiology of the plant (Gupta & Shaw, Citation2021). This method, helps to identify rice cultivars that exhibit desirable level of tolerance and high productivity in saline environments. Thus, this method is very crucial for validating results obtained from other screening methods such as hydroponic and pot screening taken under controlled environments (Zeng et al., Citation2001).
3.2. Mechanisms employed by rice plants to cope with salinity stress
Salinity induces stress in rice through three main pathways: Osmotic stress, ionic stress, and oxidative stress (İbrahimova et al., Citation2021). In order to address the salinity problem for rice production, the understanding of how rice genotypes adapt to excess salt is important for developing strategies to address rice production problems for salt prone areas (Tabassum et al., Citation2021). Salt affected soils affect plants in different ways such as through osmotic stress, ion imbalance, and oxidative damage (Hussain et al., Citation2017). Under salinity stress, rice plants respond by adjusting various morpho-physiological, biochemical, anatomical, and molecular activities (Arif et al., Citation2020). Also, plant response to salt stress varies between rice genotypes and between growth stages (Stavridou et al., Citation2017). The highest sensitivity of rice to salinity has been reported at early seedling and at reproductive growth stage, while germination, tillering, and maturity growth stages are considered relatively tolerant stages (Singh et al., Citation2021). Salt stress has been reported to cause a significant decline in plant biomass, for example, (46%) reduction in biomass was observed in susceptible rice genotypes, and less biomass reduction (18%) was observed in salt tolerant genotypes such as IR632 and IR651 when evaluated at 12 dSm−1 salt (NaCl) concentration (Moradi & Ismail, Citation2007).
Understanding the physiological mechanisms, set of genes, and gene products that are involved in stress tolerance could help breeders to develop salt-tolerant rice varieties (Gupta & Huang, Citation2014). It is known that plants growing in saline soils develops coping mechanism such as osmotic pressure adjustment, ion homeostasis, reactive oxygen species detoxification, and hormonal regulation (Zhao et al., Citation2020). Normally, plants respond to the negative effects caused by salinity through triggering the biochemical mechanisms such as osmolytes accumulation and synthesis, reactive oxygen species scavenging and intercellular ion homeostasis maintenance (Liu et al., Citation2023) ().
Figure 1. The common mechanisms involved in rice salt tolerance stress response (Liu et al., Citation2023).
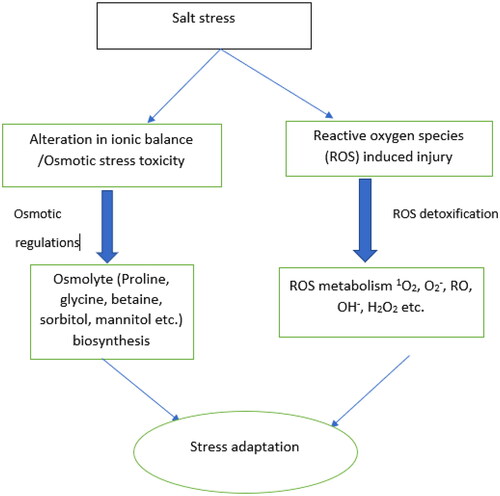
3.2.1. Osmotic pressure adjustment
In plants, osmotic regulation involves the opening of stomata which is triggered by the movement of osmolytes. Salinity stress causes plants to lower the water potential in order to facilitate water uptake by the roots (Munns, Citation2002). When exposed to high levels of salt, plants experience water deficit due to the reduced water in the soil, making it difficult for plant roots to absorb water from the rhizosphere. Additionally, when the extracellular water potential is significantly decreased, causes intracellular water discharge, causing cellular water loss (Garg et al., Citation2002). In response, plants activate osmotic adjustment mechanisms to maintain cellular water balance. Osmotic adjustment involves absorption and accumulation of compatible solutes, such as proline, glycine-betaine, and sugars, which help maintain cell turgor pressure and prevent water loss (Sharma et al., Citation2019).
Osmolytes are small organic molecules, water-soluble organic molecules that differ from inorganic ions in that they are not harmful to cells and do not interfere with cellular metabolism (Slama et al., Citation2015). They play a crucial role in maintaining cellular homeostasis and are important for the survival of cells in harsh environments (Fulda et al.,Citation2010). Proline and glycine betaine are particularly important osmolytes (Tang et al., Citation2014). Proline possesses antioxidant capabilities and, when the cell is dehydrated, functions as a chaperone, protecting the structure of macromolecules from damage (Liang et al., Citation2013). On the other hand, glycine betaine, is an organic quaternary ammonium compound, which is normally found in chloroplasts and serves as a regulator for the maintenance of the thylakoid membrane (İbrahimova et al., Citation2021).
Thus, when exposed to salt stress, plants can alter their osmotic pressure by absorbing inorganic ions from the rhizosphere, such as K+ and Ca2+ and other organic molecules that can effectively adjust plants osmotic pressure (Sripinyowanich et al., Citation2013). Plant roots are frequently associated with salt tolerant in plants because they are the primary organ that regulate the intake and transport of nutrients and salts throughout the plant, they first sense the osmotic stress (Munns, Citation2002).
3.2.2. Ion homeostasis and compartmentation
Salinity stress disrupts the balance of ions in plant tissues, leading to an excessive accumulation of toxic ions, such as sodium (Na+) and chloride (Cl-) (Zhao et al., Citation2021). To counteract this, plants employ several strategies. First, they actively exclude salt from entering the roots through selective ion uptake and transport mechanisms. Second, plants compartmentalize toxic ions in vacuoles or cell walls to prevent their harmful effects on cellular processes. Lastly, plants enhance the uptake and accumulation of essential nutrients, such as potassium (K+), calcium (Ca2+), and magnesium (Mg2+), which helps maintain ion homeostasis (Akter & Oue, Citation2018).
There are some reported effects of ion imbalance in plants, such as the observed higher spikelet sterility leading to poor seed set and lower grain yields in sensitive genotypes due to significantly higher uptake of Na+ ions by anthers of sensitive compared to tolerant genotypes (Singh et al., Citation2021). Sarhadi et al. (Citation2012) reported that in sensitive rice genotype IR64, Na+ ions concentration recorded was 21 mmol/g dry weight (dwt) in the anthers, compared with the more tolerant Cheriviruppu where Na+ ions were 0.35 mmol/g dwt. The Na+/K+ ratio in the anthers of IR64 under salt stress was more than 1.7 times higher than in plants grown under normal conditions. While, in the tolerant genotypes, Cheriviruppu, no changes was observed for the Na+/K+ ratio, signifying that the observed change in K+ ion concentration in the anthers of either IR64 or Cheriviruppu under stress and the increased Na+/K+ ratio could mainly be attributed to increased uptake of Na+ ions by IR64 under stress (Sarhadi et al., Citation2012).
Furthermore, high Na+ ion concentration has been reported to cause reduction in pollen fertility, an important parameter for salinity tolerance at the reproductive stage, which is a direct determinant of crop yield (Singh et al., Citation2021). Salt stress in soils causes plants to experience not only increased Na+ absorption but, also a reduction in K+ absorption (Zhu, Citation2003). Potassium (K+) ions are very crucial for plant development, by increasing K+ content, plants can decrease the concentration of Na+ ions to a certain level, thus decreasing the Na+/K+ ratio resulting to enhance plant growth (Zhang et al., Citation2018).
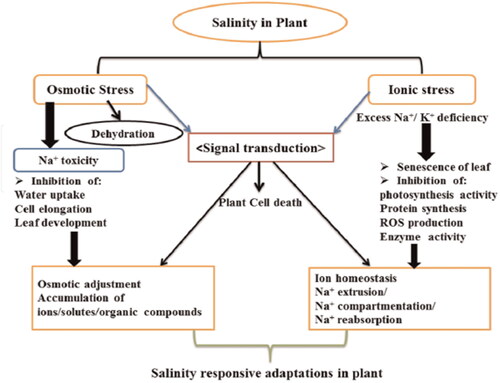
Generally, salinity appears to influence plant growth through two main pathways: water and ionic relations. Initially, plants experience water stress, leading to a reduction in leaf expansion. Then, prolonged exposure to salt stress in both soil and the plant itself results in ionic stress, particularly involving sodium (Na+) and chloride (Cl-), actually triggering premature senescence of older leaves in plants (Hussain et al., Citation2019). Plants have evolved a number of defense mechanisms against the impacts of salt stress, such as regulating ion homeostasis, ion export and compartmentalization, and osmoprotectant production (Balasubramaniam et al., Citation2023). () summarizes the effect of osmotic and ionic stress and plants adaptation mechanisms.
Figure 2. Salinity response adaptations in plants, extracted from Hussain et al. (Citation2019).
3.2.3. Reactive oxygen species (ROS) detoxification
Salinity stress often leads to increased production of reactive oxygen species (ROS), which are versatile, highly reactive molecules that plays a key role in toxic compound and signal transduction that causes environmental stress and programmed cell death in crop plants (Miller et al., Citation2008, Citation2010). To maintain optimal function, plants need a threshold level of reactive oxygen species (ROS), any deviation from this balance can damage plant physiology and lead to oxidative stress (Arif et al., Citation2020). Higher levels of salts in the growth media interrupts the antioxidant defense system through excessive production of reactive oxygen species (ROS) such as superoxide radical (O2-), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•), which subsequently results in oxidative stress (Hasanuzzaman et al., Citation2013; Mishra et al., Citation2013).
