Abstract
Nutraceuticals have generated an interest among clinicians for their applicability in the prevention and treatment of many ailments. Literature suggests the possible role of nutraceuticals in COVID-19. However, substantial uncertainty related to their safety and efficacy still exists. The aim of this study is to assess the efficacy and safety of nutraceuticals in preventing or treating COVID-19. We searched electronic databases, registries, websites, and e-libraries of development agencies. We included randomized controlled trials (RCTs), quasi-experiments, pre-post studies, and other experimental study designs. We assessed the risk of bias (RoB) using the RoB2 tool for RCTs and the ROBINS-I tool for non-RCTs. We assessed the overall certainty of the evidence using GRADEpro GDT. The outcomes assessed were the number of COVID-19 cases, change in disease severity, days of hospitalization, deaths, and adverse events. We performed the last search on 13th December 2023. After screening 481 studies, our analysis of five revealed one study with ‘low risk’ and four studies with ‘some concerns’ regarding bias. Our findings indicated that nutraceuticals did not significantly reduce COVID-19 mortality; their effect on hospitalization duration was uncertain. Furthermore, our assessment found no substantial evidence of increased adverse effects with probiotic use (RR 0.11, 95% CI 0.01 to 1.89, P = 0.13). Importantly, none of the studies investigated nutraceuticals’ preventive effects or their role in mitigating COVID-19 severity. There is limited evidence available on the use of nutraceuticals (probiotics, prebiotics, or synbiotics) for the treatment and prevention of COVID-19. Conducting methodologically robust RCTs with large population samples is recommended.PROSPERO REGISTRATION ID CRD42021284923
REVIEWING EDITOR:
Introduction
Literature suggests the possible role of nutraceutical intervention in boosting immunity against COVID-19 (Akour, Citation2020; Infusino et al., Citation2020; Khaled, Citation2021; Morais et al., Citation2020; Olaimat et al., Citation2020; Sundararaman et al., Citation2020). Probiotics improve the mucosal-barrier function, antagonize pathogens, inhibit pathogen adherence, and enhance the immunity angulating the central nervous system (Stavropoulou & Bezirtzoglou, Citation2020). When the microbiota is disrupted, infectious diseases, autoimmune diseases, allergic conditions, and other diseases may arise. Probiotics can prevent and limit the symptoms of such diseases when administered as an adjuvant by conserving the intestinal microbiota and boosting the immune system (Stavropoulou & Bezirtzoglou, Citation2020).
In a recent research conducted by McCarty and colleagues, it has been suggested that certain nutraceuticals could potentially alleviate the effects of encapsulated RNA viruses in infected individuals, such as influenza and coronavirus, by boosting immune responses (McCarty & DiNicolantonio, Citation2020). Probiotics are viable, non-pathogenic bacteria that, when consumed, improve host health or physiology (George Kerry et al., Citation2018; Maldonado Galdeano et al., Citation2019). Ingestion of probiotics has been linked to a variety of positive impacts on human health as well as changes in the physiological homeostasis of the gut flora (Ballan et al., Citation2020; Bermudez-Brito et al., Citation2012; Markowiak & Śliżewska, Citation2017). Prebiotics are ideal fermented components that offer the potential for specific biological modifications, influencing the microbiota’s composition and/or function in the gastrointestinal (GI) tract, thereby promoting health benefits for the host (Khaled, Citation2021). The beneficial impacts of prebiotics on the gastrointestinal (GI) tract, including pathogen inhibition and immune system stimulation, stem from their ability to modulate the composition and function of the human microbiota (Olaimat et al., Citation2020).
On the other hand, the term ‘synbiotic’ describes a combination of probiotics and prebiotics that collaboratively enhance each other’s effects. They possess properties of both probiotics and prebiotics and were developed to address potential challenges in the survival of probiotics within the GI tract. Incorporating nutraceuticals (probiotics, prebiotics, or synbiotics) probiotics, prebiotics, or synbiotics into the human diet is advantageous for the intestinal microbiota (Markowiak & Śliżewska, Citation2017). The finest evidence for the effectiveness of certain probiotic strains derived from RCTs is there for treating or preventing antibiotic-associated disorders, gastroenteritis, acute diarrhoea, and relieving lactose intolerance (Aponte et al., Citation2020; Nazir et al., Citation2018). Probiotic microbial agents and their components defend the body through several mechanisms. Probiotic organisms either directly or indirectly inhibit the growth of traditional or potential microorganisms, along with their epithelial adhesion and invasion, by secreting antimicrobial compounds or by encouraging host expression of defensive molecules (Plaza-Diaz et al., Citation2019). Furthermore, increasing probiotic levels may induce a ‘barrier’ effect against common pathogens (Maldonado Galdeano et al., Citation2019). They can influence the host production of immunosuppressive molecules that reduce the inflammatory response in the host or, conversely, activate host protective immunologic processes that can prevent or hasten the clearance of pathogenic infections (Ewald & Sumner, Citation2018).
Prebiotics are resistant to digestion by host enzymes. They reach the digestive system largely unchanged and undergo fermentation by saccharolytic bacteria like those from the Bifidobacterium genus in the colon (Markowiak & Śliżewska, Citation2017). The intake of prebiotics has a notable impact on the composition and metabolic functions of the intestinal microbiota. The main goal is to support the growth and functionality of beneficial bacteria, or probiotics, in the gastrointestinal (GI) tract, ultimately bestowing health advantages upon the host (Markowiak & Śliżewska, Citation2017). Commonly include prebiotics are fructans, oligosaccharides, isomaltooligosaccharides, arabinooligosaccharides, xylooligosaccharides, lactosucrose, lactobionic acid, resistant starch, psyllium, galactomannan, polyunsaturated fatty acids, and polyphenols (Markowiak & Śliżewska, Citation2017; Olaimat et al., Citation2020). The health advantages that prebiotics bring to the GI tract, such as pathogen inhibition and immune system stimulation, result from their capacity to regulate the composition and activity of the human microbiota (Olaimat et al., Citation2020). Prebiotics may also have an excellent potential effect against COVID-19 by enhancing probiotics’ growth and survivability. Furthermore, prebiotics could have a direct effect on GI symptoms caused by COVID-19 by blocking the Angiotensin-converting enzyme (ACE) enzymes (Olaimat et al., Citation2020).
On the other hand, the impact of synbiotics on metabolic health remains uncertain, and it is worth noting that the health effects of synbiotics are likely linked to the specific combination of a probiotic and prebiotic (Markowiak & Śliżewska, Citation2017). Among synbiotic products, combinations featuring Bifidobacterium or Lactobacillus genus bacteria with fructooligosaccharides appear to be particularly popular (Markowiak & Śliżewska, Citation2017).
Probiotics have recently earned the interest of physicians or clinicians due to their utilization for treating and preventing several disorders. The existing understanding indicates a connection between the gut microbiota and the liver, recognized as the ‘microbiota-gut-liver axis.’ Additionally, there is an acknowledged interaction between the intestinal microbiota and the central nervous system, referred to as the ‘microbiota-gut-brain axis.’ Moreover, there is emerging evidence supporting bidirectional communication between the lungs and the gut, termed the ‘gut-lung axis. Some people suffering from COVID-19 can present fatigue, such as ‘brain fog’, pain, even after several months, signifying chronic association of the CNS in COVID-19. In addition, gut microbiome disturbances can severely affect the brain, and its functions and restoration could have a beneficial effect. Probiotic supplementation has been observed to improve metabolic homeostasis and prevent fatigue development by enhancing the metabolites concerned with the utilization of glucose and energy (Santinelli et al., Citation2021). The significance of the ‘gut-brain’ axis has been connected to overall mental health (Dhar, Citation2021). Probiotics can have anxiolytic effects via alteration of gut microbiota (Dhar, Citation2021). Thus, gut microbiome disturbances can have a severe adverse impact on the brain and its function, and their restoration can benefit people suffering from these conditions.
Despite the elevated prevalence of COVID-19, effective medicines or therapies remain few, and no definite pharmaceutical treatment is available to target the disease’s relevant components. Several studies have demonstrated the positive and encouraging effects of probiotics, prebiotics, or synbiotics in treating or preventing COVID-19 (Akour, Citation2020; Giannoni et al., Citation2020; Infusino et al., Citation2020; Mak et al., Citation2020; Morais et al., Citation2020; Sundararaman et al., Citation2020). The positive effect of nutraceuticals (probiotics, prebiotics, or synbiotics) cannot be disregarded, but substantial uncertainty related to their safety and efficacy in clinical practice still exists (Pandey et al., Citation2022). Furthermore, the safety and effectiveness of the same in COVID-19 patients have not been well studied or systematically reviewed. There is a need to synthesize the information or evidence so that patients, practitioners, and policymakers may determine if these nutraceuticals can be used to prevent or treat COVID-19 and, if data permits, investigate the best pharmacological program for this group of patients. With this background, the objective of this systematic review is to assess the safety and efficacy of nutraceuticals (probiotics, prebiotics, or synbiotics) in preventing or treating COVID-19.
Methods
The systematic review was conducted using a standard methodology suggested in the Cochrane Handbook of Systematic Reviews (Higgins et al., Citation2021). CRD42021284923 is the registration number of the proposed protocol submitted in PROSPERO. The International Life Sciences Institute (ILSI), India, funded this work.
Inclusion and exclusion criteria
We included randomized controlled trials (RCTs), quasi-experiments, pre-post studies, and other experimental study designs reporting the efficacy of nutraceuticals (probiotics, prebiotics, or synbiotics) to prevent or treat COVID-19 and excluded case reports, observational studies, clinical observations, systematic reviews, and narrative reviews. We did not include grey literature, reports, abstracts, or conference proceedings. We restricted the language of publishing and included articles published only in English.
We included studies investigating participants affected/exposed to COVID-19 infection, regardless of age, gender, ethnicity, and setting (inpatients and outpatients), and excluded severely ill patients.
We included studies on supplementation of nutraceuticals (probiotics, prebiotics, or synbiotics) as an adjunct to standard treatments, irrespective of the form, dose, duration, or frequency. Comparators could consist of the standard of care without supplementation of probiotics, prebiotics, synbiotics, or any active comparator (such as appetizers, nutritional supplements, etc) and placebo. We also included studies that administered other adjunct interventions to the standard treatment (such as ozone oxygen therapy).
Following outcomes were considered in the review:
Primary outcomes
Number of cases of COVID-19
Change in disease severity
Days of hospitalization
Secondary outcomes
Number of deaths
Adverse events
Free of fatigue (Post-Covid fatigue)
Search methods for identification of studies
We searched for any probiotics, prebiotics, or synbiotics intervention provided to COVID-19. We searched Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Library; 2021, issue 9), MEDLINE (via PubMed), Web of Science, WHO COVID-19 database (https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/), WHO International Clinical Trials Registry (ICTRP) (apps.who.int/trialsearch/), clinicaltrials.gov (www.clinicaltrials.gov) for ongoing trials. We also searched Google Scholar, the WHO Regional Journals databases from Latin America, Africa, and South-East Asia, websites, and e-libraries of development agencies (WHO Department of Nutrition for Health and Development, UNICEF, Nutritional International, International Food Policy Research Institute (IFPRI). Additionally, we checked the reference lists of reviews and retrieved articles.