In order to prevent oxidative damage, due to salinity stress, plants trigger antioxidant protection mechanisms. This is done by enhancing the production and accumulation of antioxidant enzymes such as superoxide dismutase, catalase, peroxidase and ascorbate peroxidase, and non-enzymatic antioxidant such as glutathione, ascorbate and its derivatives and photosynthetic accessory pigments like carotenoids (Mbarki et al., Citation2018).
For instance, salt tolerant rice cultivar such as Pokkali have been reported to have higher activity of reactive oxygen species scavenging enzymes such as catalase and enhanced levels of antioxidants including ascorbate and glutathione, compared to the salt sensitive rice cultivar like Pusa Basmati during salinity stress (Hoang et al., Citation2016). Also, Dionisio-Sese and Tobita (Citation1998), indicated that salt tolerant rice cultivars showed protection mechanisms against enhanced radical production under saline stress condition by stimulating and sustaining the specific activity of antioxidant enzymes.
3.2.4. Hormonal regulation
Plant hormones, or pytohormones, are small chemicals that plays a crucial role in plant growth and development. Naturally occurring plant hormone such as, ethylene, abscisic acid (ABA), jasmonic acid (JA) and salicylic acid (SA) are associated with stress response in plants, while auxin, gibberellin (GA), cytokinins (CKs), brassinosteroids (BRs), and strigolactones (SLs) are considered as growth promotion hormones (Verma et al., Citation2016). For example, ABA regulates stomatal closure, reducing water loss, and promotes osmoprotectant synthesis, thus helping plants adapt to saline environments (Ma et al., Citation2020).
Moreover, other hormones, such as ethylene, jasmonic acid, and salicylic acid, indole acetic acid (IAA) and gibberellins (GA) contributes to stress responses by modulating various physiological processes (Rhaman et al., Citation2020). However, many studies have shown that each plant hormone does not play a single biological role in plants, nevertheless, plays sophisticated and efficient roles at different stages, in different tissues, or under different environmental conditions (Ku et al., Citation2018; Cortleven et al., Citation2019; Yu et al., Citation2019).
Plant hormones for a long time have been considered as essential endogenous molecules involved in regulating plant development and tolerance or susceptibility of diverse stresses including salinity stress. The different regulation of endogenous plant hormone levels during osmotic shock or salt stress have been observed and reported that they are highly correlated with salt stress tolerant (Ryu & Cho, Citation2015). Collective evidence indicates that phytohormones, besides controlling plant growth and development under normal conditions, also mediate various environmental stresses, including salt stress, helping to regulate plant growth adaptation (Yu et al., Citation2020).
4. Approaches for developing stress tolerance rice cultivars
The most effective way to utilize salt-affected land is through breeding salt-tolerant rice cultivars, which is the most economically feasible and environmentally friendly strategy (Qin et al., Citation2020). Sing et al. (Citation2021) reported that rice shows a strong relationship between salt tolerance at the reproductive stage and grain yield, tolerant genotypes maintained a higher number of fertile florets that contributes to seed set and, thus grain yields. Therefore, it has been established that in salt-affected soils, using genetic resources for developing salt-affected tolerant genotypes is seen as an important technique to increase productivity and enhance agricultural sustainability.
It is therefore envisioned that breeding for salt-tolerant crops that can adapt to saline conditions remains a realistic possibility with strong track record, is easy to use, affordable, and reduces environmental degradation (Jaiswal et al., Citation2019). Presently, salt-tolerant rice varieties have been developed in different parts of the world through conventional and molecular breeding approaches. They have increased the productivity of salt-affected soils for resource-poor farmers. The developed salt-tolerant rice varieties have been found to have yield advantages in salt-stressed environments. There has been a reported yield advantage of salt-tolerant rice varieties, which yields up to 4.0 t/ha in salt-stress situations (Krishnamurthy et al., Citation2022).
4.1. Conventional breeding approaches
Conventional breeding methods have been used in developing salt-tolerant rice varieties for many years. Some strategies include hybridization and progeny screening under stress conditions followed by selection (Singh et al., Citation2021). Conventional breeding methods combined with molecular and genomic methods have largely supported the improvement of rice for salt stress tolerance. Plant breeders have developed many crop varieties through selection of parental lines followed by controlled crossing.
The use of traditional breeding approaches such as hybridization, backcrossing, pedigree selection, and recurrent selection, together with the induced mutation, have been useful in developing stress-tolerant rice varieties (Haque et al., Citation2021). Conventional breeding methods have been extensively used by breeders worldwide to develop salt-tolerant rice varieties (Kurokawa et al., Citation2016). For example, since 1970, the International Rice Research Institute (IRRI) has been able to develop many salt-tolerant rice varieties using traditional breeding methods which are available for use in many countries (Haque et al., Citation2021). Furthermore, plant breeders are continuously working on developing stress-tolerant rice varieties adapted to salt-prone environments through traditional breeding approaches (Singh et al., Citation2015). For a long time, traditional rice varieties and local landraces have been used as donors for resistance or tolerance genes for the development of salt-tolerant genotypes and other important abiotic stresses. shows a list of some identified salt-tolerant rice genotypes developed in different African countries.
Table 4. Rice genotypes tolerant to salinity identified in different Africa countries.
4.2. Molecular approaches
Molecular approaches have also played a crucial role in breeding rice for salinity tolerance. Here are some key molecular techniques and strategies used in this process.
4.2.1. Marker-assisted selection (MAS)
Advances in molecular biology have led to the development of molecular markers that can find DNA polymorphisms in plant genomes (Gupta & Varshney, Citation2000). Molecular markers are specific deoxyribonucleic acid (DNA) fragments that can be studied by comparing polymorphism in the studied organism’s nucleotide sequence (Hassani et al., Citation2021). Researchers use genetic markers to understand heredity and genomic variations (Kordrostami & Rahimi, Citation2015). DNA markers are widely regarded as a beneficial tool for breeding crops like rice, wheat, forage species and many other plants (Kordrostami & Rahimi, Citation2015).
Some of the most used molecular markers for crop improvement are simple sequence repeats or microsatellite (SSR) and single nucleotide polymorphism (SNPs) markers (Nadeem et al., Citation2018). Molecular markers allow greater selection accuracy with less labour and time input while being able to help in combining different traits controlled by many genes into one cultivar. Microsatellite markers, for example, have been very useful for mapping quantitative trait loci (QTLs) for salt tolerance in rice (Sing et al., Citation2007). High throughput sequencing techniques on the other hand have advanced significantly and led to the detection of many SNPs used in the study of omics of rice (Kim et al., Citation2022). Single nucleotide polymorphisms are the most efficient and insightful genetic marker for revealing functional variations underlining important traits for crop improvement (Kim et al., Citation2022).
Marker-assisted backcrossing (MABC) is one of the most promising methods for applying molecular markers in plant breeding to determine and select genes controlling traits of interest, such as those for biotic and abiotic stress tolerance (Gouda et al., Citation2021). MABC involves using molecular markers to select target loci by reducing the size of the donor parent genome and enhancing the recovery of the recurrent parent genome (Hospital, Citation2001; Gouda et al., Citation2021). MABC can efficiently complement the classic breeding efforts in developing stress-tolerant genotypes adapted to climate change (Alpuerto et al., Citation2009).
There are numerous examples where Saltol quantitative trait locus (QTL) has been exactly introgressed into elite rice varieties through a marker-assisted breeding approach to offer a salt tolerance (Krishnamurthy et al., Citation2020). FL478 has been used as a donor of Saltol QTL because it is photoperiod insensitive, short time flowering compared to Pokkali, and offers a seedling stage salinity tolerance (Krishnamurthy et al., Citation2020; Waziri et al., Citation2016). Examples of recipient rice varieties that have been introgressed with Saltol QTL using SSR markers are PB112, PB6, AS996, BT7, Bacthom 7, Q5DB, BRRI-dhan 49 and Novator (Krishnamurthy et al., Citation2020; Waziri et al., Citation2016).
4.2.2. Quantitative trait loci (QTL) mapping
QTL mapping studies aim to find molecular markers which are naturally associated with genes controlling quantitative traits (Agarwal et al., Citation2008). There are generally two ways to dissect QTLs in plants; 1) QTL mapping/linkage analysis; 2) association or linkage disequilibrium mapping. QTL mapping involves the development of segregating mapping population, the construction of a genetic map using suitable markers, and the phenotyping of progenies to determine the segregation of the traits of interest and selection of polymorphic markers based on mapping population (Xu et al., Citation2017). Association mapping or linkage disequilibrium mapping is a method of mapping quantitative trait loci (QTL) that depends on linkage disequilibrium (LD) to determine the relationship between genotypic and phenotypic variation in natural population or germplasm collections (Breseghello & Sorrells, Citation2006). The QTL detection uses both the genotypic data and the phonotypic information (Collard et al., Citation2005).