As the COVID-19 pandemic emerged in December 2019, we considered including studies between 2019 to 2023 and the last search was performed on 13th December 2023. The search included relevant keywords, free-text terms, and Medical Subject Headings (MeSH). The search strategy for PubMed, CENTRAL, and WHO COVID-19 databases are presented in Appendix 1:
Selection of studies
After removing duplicates, two investigators (SU and MW) independently screened all the articles retrieved from the searches using the Covidence screening tool (Covidence systematic review software, Citation2019) in two phases. In the first phase, we screened all articles against the inclusion criteria based on titles and abstracts. The studies found eligible in the first screening phase were then screened based on full texts by two investigators. Disagreements were resolved by consensus. We recorded the flow of studies in the systematic reviews in the form of a PRISMA-flow diagram as suggested in ‘Cochrane Handbook for Systematic Reviews of Intervention’ (Higgins & Green, Citation2011).
Data extraction and management
Two reviewers (SU and MW) extracted data from each eligible study using a pilot-tested data extraction form, including study design, country, sample size details, characteristics of study participants (age, sex, race/ethnicity, health status), intervention details, dependent variables, and their measurement, and funding sources. One investigator reviewed all the extracted data for accuracy.
Assessment of risk of bias in included studies
Two investigators (APS and MNK) assessed the risk of bias for RCTs using Cochrane’s Risk of Bias Tool 2 (RoB2) and Non-RCTs using the ROBINS-I tool (Higgins & Green, Citation2011). Discrepancies amongst investigators were resolved through consensus and in consultation with a third reviewer.
Measures of treatment effect
One reviewer inputted the results of each trial into Review Manager 5 (Review Manager, Citation2014) for the computation of treatment effects. We intended to perform meta-analyses selectively, focusing on situations where combining results made sense, specifically when participants, interventions, and the underlying clinical question were sufficiently similar to justify pooling.
We classified the studies based on the outcomes reported. For dichotomous outcomes (deaths and adverse events), we expressed the results as risk ratios (RRs) with 95% confidence intervals (CIs) for analyses. We had planned to use mean differences (MDs) and 95% CIs or standardized mean differences (SMDs) with 95% CIs to express continuous variables (days of hospitalization). We provided a narrative description of the results when the data were inadequate. For each outcome, we examined the reported effect sizes and the heterogeneity (I2 statistic) across studies. We designated an I2 statistic ranging from 0% to 40% as indicative of low heterogeneity, 41% to 60% as moderate heterogeneity, 61% to 90% as substantial heterogeneity, and values exceeding 91% as representing considerable heterogeneity.
We had planned to use fixed‐effect analysis unless there was significant heterogeneity (P < 0.1) as assessed by the Chi2 test, where we had planned to use random‐effect analysis. Summary of the result of dichotomous and continuous outcomes was mentioned in Appendix 2 as A2 Table 1 and A2 Table 2. Individual participants in each clinical trial were the unit of analysis.
Regarding strategies for dealing with missing data, we adapted the Cochrane Handbook for Systematic Reviews of Interventions recommendations (Higgins et al., Citation2021). We contacted the investigators for clarification when the missing data was not explicit. If the outcome data for standard deviations (SD) was missing, we calculated it from 95% confidence intervals as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., Citation2021).
Other methods, including assessment of heterogeneity, reporting biases, subgroup analysis, sensitivity analysis and investigation of heterogeneity, have been taken from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., Citation2021).
Two review authors (SZQ and MNK) independently assessed the overall certainty of the evidence for three outcomes and graded using the GRADE approach (GRADEpro GDT, Citation2021). We reported an overall assessment of the certainty of the evidence for the number of deaths, number of adverse events, and days of hospitalizations in Summary of Findings (SoF) Table using GRADE pro-GDT (GRADEpro GDT, Citation2021).
Results
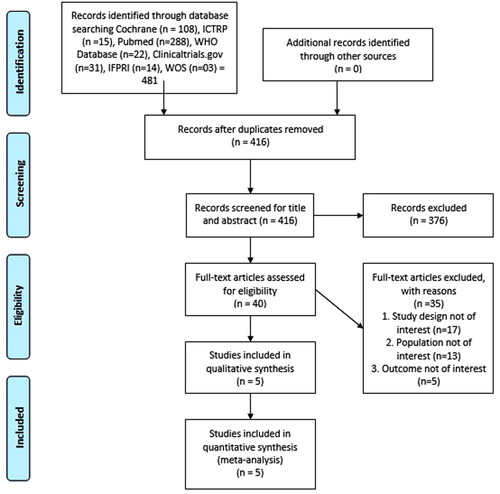
We identified 481 potential studies from different electronic databases. After removing duplicates, two reviewers independently examined 416 studies and, after initial screening (based on title and abstract), removed 376 studies. We then independently assessed the full texts of 40 potentially relevant studies and excluded 35 studies based on different study designs (n = 17), participants other than those with COVID-19 (n = 13) and outcomes not of interest (n = 5). We identified five studies for potential inclusion (Araimo et al., Citation2021; Gutiérrez-Castrellón et al., Citation2022; Ivashkin et al., Citation2023; Rathi et al., Citation2021; Reino-Gelardo et al., Citation2023). All five studies (Araimo et al., Citation2021; Gutiérrez-Castrellón et al., Citation2022; Ivashkin et al., Citation2023; Rathi et al., Citation2021; Reino-Gelardo et al., Citation2023) were included for quantitative synthesis (meta-analysis). We have presented a flow diagram detailing the selection of studies in .
We included three single-centric and one multicentric RCT published between 2019 and 2023 year (). The studies included were two open labelled (Araimo et al., Citation2021; Ivashkin et al., Citation2023), one double-blinded (Rathi et al., Citation2021), one quadruple-blinded (Gutiérrez-Castrellón et al., Citation2022), and one non-blinded (Reino-Gelardo et al., Citation2023). The studies were conducted in Spain (Reino-Gelardo et al., Citation2023), Mexico (Gutiérrez-Castrellón et al., Citation2022), Italy (Araimo et al., Citation2021), Moscow (Ivashkin et al., Citation2023), and India (Rathi et al., Citation2021).
Table 1. Characteristics of included studies.
The total number of participants included in the five trials were 860, including both males and females (Araimo et al., Citation2021; Gutiérrez-Castrellón et al., Citation2022; Ivashkin et al., Citation2023; Rathi et al., Citation2021; Reino-Gelardo et al., Citation2023). The age of the participants ranged between 18 to 75 years. All the 860 participants were diagnosed with COVID-19. Further, Araimo et al.(Araimo et al., Citation2021) included participants of COVID stage III admitted to the hospital wards andexcluded participants who were suffering from Stage IV-VI COVID-19 and were in ICU.
All the studies included had two treatment arms. In three trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023), probiotics were used as an adjuvant treatment to the standard care compared with standard treatment alone. Bacterial strains used as probiotics in these trials were species of Bifidobacterium (Bifi.), lactobacillus, and Streptococcus. In another two trials (Gutiérrez-Castrellón et al., Citation2022; Rathi et al., Citation2021), probiotics were given as asupplement or as an adjuvant to the immuno booster therapy compared to a placebo containing maltodextrin.
In a trial conducted by Reino-Gelardo et al., (Reino-Gelardo et al., Citation2023), food supplement (Gasteel Plus that includes Bifidobacterium. Lactis BPL1, lactobacillus rhamnosus CNCM I-4036, Bifi. longum ES1) was provided along with the standard treatment that was based on the international guidelines available during the study considering the severity and complexities of the disease. In one trial (Araimo et al., Citation2021), participants were given standard care consisting of antibiotics (azithromycin), anti-inflammatory (Hydroxychloroquine sulphate), and anti-cytokines (Tocilizumab) along with probiotics supplementation of SivoMixx 200 billion six sachets twice a day. However, in the other trial (Ivashkin et al., Citation2023), standard care encompassed steroids (Dexamethasone), antiviral (Favipiravir), and anticoagulant (enoxaparin) with probiotics supplementation comprising Florasan-D, Bifidobacterium bifidum PDV 0903, Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium longum PDV 2301, Bifidobacterium longum subsp, Infantis PDV 1911 for three times a day for 14 days. A trial conducted by Rathi et al. (Rathi et al., Citation2021) evaluated the efficacy of ImmunoSEB (multi-enzyme formulation of Peptizyme SP, bromelain, amylase, lysozyme, peptidase, catalase, papain, glucoamylase and lactoferrin) and ProbioSEB CSC3 (5 billion CFUs/capsule) that included Bacillus coagulans LBSC, B. subtilis PLSSC and B. clausii 088AE) prescribed twice daily in patients previously suffering from COVID-19 compared with placebo. a Further, another trial conducted by Gutierrez-Castrellon et al. (Gutiérrez-Castrellón et al., Citation2022) assessed the efficacy of AB21 probiotic capsules plus maltodextrin as a carrier compared with placebo containing only maltodextrin in symptomatic COVID-19 patients. We did not find any study where nutraceuticals (probiotics, prebiotics, or synbiotics) were given to normal subjects to prevent COVID-19. In addition, no studies reported data about the efficacy of nutraceuticals (probiotics, prebiotics, or synbiotics) in changing the severity of COVID-19. The outcome measures mainly addressed in the included studies were the number of deaths, adverse events, and days of hospitalization. Only Ivashkin et al. and Reino-Gelardo et al. (Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023) reported the number of hospitalization days. Similarly, Araimo et al. (Araimo et al., Citation2021) and Gutiérrez-Castrellón (Gutiérrez-Castrellón et al., Citation2022) reported adverse events. Ivashkin et al. (Ivashkin et al., Citation2023) and Araimo et al. (Araimo et al., Citation2021) reported the number of deaths due to COVID-19, whereas Rathi et al. (Rathi et al., Citation2021) reported data on post-covid fatigueAdverse events were reported in three trials (Araimo et al., Citation2021; Gutiérrez-Castrellón et al., Citation2022; Ivashkin et al., Citation2023).
Risk of bias in included studies
One trial (Gutiérrez-Castrellón et al., Citation2022) was at ‘low risk’ of bias, whereas the other four trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023) were at ‘Some concerns’ for risk of bias. We have presented a review authors’ judgment for each risk of bias for each study in the ‘Risk of bias’ summary ().
Figure 2. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
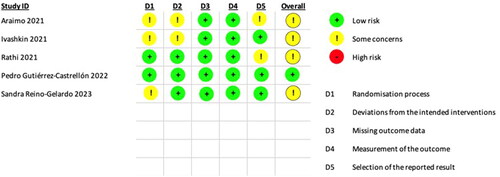
Domain 1: D1: Randomisation process
There were ‘some concerns’ in the randomization process in three trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023) and ‘low risk’ for two trials (Gutiérrez-Castrellón et al., Citation2022; Rathi et al., Citation2021). In three trials (Gutiérrez-Castrellón et al., Citation2022; Rathi et al., Citation2021; Reino-Gelardo et al., Citation2023) randomization method was mentioned whereas the only information about randomization methods in two trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023) with ‘some concerns’ is a statement that the trial is randomized. Also, no information is available about the allocation concealment in three trials with ‘some concerns’ (Araimo et al., Citation2021; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023). Whereas two trials (Gutiérrez-Castrellón et al., Citation2022; Rathi et al., Citation2021) judged to be at ‘low risk’ of bias used randomization method, generated the allocation sequence and used concealed envelopes for treatment allocation.