There are few studies conducted in Africa related to QTL mapping for salinity tolerance in rice; for example, thirteen QTLs related to shooting length, shoot dry weight, root length, and salt injury score were mapped on chromosomes 2,3,4,7,10, and 12 of the salt-tolerant parents Madina Koyo (Amoah et al., Citation2020). The reported QTLs are independent of the known major QTL (Saltol) for salinity tolerance in rice. Identifying salinity stress tolerance QTLs on different chromosomes suggests that more other QTLs also control salinity tolerance in rice (Nakhla et al., Citation2021). This information helps breeders target and introgress the desirable traits into new rice varieties.
Further studies by Bimpong et al. (Citation2015) identified numerous QTLs for salinity tolerance in rice using F2 population under field condition in west Africa (Senegal). Mapping population was developed using three African cultivars (‘NERICA-L-19’, ‘Sahel 108’, and ‘BG90-2’) as recipient parents and the salt-tolerant landrace ‘Hasawi’ as the donor parent. Salinity tolerance phenotyping was done under saline field condition, grain yield and other traits were assessed on F2 and F2:3 population. 75 QTLs for various traits were discovered through QTL mapping (Bimpong et al., Citation2015). The study revealed some important QTLs related to yield and some QTLs with large effect were shown to be strongly related with salinity tolerance representing potential candidates for MAS.
There are some important QTLs for salinity tolerance in rice, which have been mapped using stable rice populations. For instance, Saltol, a major quantitative trait locus (QTL) responsible for salinity tolerance at seedling stage, has been mapped on chromosome 1 of rice genome using recombinant inbred lines (RILs) population (Gregorio, Citation1997). The SKC1, located within a Saltol region, is another important QTL, and it has shown favourable results in improving salinity tolerance in rice (Songtoasesakul et al., Citation2023).
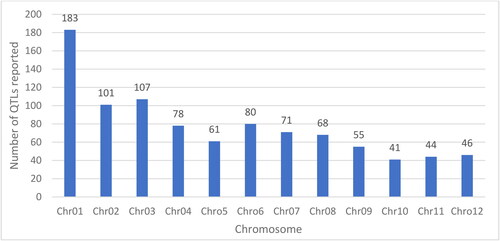
Singh et al. (Citation2021) summarized QTLs reported from various studies, about 935 QTLs from 46 different studies conducted in different countries around the world. The reported QTLs are associated with tolerant at seedling and reproductive growth stages. All the QTLs are summarized in () whereby the studies were conducted in biparental mapping populations. These QTLs can further be studied or validated in saline environments of Africa in order to find the most important QTLs for marker assisted selection.
Figure 3. Chromosome wise reported quantitative trait loci (QTL) for salt tolerance (Singh et al., Citation2021).
4.2.3. Genetic transformation techniques
Genetic transformation techniques, such as gene editing or gene transfer allows genes linked to stress tolerance to be directly introduced or modified for enhanced stress adaptation. Gene transfer or editing enables the development of rice varieties with enhanced salt tolerance by improving their adaptation under saline conditions. Rice crop genome has recently been effectively edited using CRISPR-directed evolution, CRISPR-Cas12a, and base editors, among other methods (Zafar et al., Citation2020).
These techniques have increased the potential for crop improvement. shows some of the improved traits in rice through genome editing methods. To increase rice’s resistance to salt, OsRR22 was targeted using CRISPR-Cas9, and the resulting mutant lines were examined for agronomic traits and seedling stage salinity tolerance. They performed better than even the wild types (Zafar et al., Citation2020).
Table 5. The CRISPR/Cas9 system for enhancing salinity tolerance in rice.
Furthermore, genetic engineering techniques which permit the introduction of the desired gene into elite cultivars without losing other desired traits have been used to develop rice genotypes tolerant to salt stress. The development of an efficient agrobacterium-mediated transformation method has substantially contributed to rice’s genetic advancement. Salinity tolerance in rice has been achieved through genetic modification focusing on genes encoding heat-shock and abundant proteins in late embryogenesis, transcription and signal transduction factors, programmed cell death, ROS (antioxidants) detoxification, compatible organic solutes, and ion transport (Liao et al., Citation2016).
Additionally, genetic engineering has been applied to generate rice with salinity tolerance using the known strategies employed by rice and plant breeders to cope with salt stress. shows rice cultivars that have been improved through genetic engineering. For rice to be tolerant to salinity, salt-responsive genes such as phosphatase 1a (OsPP1a) must be expressed. When exposed to salinity, the buildup of Na+/H+ in both shoots and roots makes transgenic rice salt tolerant. One of the potential gene families in rice that exhibits a variety of stress reactions under abiotic stress circumstances is sodium proton antiporter. OsNHX1 genes, vascular Na+/H+ antiporter genes derived from landrace Pokkali, its overexpression in rice improves the ability to tolerate salt for transgenic rice (Chen et al., Citation2007; Haque et al., Citation2021).
Table 6. Improving salinity stress tolerance in rice using a genetic engineering approach.
5. Challenges, conclusions and future projections
5.1. Challenges in breeding rice for salt affected soils in Africa
Rice has a narrow genetic base, which hinders the effectiveness of breeding rice for tolerant to salinity and other stresses (Rasheed et al., Citation2022). The limited genetic diversity of local rice varieties could be one of the problems affecting many breeding programs involved in developing salt-tolerant rice varieties. Also, the lack of diverse genetic resources or continuous supply of genetic resources for salinity-tolerant genes or gene-complexes has limited progress in selecting and breeding rice for salt-tolerance. Although, some efforts have been made to explore and utilize the genetic diversity of rice for salinity tolerance through collecting and preserving diverse rice germplasm from different regions and ecotypes within Africa and beyond. Still, there is little progress in developing tolerant rice genotypes due few parent materials available, which restricts the development of salt-tolerant rice cultivars (Xie et al., Citation2021).
Salinity tolerance is a complex trait controlled by many genes; it is difficult to improve through conventional breeding process. It is influenced by several genes and is one of the most complex physiological traits (Ismail & Horie, Citation2017). Evaluation of salt tolerance in rice is equally complex and traditionally, phenotypic traits have been used to evaluate for salt tolerance in rice (Jaiswal et al., Citation2019). However, this approach lacks completeness and accuracy and can lead to an evaluation of salt tolerance which is not in line with actual field performance (Qin et al., Citation2020). While many genes associated with salt tolerance in rice have been identified using different mapping populations, but only few important salt tolerance QTLs have been isolated. Nevertheless, the identified genes have limited applicability in breeding programs due to inconsistency caused by variability in genetic backgrounds and environments (Qin et al., Citation2020).
Moreover, there is a problem with the adaptation of new tolerant rice cultivars to local conditions. Rice varieties developed for salinity tolerance in one region does not perform optimally in different agroecological zones within Africa. Breeding programs need to consider the specific environmental conditions and adaptability of the developed varieties to ensure their success in different regions. Also, farmer acceptance and adoption for developed salt-tolerant rice varieties, has been a challenge. Farmers need to be exposed to the agronomic practices associated with the salt tolerant rice varieties to encourage their adoption for integration into existing farming systems. Variety development need as well to align to product market and requirement in order to attract farmers adoption.
There is also, a problem with limited resources and infrastructure for screening rice for salinity tolerance as well as lack of high-throughput genotyping platforms to speed up gene identification, and gene mapping process. Low funding for research activities, inadequate screening facilities, and skilled personnel have been an obstacle to developing salinity tolerant rice cultivars. Limited resources and infrastructure are among the hindering factors to breeding efforts for salinity tolerance cultivars in the region. Also, effective phenotyping method is one of the limiting factors, hence there is a need to improve the phenotyping methods to effectively evaluate the performance of rice plants under saline conditions.
5.2. Conclusions and future projections
In most of the sub-Saharan African countries, land and soil degradation undermines efforts toward sustainable agricultural production and poses a major threat to the future of agriculture. Soil salinity which is among the causes of agricultural land degradation in Africa, is one of the most severe and common abiotic stress that affects rice productivity. There are several approaches for the management of salt-affected soils. However, one of the best approaches to overcoming salt stress problems in Africa could be through breeding crops with stress-tolerant genes so that they can be grown in salt-prone areas. Salt tolerance rice genotypes have been identified using conventional breeding and modern biotechnology approaches. Furthermore, quantitative trait loci (QTLs) associated with salt stress tolerance have been mapped on different regions of the rice genome. The identified genes and genotypes are the potential donors in the development of new tolerant rice cultivars adapted to local environments.
Conventional breeding has been a successful method; however, the approach has been time-consuming as it requires many years to develop a new variety. Biotechnology approaches are more advantageous, offer an improved alternative for transferring salt tolerance genes (QTLs), and provide a rapid way to develop new tolerant rice cultivars that could help reduce the consequences caused by soil salinity effects in Africa. By using the reviewed modern breeding methods, development of salt tolerant rice varieties is guaranteed and that ensures increased and sustainable agricultural productivity.
To address the problems, highlighted by the study, the following can be taken into account for future study on salinity stress tolerance. Plant stress tolerance is so complex, it is necessary to combine many approaches, including physiological, biochemical, soil, agronomical, and molecular methods, in order to attain salt stress tolerance.
Therefore, it is very crucial to expand the genetic diversity of rice genotypes used in breeding programs. This could involve exploring and incorporating genes from wild rice relatives or other salt-tolerant rice genotypes from different regions. Through broadening the genetic diversity, breeders can access a wider range of traits and potential parental lines that serve as sources of salinity tolerance. It is projected for enhanced collaboration between national research institutes and the international research institutes such as the Africa Rice Center (AfricaRice), and International Rice Research Institute (IRRI) for germplasm exchange, knowledge sharing, and conduct joint research work on improving salinity tolerance in rice. The efforts could lead to development of some new rice cultivars with enhanced salinity tolerance, and well adapted to the diverse agroecological conditions across Africa.
Also, breeding programs in Africa could deploy already proven technologies most of which have been discussed in this review such as use of molecular markers and genomic selection to help in accelerating the breeding process. This could help the breeding programs to identify specific genes associated with salinity tolerance and individual plants possessing the tolerant genes for use in their local conditions. MAS allows breeders to select plants with desired traits more efficiently, reducing the time and resources required for traditional breeding methods (Collard & Mackill, Citation2008). Another related technology is the genomic selection which involves using genomic data to predict the performance of rice plants. By analyzing the entire genome, breeders can identify markers associated with salinity tolerance and select plants with the highest potential for success (Nongpiur et al., Citation2016). This approach can accelerate the breeding process and improve the accuracy of trait selection.
Moreover, considering the impact of climate change is essential, and breeding programs should focus on developing rice varieties that not only tolerate current salinity levels but also have the potential to adapt to future changes in climate and salinity conditions. This can involve incorporating traits related to drought tolerance, heat tolerance, and resilience to other environmental stresses. To maximize the productivity of crop plants under adverse conditions, there is an urgent need to look for sources of diverse genetic variation that can be used for developing new cultivars with greater yield potential and stability over seasons. Salinity typically coexists with other environmental stresses, and it is hard to predict what will occur in the future, therefore, developing climate-resilient varieties that can withstand different abiotic stress such as salinity, drought and many others will provide a suitable solution (Rivero et al., Citation2022).
Thus, this study provides an overview of the degree of salinity problems in some of the African countries, the screening methods, mechanisms associated with stress responses, and the available rice genotypes that can withstand salt stress. In an effort to better present the current state and help future scientists better understand the challenges and focus their efforts toward developing salt-tolerant rice varieties, we have attempted to provide an overview of the various approaches, that can be employed in order to attain salt stress tolerance in rice.
Acknowledgement
The support from climate-smart African rice research project for financial support through DANIDA is highly appreciated. Tanzania Agricultural Research Institute (TARI) is recognized for granting study leave to the first author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Nafeti Titus Mheni
Nafeti Titus Mheni, is a research Scientist at Tanzania Agricultural Research Institute (TARI), under the Ministry of Agriculture in Tanzania. Currently, he is a Ph.D. student at Sokoine University of Agriculture (SUA) in the department of Crop Science and Horticulture. His research work is on “Quantitative Trait Loci Mapping (QTL) for salinity tolerance of rice”. This review paper is aimed to provide information on the problems of soil salinity in sub Saharan Africa and the available challenges which limits research on breeding rice for salt tolerance in the region. Research areas of interest includes crop improvement for abiotic and biotic stress through innovative integration of basic and applied research. He has a great interest on quantitative genetics, molecular plant breeding, genomic selection and quantitative trait loci mapping. With the current changes in climatic conditions, this paper aims to provide information regarding the extent of soil salinity problem in African countries and the available management options. The information also will be valuable to rice breeders across the continent, as some of the suggested breeding materials, techniques and the already identified genes for salinity tolerant can be used to speed up the development of tolerant rice cultivars adapted to African rice growing conditions.
References
- Acosta-Motos, J., Ortuño, M., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M., & Hernandez, J. (2017). Plant responses to salt stress: Adaptive mechanisms. Agronomy, 7(1), 1. https://doi.org/10.3390/agronomy7010018
- Adhanom, O. M. (2019). Salinity and sodicity hazard characterization in major irrigated areas and irrigation water sources, Northern Ethiopia. Cogent Food and Agriculture, 5(1), 1673110. https://doi.org/10.1080/23311932.2019.1673110
- Agarwal, M., Neeta, S., & Harish, P. (2008). Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Reports, 27(4), 617–19. https://doi.org/10.1007/s00299-008-0507-z
- Agegnehu, G., Amede, T., Erkossa, T., Yirga, C., Henry, C., Tyler, R., Nosworthy, M. G., Beyene, S., & Sileshi, G. W. (2021). Extent and management of acid soils for sustainable crop production system in the tropical agroecosystems: A review. Acta Agriculturae Scandinavica, Section B—Soil & Plant Science, 71(9), 852–869. https://doi.org/10.1080/09064710.2021.1954239
- Akter, M., & Oue, H. (2018). Effect of saline irrigation on accumulation of Na+, K+, Ca2+, and Mg2+ ions in rice plants. Agriculture (Switzerland,), 8(10), 164. https://doi.org/10.3390/agriculture8100164
- Ali, M. N., Yeasmin, L., Gantait, S., Goswami, R., & Chakraborty, S. (2014). Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiology and Molecular Biology of Plants: An International Journal of Functional Plant Biology, 20(4), 411–423. https://doi.org/10.1007/s12298-014-0250-6
- Alpuerto, V. L. E. B., Norton, G. W., Alwang, J., & Ismail, A. M. (2009). Economic impact analysis of marker-assisted breeding for tolerance to salinity and phosphorous deficiency in rice. Review of Agricultural Economics, 31(4), 779–792. http://www.jstor.org/stable/40588528 https://doi.org/10.1111/j.1467-9353.2009.01466.x
- Amoah, N. A., Richard, A., Alex, W., Baboucarr, M., Ibnou, D., & Isaac, K. (2020). Mapping QTLs for tolerance to salt stress at the early seedling stage in rice (Oryza Sativa L.) using a newly identified donor ‘Madina Koyo. Euphytica, 216(10), 1–23. https://doi.org/10.1007/s10681-020-02689-5
- Anwar, A., & Kim, J. (2020). Transgenic breeding approaches for improving abiotic stress tolerance: recent progress and future perspectives. International Journal of Molecular Sciences, 21(8), 2695. https://doi.org/10.3390/ijms21082695
- Anyomi, W. E., Ashalley, R., Amoah, N. A., Blay, E. T., & K, O. (2018). Hydroponic screening of rice seedlings for salinity tolerance. Researchjournali’s Journal of Agriculture, 5(6), 1–15.
- Arif, Y., Singh, P., Siddiqui, H., Bajguz, A., & Hayat, S. (2020). Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiology and Biochemistry: PPB, 156, 64–77. https://doi.org/10.1016/j.plaphy.2020.08.042
- Arouna, A., Devkota, K. P., Yergo, W. G., Saito, K., Frimpong, B. N., Adegbola, P. Y., Depieu, M. E., Kenyi, D. M., Ibro, G., Fall, A. A., & Usman, S. (2021). Assessing rice production sustainability performance indicators and their gaps in twelve sub-Saharan African countries. Field Crops Research, 271(3), 108263. https://doi.org/10.1016/j.fcr.2021.108263
- Asamoah, A., Antwi-Boaasiako, C., Frimpong-Mensah, K., & Soma Dohan, M. (2013). Adoptable technique(s) for managing Ghanaian saline soils. Octa Journal of Environmental Research, 1(1), 48–51.
- Baker, N. R. (2008). Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annual Review of Plant Biology, 59(1), 89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
- Balasubramaniam, T., Shen, G., Esmaeili, N., & Zhang, H. (2023). Plants’ response mechanisms to salinity stress. Plants (Basel, Switzerland), 12(12), 2253. https://doi.org/10.3390/plants12122253
- Bhowmik, S. K., Titov, S., Islam, M. M., Siddika, A., Sultana, S., & Haque, M. D. S. (2009). Phenotypic and genotypic screening of rice genotypes at seedling stage for salt tolerance. African Journal of Biotechnology, 8(23), 6490–6494. http://www.academicjournals.org/AJB
- Bimpong, I. K., Manneh, B., Sock, M., Diaw, F., Amoah, N. K. A., Ismail, A. M., Gregorio, G., Singh, R. K., & Wopereis, M. (2015). Improving salt tolerance of lowland rice cultivar ‘Rassi’ through marker-aided backcross breeding in West Africa. Plant Science: An International Journal of Experimental Plant Biology, 242, 288–299. https://doi.org/10.1016/j.plantsci.2015.09.020
- Bjornlund, V., Bjornlund, H., & Rooyen Van, A. F. (2020). Why agricultural production in sub-Saharan Africa remains low compared to the rest of the world – a historical perspective. International Journal of Water Resources Development, 36(sup1), S20–S53. https://doi.org/10.1080/07900627.2020.1739512
- Breseghello, F., & Sorrells, M. E. (2006). Association mapping of kernel size and milling quality in wheat (Triticum Aestivum L.) cultivars. Genetics, 172(2), 1165–1177. https://doi.org/10.1534/genetics.105.044586
- Calone, R., Sanoubar, R., Lambertini, C., Speranza, M., Vittori Antisari, L., Vianello, G., & Barbanti, L. (2020). Salt tolerance and Na allocation in sorghum bicolor under variable soil and water salinity. Plants (Basel, Switzerland), 9(5), 561. https://doi.org/10.3390/plants9050561
- Calvert, L., Sanint, L., Châtel, M., & Izquierdo, J. (2004). Rice production in Latin America at critical crossroads. Tropical Agriculture, 55, 65–73. https://hdl.handle.net/10568/65911.