Domain 2: Deviations from the intended effect
‘Some concerns’ were reported in this domain in two trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023) and ‘low risk’ for three trials (Gutiérrez-Castrellón et al., Citation2022; Rathi et al., Citation2021; Reino-Gelardo et al., Citation2023). Two trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023) had ‘some concerns,’ as no information was available on whether the participants, caregivers, and/or people delivering the interventions were aware of their assigned intervention during the trial. In another three trials, one trial (Rathi et al., Citation2021) with ‘low risk’ was a double-blind trial wherein the participants and people delivering the interventions were blinded to their assigned intervention during the trial. The investigators have undertaken intention-to-treat (ITT) analyses. Another trial (Gutiérrez-Castrellón et al., Citation2022) was quadruple-blinded, where all participants and caregivers were unaware of the treatment, whereas the other trial (Reino-Gelardo et al., Citation2023) with ‘low risk’.
Domain 3: Missing outcome data
All the five included trials (Araimo et al., Citation2021; Gutiérrez-Castrellón et al., Citation2022; Ivashkin et al., Citation2023; Rathi et al., Citation2021; Reino-Gelardo et al., Citation2023) were at ‘low risk’ for missing outcome data. A trial conducted by Reino-Gelardo et al. (Reino-Gelardo et al., Citation2023) reported a 5% loss to followup, and another trial (Gutiérrez-Castrellón et al., Citation2022) reported a < 10% loss to follow up. Only two participants in the intervention group were lost to follow-up in one (Ivashkin et al., Citation2023) of the included trials. The other two trials (Araimo et al., Citation2021; Rathi et al., Citation2021) reported the participants’ 100% follow-up compliance.
Domain 4: Measurement of the outcome
All the five included trials were at ‘low risk’ of bias for outcome measurement. The methods of measurement of outcomes included in this review (days of hospitalization, number of deaths, and number of adverse events) were appropriate. They probably may not have differed between intervention groups in all the included trials. The outcome assessors were not blinded in the open-labelled trial (Ivashkin et al., Citation2023) and were blinded in the two trials (Gutiérrez-Castrellón et al., Citation2022; Rathi et al., Citation2021). The trial by Araimo et al., (Citation2021) did not provide information about the blinding status of the outcome assessors, whereas Reino-Gelardo et al. (Reino-Gelardo et al., Citation2023) conducted a non-blinded study.
Domain 5: Selection of the reported results
Two trials (Araimo et al., Citation2021; Rathi et al., Citation2021) had ‘some concerns’, while three trials (Gutiérrez-Castrellón et al., Citation2022; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023) were at ‘low risk’ for this domain. The pre-specified analysis plans reported in the protocols of the two included trials (Araimo et al., Citation2021; Rathi et al., Citation2021) were not available in sufficient detail to compare with those presented in the published report(s). In the other three trials (Gutiérrez-Castrellón et al., Citation2022; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023), the protocol was available with a registered ID.
Effects of interventions
Comparison 1: Nutraceuticals (Probiotics or prebiotics or synbiotics) as a adjunct to standard treatments verses Standard of care without supplementation of probiotics or prebiotics or synbiotics
We did not find any study that assessed, the number of cases of COVID-19, change in disease severity, and fatigue in this comparison group.
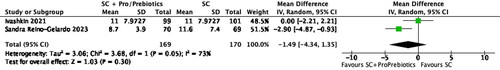
Days of hospitalization
Two studies (Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023) reported data on days of hospitalization. The pooled analysis shows reduction in the days of hospitalization with probiotics and prebiotics with standard care as compared to only standard care (two studies, 339 participants, MD -1.49, 95% CI -4.34 to 1.35, P = 0.30, I2=73%) ().
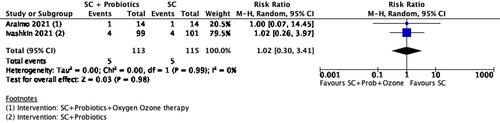
Figure 3. Forest plot of comparison: Standard care + Probiotics verses Standard care, outcome: Days of hospitalization Number of deaths.
In a trial by Araimo et al. (Araimo et al., Citation2021), assessing probiotics and ozone therapy alongside standard care versus standard care alone, no significant difference in the risk of death was observed between the intervention and comparison groups. Similarly, in a trial by Ivashkin et al. (Ivashkin et al., Citation2023), comparing probiotics as an adjunct to standard care with standard care alone, the risk of death in both groups was found to be nearly identical. The pooled analysis does not show any reduction in the number of deaths with prebiotics (two studies, 228 participants, RR 1.02, 95% CI 0.3 to 3.41, P = 0.98, I2=0%) ().
Adverse events
One trial (Araimo et al., Citation2021) compared the efficacy of probiotics and ozone therapy given as an adjunct to standard care verses standard care (without probiotics and ozone therapy). No adverse events were reported in the intervention group while four participants (30%) reported diarrhoea (one study, 28 participants, RR 0.11,95% CI 0.01 to 1.89, P = 0.13).