- Chen, M., Chen, Q.-J., Niu, X.-G., Zhang, R., Lin, H.-Q., Xu, C.-Y., Wang, X.-C., Wang, G.-Y., & Chen, J. (2007). Expression of OsNHX1 gene in maize confers salt tolerance and promotes plant growth in the field. Plant, Soil and Environment, 53(11), 490–498. https://doi.org/10.17221/2302-PSE
- Collard, B. C. Y., Jahufer, M. Z. Z., Brouwer, J. B., & Pang, E. C. K. (2005). An introduction to markers, quantitative trait loci (QT L) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica, 142(1–2), 169–196. https://doi.org/10.1007/s10681-005-1681-5
- Collard, B. C. Y., & Mackill, D. J. (2008). Marker-assisted selection : An approach for precision plant breeding in the twenty-first century. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1491), 557–572. https://doi.org/10.1098/rstb.2007.2170
- Cortleven, A., Leuendorf, J. E., Frank, M., Pezzetta, D., Bolt, S., & Schmülling, T. (2019). Cytokinin action in response to abiotic and biotic stresses in plants. Plant, Cell & Environment, 42(3), 998–1018. https://doi.org/10.1111/pce.13494
- Daba, A. W., & Qureshi, A. S. (2021). Review of soil salinity and sodicity challenges to crop production in the lowland irrigated areas of Ethiopia and its management stategies. Land, 10(12), 1377. https://doi.org/10.3390/land10121377
- Dasgupta, S., Hossain, M., & Huq, M. (2018). Climate change, salinization and high-yield rice production in coastal Bangladesh. AMBIO: A Journal of the Human Environment, 1(April), 66–89. https://doi.org/10.1596/1813-9450-7140
- Diao, X., Hazell, P., & Thurlow, J. (2010). The role of agriculture in African development. World Development, 38(10), 1375–1383. https://doi.org/10.1016/j.worlddev.2009.06.011
- Dionisio-Sese, M. L., & Tobita, S. (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Science, 135(1), 1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
- Ehtaiwesh, A. (2022). The effect of salinity on nutrient availability and uptake in crop plants. Scientific Journal of Applied Sciences of Sabratha University, 55–73. https://doi.org/10.47891/sabujas.v0i0.55-73
- EL Sabagh, A., Çiğ, F., Seydoşoğlu, S., Leonardo, Battaglia, M. L., Javed, T., Iqbal, M. A., Mubeen, M., Ali, M., Ali, M., Bengisu, G., Konuşkan, Ö., Barutcular, C., Erman, M., Açikbaş, S., Hossain, A., Islam, M. S., Wasaya, A., Ratnasekera, D., Arif, M., & Ahmad, Z. (2021). Salinity stress in maize: Effects of stress and recent developments of tolerance for improvement. Cereal Grains, 1–20. https://doi.org/10.5772/intechopen.98745
- Fageria, N. K., Gheyi, H. R., & Moreira, A. (2011). Nutrient bioavailability in salt affected soils. Journal of Plant Nutrition, 34(7), 945–962. https://doi.org/10.1080/01904167.2011.555578
- FAO. (2000). Land resource potential and constraints at regional and country levels. World Soil Resources Reports (Vol. 9). FAO.
- FAO. (2005). Saline soils and their management. FAO.
- FAO. (2022). Halt soil salinization, boost soil productivity. Proceeding of the Global Symposium on Salt -Affected Soils, 20–22 October 2021, Rome. https://doi.org/10.4060/cb9565en
- Fulda, S., Gorman, A. M., Hori, O., & Samali, A. (2010). Cellular stress responses: cell survival and cell death. International Journal of Cell Biology, 2010, 214074. https://doi.org/10.1155/2010/214074
- Garg, A. K., Kim, J., Owens, T. G., Ranwala, A. P., Choi, Y. D., Kochian, L. V., & Wu, R. J. (2002). Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proceedings of the National Academy of Sciences of the United States of America, 99(25), 15898–15903. https://doi.org/10.1073/pnas.252637799
- Gomiero, T. (2016). Soil degradation, land scarcity and food security: reviewing a complex challenge. Sustainability, 8(3), 281. https://doi.org/10.3390/su80302811–41
- Gouda, A. C., Warburton, M. L., Djedatin, G. L., Kpeki, S. B., Wambugu, P. W., Gnikoua, K., & Ndjiondjop, M. N. (2021). Development and validation of diagnostic SNP markers for quality control genotyping in a collection of four rice (Oryza) species. Scientific Reports, 11(1), 18617. https://doi.org/10.1038/s41598-021-97689-3
- Grattan, S. R., Shannon, M. C., & Roberts, S. R. (2002, December). Field study with metallic rings. 189–195.
- Gregorio, G. B. (1997). No. 30 IRRI Discussion Paper Series. Development, 22(30).
- Gregorio, G. B., Senadhira, D., & Mendoza, R. D. (1997). Screening rice for salinity tolerance (IRRI Discussion Paper Series No. 22). lnternational Rice Research Institute.
- Gregorio, G. B., Islam, R., Vergara, G. V., & Thirumeni, S. (2013). Recent advances in rice science to design salinity and other abiotic stress tolerant rice varieties. SABRAO Journal of Breeding and Genetics, 45(1), 31–41.
- Gupta, B., & Huang, B. (2014). Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomics, 2014, 701596–701518. https://doi.org/10.1155/2014/701596
- Gupta, A., & Shaw, B. P. (2021). Field-and laboratory-based methods of screening salt tolerant genotypes in field- and laboratory-based methods of screening salt tolerant genotypes in rice. Crop and Pasture Science, 72(2), 85. https://doi.org/10.1071/CP20393
- Gupta, P. K., & Varshney, R. K. (2000). The development and use of microsatellite markers for genetic analysis and plant breeding with emphasis on bread wheat. Euphytica, 113(3), 163–185. https://doi.org/10.1023/A:1003910819967
- Haque, M. A., Rafii, M. Y., Yusoff, M. M., Ali, N. S., Yusuff, O., Datta, D. R., Anisuzzaman, M., & Ikbal, M. F. (2021). Advanced breeding strategies and future perspectives of salinity tolerance in rice. Agronomy, 11(8), 1631. https://doi.org/10.3390/agronomy11081631
- Hasanuzzaman, M., Nahar, K., & Fujita, M. (2013). Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In P. Ahmed, M. M. Azooz, & M. N. V. Prasad (Eds.), Ecophysiology and responses of plants under salt stress (pp. 25–87). Springer. https://doi.org/10.1007/978-1-4614-4747-4_2
- Hassani, A., Azapagic, A., & Shokri, N. (2021). Global predictions of primary soil salinization under changing climate in the 21st century. Nature Communications, 12(1), 6663. https://doi.org/10.1038/s41467-021-26907-3
- Hussain, S., Shaukat, M., Ashraf, M., Zhu, C., Jin, Q., & Zhang, J. (2019). Salinity stress in arid and semi-arid climates: Effects and management in field crops. Climate Change and Agriculture, 12, 123–145. https://doi.org/10.5772/intechopen.87982
- Hoang, T. M. L., Tran, T. N., Nguyen, T. K. T., Williams, B., Wurm, P., Bellairs, S., & Mundree, S. (2016). Improvement of salinity stress tolerance in rice: challenges and opportunities. Agronomy, 6(4), 54. https://doi.org/10.3390/agronomy6040054
- Hoshida, H., Tanaka, Y., Hibino, T., Hayashi, Y., Tanaka, A., Takabe, T., & Takabe, T. (2000). Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Molecular Biology, 43(1), 103–111. https://doi.org/10.1023/A:1006408712416
- Hospital, F. (2001). Size of donor chromosome segments around introgressed loci and reduction of linkage drag in marker-assisted backcross programs. Genetics, 158(3), 1363–1379. https://doi.org/10.1093/genetics/158.3.1363
- Hussain, S., Jun-Hua, Z., Chu, Z., Lian-Feng, Z., Xiao-Chuang, C., Sheng-Miao, Y., James, A. B., Ji-Jie, H., & Qian-Yu, J. (2017). Effects of salt stress on rice growth, development characteristics, and the regulating ways: a review. Journal of Integrative Agriculture, 16(11), 2357–2374. https://doi.org/10.1016/S2095-3119(16)61608-8
- Hussain, S., Shaukat, M., Ashraf, M., Zhu, C., Jin, Q., & Zhang, J. (2019). Salinity stress in arid and semi-arid climates: effects and management in field crops. Climate Change and Agriculture, 12, 123–145. https://doi.org/10.5772/intechopen.87982
- İbrahimova, U., Kumari, P., Yadav, S., Rastogi, A., Antala, M., Suleymanova, Z., Zivcak, M., Tahjib-Ul-Arif, M. D., Hussain, S., Abdelhamid, M., Hajihashemi, S., Yang, X., & Brestic, M. (2021). Progress in understanding salt stress response in plants using biotechnological tools. Journal of Biotechnology, 329, 180–191. https://doi.org/10.1016/j.jbiotec.2021.02.007
- Ismail, A. M., & Horie, T. (2017). Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annual Review of Plant Biology, 68(1), 405–434. https://doi.org/10.1146/annurev-arplant-042916-040936
- Ismail, A. M., & Thomson, M. J. (2011). Molecular breeding of rice for problem soils. In A. Costa de Oliveira & R. Varshney (Eds.), Root genomics (pp. 289–311). Springer.