Comparison 2: Nutraceuticals (Probiotics orprebiotics or synbiotics)an adjunct to standard treatments versus Any active comparator (such as appetizers, nutritional supplements, etc.)
We did not find any study comparing nutraceuticals (probiotics, prebiotics, or synbiotics) adjuncts to standard treatments with an active comparator such as appetizers or nutritional supplements, etc.
Comparison 3: Nutraceuticals (Probiotics or prebiotics or synbiotics) as an adjunct to standard treatments verses placebo
Except for the adverse events, we did not find any study that assessed any of the other outcomes in this comparison group.
Adverse events
One study (Gutiérrez-Castrellón et al., Citation2022) compared the adverse events in probiotics and prebiotics versus placebo and found that the risk of events was 35% lesser with probiotics and prebiotics as compared to placebo (one study, 300 participants, RR 0.65, 95% CI 0.47 to 0.90, P = 0.009) Fatigue.
We found only one study (Rathi et al., Citation2021) that addressed this outcome. In this study, fatigue evaluation utilized a validated Chalder Fatigue Scale (CFQ-11), an 11-item self-report tool (Rathi et al., Citation2021). A cumulative score of four or higher indicates meeting the fatigue criteria.
The supplemental administration of prebiotics and immune boosters made patients free of fatigue in a significantly greater percentage of subjects in the intervention group compared to the control arm on day 14 (one study, 200 participants, RR 6.07, 95% CI 3.79 to 9.71). Positive outcomes were observed at earlier time intervals, revealing that a higher percentage of patients in the experimental group experienced relief from fatigue on days 4 and 8 compared to those who received the intervention at later stages.
Quality of evidence
The quality of evidence (QoE) was ‘low’ for a number of deaths and adverse events, and ‘very low’ for Days of hospitalization for the comparison ‘Standard care + Nutraceuticals (Probiotics/Prebiotics) compared to Standard care for Covid-19’.The QoE was downgraded for the reasons mentioned in the explanatory notes of .
Table 2. Summary of findings table for comparison: standard care + nutraceuticals (probiotics/prebiotics/synbiotics) compared to standard care.
The quality of evidence (QoE) was ‘moderate’ for adverse events for the comparison of ‘Nutraceuticals (Probiotics/Prebiotics/Synbiotics) versus placebo for COVID-19’. The QoE is downgraded by one level as the sample size is less than the Optimal Information Size (OIS) ().
Table 3. Summary of findings table for comparison: Nutraceuticals (Probiotics/Prebiotics/Synbiotics) verses placebo.
Excluded studies
A total of 35 trials were excluded from this review. A total of 13 trials did not include participants of interest, and 17 studies had study designs other than RCTs, quasi-experiments, trials, pre-post studies, and other experimental study designs, and in five studies, outcomes not of interest were reported. We presented the list of excluded studies in .
Table 4. Characteristics of excluded studies.
Characteristics of ongoing studies
We came across 29 ongoing trials on nutraceuticals (probiotics, prebiotics, or synbiotics) for COVID-19. Recruitment status is completed in 9 trials, Recruiting in 12 trials, and not yet recruiting in 6 trials. Two trials are currently suspended. The trials are being conducted in Mexico (n = 1), Pakistan (n = 1), Canada (n = 3), Ukraine (n = 1), Japan (n = 1) India (n = 1), Austria (n = 1), Belgium (n = 1), China (n = 1), Italy (n = 1), New Zealand (n = 1), Iran (n = 3), Spain (n = 4), United States (n = 3), United Kingdom (n = 2), Indonesia (n = 1), Austria (n = 1), Hong Kong (n = 2). We documented the list of ongoing studies in : Characteristics of ongoing studies.
Discussion
We identified 481 potential studies from different electronic databases. Despite undertaking a comprehensive search strategy, only five trials with 860 participants were included in this review. Participants included both adult males and females. In three trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023), probiotics were used as an adjuvant treatment to the standard care, and in other two trials (Gutiérrez-Castrellón et al., Citation2022; Rathi et al., Citation2021), probiotics (with or without prebiotics) were given as a supplement or as an adjuvant to the immunotherapy compared with placebo containing maltodextrin. One trial (Gutiérrez-Castrellón et al., Citation2022) was at ‘low risk’ of bias, whereas the other four trials (Araimo et al., Citation2021; Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023) were at ‘some concerns’ for risk of bias. The total number of participants included in the five trials was 860. None of the included studies reported the number of cases of COVID-19 and changes in the disease severity. The outcome measures were mainly focused on the number of deaths (Araimo et al., Citation2021; Ivashkin et al., Citation2023), adverse events (Araimo et al., Citation2021; Gutiérrez-Castrellón et al., Citation2022), days of hospitalization (Ivashkin et al., Citation2023; Reino-Gelardo et al., Citation2023) and fatigue (Rathi et al., Citation2021). There is limited evidence available on using nutraceuticals (probiotics/prebiotics/synbiotics) for the treatment and prevention of COVID-19, as the observed effect in minimizing deaths and the duration of hospitalization in COVID-19 patients was uncertain. Therefore, there is presently no evidence to support the use of nutraceuticals (probiotics, prebiotics, or synbiotics) in the treatment of COVID-19.
Additionally, there was no evidence that using nutraceuticals would cause more negative side effects. There are currently 29 ongoing trials () that can change the findings in the update of this review. Several uncertainties persist regarding using nutraceuticals for the prevention and treatment of COVID-19. These include the following: a small number of published studies with relatively few participants, no data on the efficacy of nutraceuticals in the prevention of COVID-19, and limited data on the number of deaths, adverse events, and days of hospitalization.
Table 5. Characteristics of ongoing studies.
Overall completeness and applicability of evidence
Some issues impede the completeness and applicability of the evidence in this review. The number of studies and participants included are limited. Studies included only adults. The findings of this review apply only to currently prescribed probiotic strains such as Lactobacillus, Bifidobacteria and Streptococcus species as a single strain or in probiotic mixtures and at different doses, frequencies, and durations. No studies were reported on non‐lactic acid bacteria. Prebiotics that were included in this review are fructooligosaccharides and maltodextrin (Gutiérrez-Castrellón et al., Citation2022; Reino-Gelardo et al., Citation2023). The lack of evidence regarding the efficacy of probiotics, prebiotics or synbiotics in preventing COVID-19 and changing the severity of COVID-19 (the primary outcomes for this review) is an issue with the completeness of the evidence.
There is insufficient evidence to confirm whether nutraceuticals (probiotics, prebiotics or synbiotics) can provide a therapeutic advantage in COVID-19 patients. Hence, it is hard to draw a definitive conclusion about their effects on the prevention and treatment of COVID-19.
Quality of the evidence
The quality of evidence (QoE) was ‘low’ for a number of deaths and adverse events, and ‘very low’ for Days of hospitalization for the comparison ‘Standard care + Nutraceuticals (Probiotics/Prebiotics/Synbiotics) compared to Standard care for Covid-19’. The QoE was downgraded by two for the risk of bias for some concerns in Domain 1 and Domain 2 of RoB2 Tool. The QoE was downgraded as the studies did not report the method of randomization and were open-labeled RCTs and also sample size was below the Optimal Information Size (OIS). The QoE was downgraded by one level due to the presence of heterogeneity (I2=73%) (). The quality of evidence (QoE) was ‘moderate’ for adverse events for the comparison of ‘Nutraceuticals (Probiotics/Prebiotics/Synbiotics) versus placebo for COVID-19’. The QoE was downgraded by one level as the sample size is less than the Optimal Information Size (OIS) ().
Potential biases in the review process
We conducted comprehensive searches to identify all the relevant studies. However, some trials may have been missed. We did not search the grey literature, thereby missing any conference proceedings/abstracts presented during the rapidly evolving pandemic situation. Three of the included trials were open-label, thus affecting the quality of the evidence. To avoid bias during the review process, two authors independently undertook screening for the selection of studies, extraction of data, and assessment of the risk of bias in the included studies. Additionally, disagreements amongst these reviewers were resolved initially through consensus and a third reviewer. None of the reviewers was involved in any of the included trials. As only five studies were analyzed in this review, it was impossible to detect the publication bias. Also, the power to identify the overall effect estimate pattern was inadequate, so a chance of coincidental findings cannot be omitted. There were no other potential biases.
Agreements and disagreements with other studies or reviews
No previous systematic review investigated the use of nutraceuticals (Probiotics/Prebiotics/Synbiotics) for COVID-19.
Conclusions
There is limited evidence available on using nutraceuticals (Probiotics/Prebiotics/Synbiotics) for the treatment and prevention of COVID-19, as the observed effect in minimizing deaths and the duration of hospitalization in COVID-19 patients was uncertain. In addition, there was no evidence of increased adverse effects with nutraceuticals use. Therefore, for the treatment of COVID-19, the use of nutraceuticals is uncertain, and it should not be considered conclusive for any implication for practice. Future studies should report/measure outcomes such as the number of cases of COVID-19 and the change in disease severity. Researchers should also consider standardizing doses/concentrations of nutraceuticals given. Methodologically robust randomized controlled trials needed to be undertaken in large samples of populations so that evaluating their therapeutic potential gives us quality evidence for their efficacy and safety in clinical practice.
Authors’ contributions
Concept and ideation: APS; Protocol development: APS and MNK; Literature search: AG and SZQ; Screening and selection of studies: SU and MW; Data extraction: SU and MW; Assessment of risk of bias of included studies: APS and MNK; Meta-analysis: AG; Assessment of Quality of Evidence: SZQ; Overall preparation of the manuscript: APS, MNK and SU with inputs from all the other reviewers.
Differences between protocol and review
In the protocol, we had planned to assess the risk of bias for RCTs using Cochrane’s Risk of Bias Tool. However, we used the RoB 2 tool, the revised tool for assessing the risk of bias in randomized trials (Sterne et al., Citation2019). We had not kept ‘Comparison 3: Nutraceuticals (Probiotics or prebiotics or synbiotics) as an adjunct to standard treatments verses placebo’ as a Comparison in the protocol. However, one trial (Rathi et al., Citation2021) reported a ‘Free of fatigue’ outcome for this comparison group. We felt that readers might be interested in understanding the efficacy of probiotics in reducing fatigue. Hence, we have added ‘Free of fatigue’’ as a secondary outcome under this comparison group.
Appendices tables and search strategies.docx
Download MS Word (25.1 KB)Acknowledgement
Dr. Rekha Sinha, International Life Sciences of India (ILSI).