- Issue, S. S., Munns, R., James, R. A., & Avenue, O. S. (2006). Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany, 57(5), 1025–1043. https://doi.org/10.1093/jxb/erj100
- Jaiswal, S., Gautam, R. K., Singh, R. K., Krishnamurthy, S. L., Ali, S., Sakthivel, K., Iquebal, M. A., Rai, A., & Kumar, D. (2019). Harmonizing technological advances in phenomics and genomics for enhanced salt tolerance in rice from a practical perspective. Rice (New York, NY), 12(1), 89. https://doi.org/10.1186/s12284-019-0347-1
- Jie, C., Jing-Zhang, C., Man-Zhi, T., & Zi-Tong, G. (2002). Soil degradation: A global problem endangering sustainable development. Journal of Geographical Sciences, 12(2), 243–252. https://doi.org/10.1007/BF02837480
- Kakar, N., Jumaa, S. H., Redoña, E. D., Warburton, M. L., & Reddy, K. R. (2019). Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice (New York, NY), 12(1), 57. https://doi.org/10.1186/s12284-019-0317-7
- Kargbo, S. S., Showemimo, F. A., Porbeni, J. B. O., & Akintokun, P. O. (2019). Response of rice genotypes to salinity under hydroponic conditions. Agro-Science, 18(3), 11. https://doi.org/10.4314/as.v18i3.3
- Kathuli, P., Itabari, J. K., Sijali, I. V., Gatuthu, J., & Kiaura, S. (2014). Diagnosis of sources of soil salinisation in selected irrigation schemes in semi-arid lands of Taita-Taveta County in Kenya. Joint proceedings of the 27th Soil Science Society of East Africa and the 6th African Soil Science Society, October 2013 (pp. 1–9).
- Kayode, O. T., Aizebeokhai, A. P., & Odukoya, A. M. (2021). Soil salinity and its implications on sustainable agriculture in southern and northcentral states of Nigeria soil salinity and its implications on sustainable agriculture in southern and northcentral states of Nigeria. IOP Conference Series: Earth and Environmental Science, 655(1), 012077. https://doi.org/10.1088/1755-1315/655/1/012077
- Kim, K. W., Bhagwat, N., Jungrye, N., S, H. C., Jungmin, H., & Yong, J. P. (2022). Development of an inclusive 580K SNP array and its application for genomic selection and genome-wide association studies in rice. Frontiers in Plant Science, 13, 1036177. https://doi.org/10.3389/fpls.2022.1036177
- Kordrostami, M., & Rahimi, M. (2015). Molecular markers in plants: concepts and applications. Syria Studies, 7(1), 37–72. https://www.jstor.org/stable/41857625
- Krishnamurthy, S. L., Gautam, R. K., Sharma, P. C., & Sharma, D. K. (2016). Effect of different salt stresses on agro-morphological traits and utilisation of salt stress indices for reproductive stage salt tolerance in rice. Field Crops Research, 190, 26–33. https://doi.org/10.1016/j.fcr.2016.02.018
- Krishnamurthy, S. L., Lokeshkumar, B. M., Rathor, S., Warraich, A. S., Yadav, S., Gautam, R. K., Sing, R. K., & Sharma, P. C. (2022). Development of salt-tolerant rice varieties to enhancing productivity in salt-affected environments. Environtal Science. Proceednins, 13, 30. https://doi.org/10.3390/environsciproc2022016030
- Krishnamurthy, S. L., Pundir, P., Warraich, A. S., Rathor, S., Lokeshkumar, B. M., Singh, N. K., & Sharma, P. C. (2020). Introgressed saltol QTL lines improves the salinity tolerance in rice at seedling stage. Frontiers in Plant Science, 11(June), 833. https://doi.org/10.3389/fpls.2020.00833
- Ku, Y., Sintaha, M., Cheung, M., & Lam, H. (2018). Plant hormone signaling crosstalks between biotic and abiotic stress responses. International Journal of Molecular Sciences, 19(10), 3206. https://doi.org/10.3390/ijms19103206
- Kumar, R., Dhansu, P., Kulshreshtha, N., Meena, M. R., Kumaraswamy, M. H., Appunu, C., Chhabra, M. L., & Pandey, S. K. (2023). Identification of salinity tolerant stable sugarcane cultivars using AMMI, GGE and some other stability parameters under multi environments of salinity stress. Sustainability, 15(2), 1119. https://doi.org/10.3390/su15021119
- Kumar, P., & Sharma, P. K. (2020). Soil salinity and food security in India. Frontiers in Sustainable Food Systems, 4, 533781. https://doi.org/10.3389/fsufs.2020.533781
- Kumar, V. V. S., Verma, R. K., Yadav, S. K., Yadav, P., Watts, A., Rao, M. V., & Chinnusamy, V. (2020). CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in Indica mega rice cultivar MTU1010. Physiology and Molecular Biology of Plants: An International Journal of Functional Plant Biology, 26(6), 1099–1110. https://doi.org/10.1007/s12298-020-00819-w
- Kurokawa, Y., Noda, T., Yamagata, Y., Angeles-Shim, R., Sunohara, H., Uehara, K., Furuta, T., Nagai, K., Jena, K. K., Yasui, H., Yoshimura, A., Ashikari, M., & Doi, K. (2016). Construction of a versatile SNP array for pyramiding useful genes of rice. Plant Science: An International Journal of Experimental Plant Biology, 242, 131–139. https://doi.org/10.1016/j.plantsci.2015.09.008
- Lal, R., & Stewart, B. (2019). Soil degradation and restoration in Africa (1st ed.). CRC Press.
- Liang, X., Zhang, L., Natarajan, S. K., & Becker, D. F. (2013). Proline mechanisms of stress survival. Antioxidants & Redox Signaling, 19(9), 998–1011. https://doi.org/10.1089/ars.2012.5074
- Liao, Y. D., Lin, K. H., Chen, C. C., & Chiang, C. M. (2016). Oryza Sativa protein phosphatase 1a (OsPP1a) involved in salt stress tolerance in transgenic rice. Molecular Breeding, 36(3), 1–19. https://doi.org/10.1007/s11032-016-0446-2
- Liu, Y., Wang, F., Zhang, A., Chen, Z., Luo, X., Kong, D., Zhang, F., Yu, X., Liu, G., & Luo, L. (2023). Improvement of salinity tolerance in water-saving and drought-resistance rice (WDR). International Journal of Molecular Sciences, 24(6), 5444. https://doi.org/10.3390/ijms24065444
- Ma, Y., Dias, M. C., & Freitas, H. (2020). Drought and salinity stress responses and microbe-induced tolerance in plants. Frontiers in Plant Science, 11, 591911. https://doi.org/10.3389/fpls.2020.591911
- Machado, R. M. A., & Serralheiro, R. P. (2017). Soil salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae, 3(2), 30. https://doi.org/10.3390/horticulturae3020030
- Maïga, Y., Mawussi, G., Faye, O. N., & Fall, A. (2020). Screening of rice lines (Oryza Spp L. 1753) for salinity tolerance at vegetative stage under Senegal River valley conditions. Journal of Experimental Agriculture International, 42(4), 71–81. https://doi.org/10.9734/jeai/2020/v42i430501
- Mbarki, S., Sytar, O., Cerda, A., Zivcak, M., Rastogi, A., He, X., Zoghlami, A., Abdelly, C., & Brestic, M. (2018). Strategies to mitigate the salt stress effects on photosyntheticapparatus and productivity of crop plants. In V. Kumar, S. H. Wani, P. Suprasanna, & L.-S. P. Tran (Eds.), Salinity responses and tolerance in plants, Volume 1: Targeting sensory, transport and signaling mechanisms (pp. 85–136). Springer International Publishing. https://doi.org/10.1007/978-3-319-75671-4_4
- Meliyo, J. L., Kashenge-Killenga, S., Victor, K. M., Mfupe, B., Hiza, S., Kihupi, L., Boman, B., & Dick, W. (2016). Evaluation of salt affected soils for rice (Oryza Sativa) production in Ndungu irrigation scheme same district, Tanzania. Sustainable Agriculture Research, 6(1), 24–38. https://doi.org/10.5539/sar.v6n1p24
- Miller, G., Shulaev, V., & Mittler, R. (2008). Reactive oxygen signaling and abiotic stress. Physiologia Plantarum, 133(3), 481–489. https://doi.org/10.1111/j.1399-3054.200801090.x
- Miller, G., Suzuki, N., Ciftci-Yilmaz, S., & Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment, 33(4), 453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
- Mishra, P., Bhoomika, K., & Dubey, R. S. (2013). Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive indica rice (Oryza sativa L.) seedlings. Protoplasma, 250(1), 3–19. https://doi.org/10.1007/s00709-011-0365-3
- Mishra, A., & Tanna, B. (2017). Halophytes: Potential resources for salt stress tolerance genes and promoters. Frontiers in Plant Science, 8, 829. https://doi.org/10.3389/fpls.2017.00829
- Moatabarniya, S., Rad, A. C., Sima, N. A. K., Askari, H., Zeinalabedini, M., Hesarkhani, Z., & Ghaffari, M. R. (2022). Morphological and anatomical changes of Salicornia roots are associated with different salinity and nutrients conditions in contrasting genotypes. Rhizosphere, 24, 100629. https://doi.org/10.1016/j.rhisph.2022.100629
- Moradi, F., & Ismail, A. M. (2007). Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of Botany, 99(6), 1161–1173. https://doi.org/10.1093/aob/mcm052
- Mosier, S., Córdova, S. C., & Robertson, G. P. (2021). Restoring soil fertility on degraded lands to meet food, fuel, and climate security needs via perennialization. Frontiers in Sustainable Food Systems, 5(October), 1–18. https://doi.org/10.3389/fsufs.2021.706142
- Mukasa, N. A., Woldemichael, D. A., Salami, O. A., & Simpasa, M. A. (2017). Africa’s agricultural transformation: Identifying priority areas and overcoming challenges. Africa Economic Brief, 8(3), 1–16.
- Munns, R. (2002). Comparative physiology of salt and water stress. Plant, Cell & Environment, 25(2), 239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
- Munns, R., & Munns, R. (2005). Genes and salt tolerance: Bringing them together. The New Phytologist, 167(3), 645–663. https://doi.org/10.1111/j.1469-8137.2005.01487.x
- Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59(1), 651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
- Muthayya, S., Sugimoto, J. D., Montgomery, S., & Maberly, G. F. (2014). An overview of global rice production, supply, trade, and consumption. Annals of the New York Academy of Sciences, 1324(1), 7–14. https://doi.org/10.1111/nyas.12540
- Nadeem, M. A., Nawaz, M. A., Shahid, M. Q., Doğan, Y., Comertpay, G., Yıldız, M., Hatipoğlu, R., Ahmad, F., Alsaleh, A., Labhane, N., Özkan, H., Chung, G., & Baloch, F. S. (2018). DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnology & Biotechnological Equipment, 32(2), 261–285. https://doi.org/10.1080/13102818.2017.1400401
- Nakhla, W. R., Wenqiang, S., Kai, F., Kang, Y., Chaopu, Z., & Sibin, Y. (2021). Identification of qtls for salt tolerance at the germination and seedling stages in rice. Plants (Basel, Switzerland), 10(3), 428. https://doi.org/10.3390/plants10030428
- Nongpiur, R. C., Singla-Pareek, S. L., & Pareek, A. (2016). Genomics approaches for improving salinity stress tolerance in crop plants. Current Genomics, 17(4), 343–357. https://doi.org/10.1111/nyas.12540
- Obalum, S. E., Buri, M. M., Nwite, J. C., Watanabe, Y., Igwe, C. A., Wakatsuki, T., & Hermansah. (2012). Soil degradation-induced decline in productivity of sub-Saharan African soils : The prospects of looking downwards the lowlands with the sawah ecotechnology. Applied and Environmental Soil Science, 2012, 1–10. 2012 https://doi.org/10.1155/2012/673926
- Obata, T., Kitamoto, H. K., Nakamura, A., Fukuda, A., & Tanaka, Y. (2007). Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiology, 144(4), 1978–1985. https://doi.org/10.1104/pp.107.101154
- Osman, K. T. (2013). Saline and sodic soils. In K. T. Osman (Ed.), Management of soil problems (pp. 255–298). Springer International Publishing. https://doi.org/10.1007/978-3-319-75527-4.63
- Phang, T. H., Shao, G., & Lam, H. M. (2008). Salt tolerance in soybean. Journal of Integrative Plant Biology, 50(10), 1196–1212. https://doi.org/10.5772/intechopen.102835
- Pratiwi, H., Nugrahaeni, N., & Taufiq, A. (2021). Identification of peanut germplasm tolerance to salinity stress. Buletin Palawija, 19(1), 1. https://doi.org/10.21082/bulpa.v19n1.2021.p1-9
- Qin, H., Li, Y., & Huang, R. (2020). Advances and challenges in the breeding of salt-tolerant rice. International Journal of Molecular Sciences, 21(21), 8385. https://doi.org/10.3390/ijms21218385
- Rad, H. E., Aref, F., & Rezaei, M. (2012). Response of rice to different salinity levels during different growth stages. Research Journal of Applied Sciences, Engineering and Technology, 4(17), 3040–3047. https://doi.org/10.1016/S2095-3119(16)61608-8
- Rahman, A. N. M. R. (2022). Trends in rice research : 2030 and beyond, 1–17. https://doi.org/10.1002/fes3.390
- Rasheed, A., Li, H., Nawaz, M., Mahmood, A., Hassan, M. U., Shah, A. N., Hussain, F., Azmat, S., Gillani, S. F. A., Majeed, Y., Qari, S. H., & Wu, Z. (2022). Molecular tools, potential frontiers for enhancing salinity tolerance in rice: A critical review and future prospective. Frontiers in Plant Science, 13(7), 966749. https://doi.org/10.3389/fpls.2022.966749
- Rhaman, M. S., Imran, S., Rauf, F., Khatun, M., Baskin, C. C., Murata, Y., & Hasanuzzaman, M. (2020). Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants, 10(1), 37. https://doi.org/10.3390/plants10010037
- Rivero, R. M., Mittler, R., Blumwald, E., & Zandalinas, S. I. (2022). Developing climate-resilient crops: Improving plant tolerance to stress combination. The Plant Journal: For Cell and Molecular Biology, 109(2), 373–389. https://doi.org/10.1111/tpj.15483
- Ryu, H., & Cho, Y. (2015). Plant hormones in salt stress tolerance. Journal of Plant Biology, 58(3), 147–155. https://doi.org/10.1007/s12374-015-0103-z
- Sahoo, S., & Baranda, B. (2018). Salinity tolerance in wheat. Marumegh, 3(1), 61–65. https://doi.org/10.3390/plants12183330
- Saijo, Y., Hata, S., Kyozuka, J., Shimamoto, K., & Izui, K. (2000). Over-expression of a single Ca 2 + -dependent protein kinase confers both cold and salt/drought tolerance on rice plants. The Plant Journal: For Cell and Molecular Biology, 23(3), 319–327. https://doi.org/10.3389/fpls.2022.1043757
- Salih, S. A., & Elsheik, M. A. (2014, March 17–18). The nature and properties of salt affected soil in South Khartoum [Paper presentation]. International Conference on Biological, Civil and Environmental Engineering (BCEE-2014)]. https://doi.org/10.15242/IICBE.C0314140
- Sarhadi, E., Bazargani, M. M., Sajise, A. G., Abdolahi, S., Vispo, N. A., Arc-Eta, M., Nejad, G. M., Singh, R. K., & Salekdeh, G. H. (2012). Proteomic analysis of rice anthers under salt stress. Plant Physiology and Biochemistry: PPB, 58, 280–287. https://doi.org/10.1016/j.plaphy.2012.07.013
- Segal, R., & Minh, L. N. (2019). Unfair harvest. The State of Rice in Asia, March 2018 (pp. 1–3). https://doi.org/10.21201/2019.4184
- Sharif, I., Aleem, S., Farooq, J., Rizwan, M., Younas, A., Sarwar, G., & Chohan, S. M. (2019). Salinity stress in cotton: Effects, mechanism of tolerance and its management strategies. Physiology and Molecular Biology of Plants: An International Journal of Functional Plant Biology, 25(4), 807–820. https://doi.org/10.1007/s12298-019-00676-2
- Sharma, A., Shahzad, B., Kumar, V., Kohli, S. K., Sidhu, G. P. S., Bali, A. S., Handa, N., Kapoor, D., Bhardwaj, R., & Zheng, B. (2019). Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules, 9(7), 285. https://doi.org/10.3390/biom9070285
- Shahid, S. A., Zaman, M., & Heng, L. (2018). Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. Springer.