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Akbarzadeh, A., Taheri, M., Ebrahimi, B., Alirezaei, P., Doosti-Irani, A., Soleimani, M., & Nouri, F. (2022). Evaluation of Lactocare® Synbiotic administration on the serum electrolytes and trace elements levels in psoriasis patients: A randomized, double-blind, placebo-controlled clinical trial Study. Biological Trace Element Research, 200(10), 1–20. https://doi.org/10.1007/s12011-021-03020-6
- Akour, A. (2020). Probiotics and COVID‐19: is there any link? Letters in Applied Microbiology, 71(3), 229–234. https://doi.org/10.1111/lam.13334
- Angurana, S. K., & Bansal, A. (2021). Probiotics and Coronavirus disease 2019: Think about the link. The British Journal of Nutrition, 126(10), 1564–1570. https://doi.org/10.1017/S000711452000361X
- Aponte, M., Murru, N., & Shoukat, M. (2020). Therapeutic, prophylactic, and functional use of probiotics: A current perspective. Frontiers in Microbiology, 11, 562048. https://doi.org/10.3389/fmicb.2020.562048
- Araimo, F., Imperiale, C., Tordiglione, P., Ceccarelli, G., Borrazzo, C., Alessandri, F., Santinelli, L., Innocenti, G. P., Pinacchio, C., Mauro, V., Recchia, G. E., Zancla, S., Calò, A., Poscia, R., Ruberto, F., d’Ettorre, G., Bilotta, F., Mastroianni, C., & Pugliese, F. (2021). Ozone as adjuvant support in the treatment of COVID‐19: A preliminary report of probiozovid trial. Journal of Medical Virology, 93(4), 2210–2220. https://doi.org/10.1002/jmv.26636
- Baindara, P., Chakraborty, R., Holliday, Z. M., Mandal, S. M., & Schrum, A. G. (2021). Oral probiotics in coronavirus disease 2019: Connecting the gut–lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes and New Infections, 40, 100837. https://doi.org/10.1016/j.nmni.2021.100837
- Ballan, R., Battistini, C., Xavier-Santos, D., & Saad, S. M. I. (2020). Interactions of probiotics and prebiotics with the gut microbiota. In: Progress in Molecular Biology and Translational Science. [Internet] (pp. 265–300). Elsevier. [cited 2023 Dec 23]. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1877117320300430
- Bangar, S., Sonar, P., Mane, A., Sane, S., Kadam, A., Katendra, T. L., Rahane, G., Sinha, A., & Sahay, S. (2023). Prevention of recurrence of bacterial vaginosis using lactobacilli-containing vaginal tablets among women with HIV: A randomized, placebo-controlled, double-blinded phase IV trial. International Journal of Infectious Diseases: IJID: official Publication of the International Society for Infectious Diseases, 129, 197–204. https://doi.org/10.1016/j.ijid.2023.02.003
- Bermudez-Brito, M., Plaza-Díaz, J., Muñoz-Quezada, S., Gómez-Llorente, C., & Gil, A. (2012). Probiotic Mechanisms of Action. Annals of Nutrition & Metabolism, 61(2), 160–174. https://doi.org/10.1159/000342079
- Blackett, J. W., Sun, Y., Purpura, L., Margolis, K. G., Elkind, M. S. V., O’Byrne, S., Wainberg, M., Abrams, J. A., Wang, H. H., Chang, L., & Freedberg, D. E. (2022). Decreased gut microbiome tryptophan metabolism and serotonergic signaling in patients with persistent mental health and gastrointestinal symptoms After COVID-19. Clinical and Translational Gastroenterology, 13(10), e00524. https://doi.org/10.14309/ctg.0000000000000524
- Covidence systematic review software. (2019). Covidence systematic review software. Veritas Health Innovation [Internet]. Available from: www.covidence.org
- Darbandi, A., Asadi, A., Ghanavati, R., Afifirad, R., Darb Emamie, A., Kakanj, M., & Talebi, M. (2021). The effect of probiotics on respiratory tract infection with special emphasis on COVID-19: Systemic review 2010–20. International Journal of Infectious Diseases: IJID: official Publication of the International Society for Infectious Diseases, 105, 91–104. https://doi.org/10.1016/j.ijid.2021.02.011
- De Boeck, I., Cauwenberghs, E., Spacova, I., Gehrmann, T., Eilers, T., Delanghe, L., Wittouck, S., Bron, P. A., Henkens, T., Gamgami, I., Simons, A., Claes, I., Mariën, J., Ariën, K. K., Bakokimi, D., Loens, K., Jacobs, K., Ieven, M., Bruijning-Verhagen, P., … Lebeer, S. (2022). Randomized, double-blind, placebo-controlled trial of a throat spray with selected Lactobacilli in COVID-19 outpatients. Pride DT, editor. Microbiology Spectrum, 10(5), e01682-22. https://doi.org/10.1128/spectrum.01682-22
- Dhar, D. (2021). Impending mental health issues during coronavirus disease 2019—time for personalized nutrition based on the gut microbiota to tide over the crisis? Frontiers in Neuroscience, 15, 831193. https://doi.org/10.3389/fnins.2021.831193
- Ewald, D. R., & Sumner, S. C. J. (2018). Human microbiota, blood group antigens, and disease. Wiley Interdisciplinary Reviews. Systems Biology and Medicine, 10(3), e1413. https://doi.org/10.1002/wsbm.1413
- Fernández-Ferreiro, A., Formigo-Couceiro, F. J., Veiga-Gutierrez, R., Maldonado-Lobón, J. A., Hermida-Cao, A. M., Rodriguez, C., Bañuelos, O., Olivares, M., & Blanco-Rojo, R. (2022). Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the immune response of elderly subjects to COVID-19 vaccination: A randomized controlled trial. Nutrients, 14(1), 228. https://doi.org/10.3390/nu14010228
- Forsgård, R. A., Rode, J., Lobenius-Palmér, K., Kamm, A., Patil, S., Tacken, M. G. J., Lentjes, M. A. H., Axelsson, J., Grompone, G., Montgomery, S., & Brummer, R. J. (2023). Limosilactobacillus reuteri DSM 17938 supplementation and SARS-CoV-2 specific antibody response in healthy adults: a randomized, triple-blinded, placebo-controlled trial. Gut