- Shrivastava, P., & Kumar, R. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences, 22(2), 123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
- Sing, R., Gb, G., & Jain, R. (2007). QTL mapping for salinity tolerance in rice. Physiology and Molecular Biology of Plants, 13, 87–99. https://doi.org/10.1038/s41598-022-21737-9
- Singh, R. K., Kota, S., & Flowers, T. J. (2021). Salt tolerance in rice: Seedling and reproductive stage QTL mapping come of age. TAG. Theoretical and Applied Genetics. Theoretische Und Angewandte Genetik, 134(11), 3495–3533. https://doi.org/10.1007/s00122-021-03890-3
- Singh, R., Singh, Y., Xalaxo, S., Verulkar, S., Yadav, N., Singh, S., Singh, N., Prasad, K. S. N., Kondayya, K., Rao, P. V. R., Rani, M. G., Anuradha, T., Suraynarayana, Y., Sharma, P. C., Krishnamurthy, S. L., Sharma, S. K., Dwivedi, J. L., Singh, A. K., Singh, P. K., Singh, N. K., Kumar, R., & Nilanjay. (2015). From QTL to variety-harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Science: An International Journal of Experimental Plant Biology, 242, 278–287. https://doi.org/10.1016/j.plantsci.2015.08.008
- Singla-Pareek, S. L., Yadav, K., Pareek, A., Sopory, ÆM. K., & Reddy, S. K. (2008). Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Research, 17(2), 171–180. https://doi.org/10.1007/s11248-007-9082-2
- Sitta, B., Kashenge, S., Lee, S.-B., Kyung-Ho, K., Bulegeya, V., Batare, M., & Mwakapala, R. (2022). Morphogenetic response of assorted rice genotypes to salinity in Tanzania. European Journal of Agriculture and Food Sciences, 4(5), 19–27. https://doi.org/10.24018/ejfood.2022.4.5.504
- Slama, I., Abdelly, C., Bouchereau, A., Flowers, T., & Savouré, A. (2015). Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Annals of Botany, 115(3), 433–447. https://doi.org/10.1093/aob/mcu239
- Songtoasesakul, D., Aesomnuk, W., Pannak, S., Siangliw, J. L., Siangliw, M., Toojinda, T., Wanchana, S., & Arikit, S. (2023). QTL-seq identifies pokkali-derived QTLs and candidate genes for salt tolerance at seedling stage in rice. Agriculture, 13(8), 1596. https://doi.org/10.3390/agriculture13081596
- Sripinyowanich, S., Klomsaku, L. P., Boonburapong, B., Bangyeekhun, T., Asami, T., Gu, H., Buaboocha, T., & Chadchawan, E. (2013). Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): The role of Os P5CS1 and Os P5CR gene expression duringsalt stress. Environmental and Experimental Botany, 86, 94–105. https://doi.org/10.3390/ijms24065444
- Stavi, I., Thevs, N., & Priori, S. (2021). Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Frontiers in Environmental Science, 9, 712831. https://doi.org/10.3389/fenvs.2021.712831
- Stavridou, E., Hastings, A., Webster, R. J., & Robson, P. R. H. (2017). The impact of soil salinity on the yield, composition and physiology of the bioenergy grass miscanthus × giganteus. GCB Bioenergy, 9(1), 92–104. https://doi.org/10.1111/gcbb.12351
- Stepien, P., & Johnson, G. N. (2009). Contrasting responses of photosynthesis to salt stress in the glycophyte arabidopsis and the halophyte thellungiella: Role of the plastid terminal oxidase as an alternative electron sink. Plant Physiology, 149(2), 1154–1165. https://doi.org/10.1104/pp.108.132407
- Subudhi, P. K., Shankar, R., & Jain, M. (2020). Whole genome sequence analysis of rice genotypes with contrasting response to salinity stress. Scientific Reports, 10(1), 21259. https://doi.org/10.1038/s41598-020-78256-8
- Tabassum, R., Tahjib-Ul-Arif, M., Hasanuzzaman, M., Sohag, A. A. M., Islam, M. S., Shafi, S. M. S. H., Islam, M. M., & Hassan, L. (2021). Screening salt-tolerant rice at the seedling and reproductive stages: An effective and reliable approach. Environmental and Experimental Botany, 192(August), 104629. https://doi.org/10.1016/j.envexpbot.2021.104629
- Tang, X., Mu, X., Shao, H., Wang, H., & Brestic, M. (2014). Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Environmental and Experimental Botany, 8551, 1–13. https://doi.org/10.3109/07388551.2014.889080
- Thiam, S., Villamor, G. B., Faye, L. C., Henri, J., Sène, B., Diwediga, B., & Kyei, N. (2021). Monitoring land use and soil salinity changes in coastal landscape: A case study from Senegal. Environmental Monitoring and Assessment, 193(5), 259. https://doi.org/10.1007/s10661-021-08958-7
- Tully, K., Sullivan, C., Weil, R., & Sanchez, P. (2015). The state of soil segradation in sub-Saharan Africa: Baselines, trajectories, and solutions. Sustainability, 7(6), 6523–6552. https://doi.org/10.3390/su7066523
- Umego, C., Ntui, V. O., Ita, E. E., Opara, C., & Uyoh, E. A. (2020). Screening of rice accessions for tolerance to drought and salt stress using morphological and physiological parameters. American Journal of Plant Sciences, 11(12), 2080–2102. https://doi.org/10.4236/ajps.2020.1112147
- van Oort, P. A. J., & Zwart, S. J. (2018). Impacts of climate change on rice production in Africa and causes of simulated yield changes. Global Change Biology, 24(3), 1029–1045. https://doi.org/10.1111/gcb.13967
- Van Oort, P. A. J., Saito, K., Tanaka, A., Amovin-Assagba, E., Van Bussel, L. G. J., Van Wart, J., de Groot, H., van Ittersum, M. K., Cassman, K. G., & Wopereis, M. C. S. (2015). Assessment of rice self-sufficiency in 2025 in eight African countries. Global Food Security, 5, 39–49. https://doi.org/10.1016/j.gfs.2015.01.002
- Verma, V., Ravindran, P., & Kumar, P. P. (2016). Plant hormone-mediated regulation of stress responses. BMC Plant Biology, 16, 86. https://doi.org/10.1186/s12870-016-0771-y
- Wang, Y., Deng, C., Liu, Y., Niu, Z., & Li, Y. (2018). Identifying change in spatial accumulation of soil salinity in an inland river watershed, China. The Science of the Total Environment, 621, 177–185. https://doi.org/10.1016/j.scitotenv.2017.11.222
- Waziri, A., Kumar, P., & Purty, R. S. (2016). Saltol QTL and their role in salinity tolerance in rice. Austin Journal of Biotechnology & Bioengineering, 3(3), 1067.
- Xie, L., Zheng, C., Li, W., Pu, M., Zhou, G., Sun, W., Wu, X., Zhao, X., & Xie, X. (2021). Mapping and identification a salt-tolerant QT L in a salt - resistant rice. Journal of Plant Growth Regulation, 41(6), 2347–2358. https://doi.org/10.1007/s00344-021-10448-6
- Xiong, L., & Yang, Y. (2003). Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid – inducible mitogen-activated protein kinase. The Plant Cell, 15(3), 745–759. https://doi.org/10.1105/tpc.008714
- Xu, Y., Pengcheng, L., Zefeng, Y., & Chenwu, X. (2017). Genetic mapping of quantitative trait loci in crops. The Crop Journal, 5(2), 175–184. https://doi.org/10.1016/j.cj.2016.06.003
- Yu, Z., Duan, X., Luo, L., Dai, S., Ding, Z., & Xia, G. (2020). How plant hormones mediate salt stress responses. Trends in Plant Science, 25(11), 1117–1130. https://doi.org/10.1016/j.tplants.2020.06.008
- Yue, E., Cao, H., & Liu, B. (2020). OsmiR535, a potential genetic editing target for drought and salinity stress tolerance in Oryza Sativa. Plants (Basel, Switzerland), 9(10), 1337. https://doi.org/10.3390/plants9101337
- Yu, Z., Zhang, D., Xu, Y., Jin, S., Zhang, L., Zhang, S., Yang, G., Yan, K., Wu, C., Zheng, C., & Huang, J. (2019). CEPR2 phosphorylates and accelerates the degradation of PYR/PYLs in arabidopsis. Journal of Experimental Botany, 70(19), 5457–5469. https://doi.org/10.1093/jxb/erz302
- Zafar, K., Sedeek, K. E. M., Rao, G. S., Khan, M. Z., Amin, I., Kamel, R., Mukhtar, Z., Zafar, M., Mansoor, S., & Mahfouz, M. M. (2020). Genome editing technologies for rice improvement: Progress, prospects, and safety concerns. Frontiers in Genome Editing, 2, 5. https://doi.org/10.3389/fgeed.2020.00005
- Zeng, L., & Shannon, M. (2005). Salinity effects on seedling growth and yield components of different inbred rice lines. Pakistan Journal of Botany, 37(1), 131–139.
- Zeng, L., Shannon, M. C., & Lesch, S. M. (2001). Timing of salinity stress affects rice growth and yield components. Agricultural Water Management, 48(3), 191–206. https://doi.org/10.1016/S0378-3774(00)00146-3
- Zhang, Y., Fang, J., Wu, X., & Dong, L. (2018). Na +/K + balance and transport regulatory mechanisms in weedy and cultivated rice (Oryza Sativa L.) under salt stress. BMC Plant Biology, 18(1), 375. https://doi.org/10.1186/s12870-018-1586-9
- Zhang, A., Liu, Y., Wang, F., Li, T., Chen, Z., Kong, D., Bi, J., Zhang, F., Luo, X., Wang, J., Tang, J., Yu, X., Liu, G., & Luo, L. (2019). Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Molecular Breeding, 39(3), 47. https://doi.org/10.1007/s11032-019-0954-y
- Zhao, S., Zhang, Q., Liu, M., Zhou, H., Ma, C., & Wang, P. (2021). Regulation of plant responses to salt stress. International Journal of Molecular Sciences, 22(9), 1–16. https://doi.org/10.3390/ijms22094609
- Zhao, C., Zhang, H., Song, C., Zhu, J. K., & Shabala, S. (2020). Mechanisms of plant responses and adaptation to soil salinity. Innovation (Cambridge (Mass.)), 1(1), 100017. https://doi.org/10.1016/j.xinn.2020.100017
- Zhu, J. K. (2003). Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology, 6(5), 441–445. https://doi.org/10.1016/s1369-5266(03)00085-2

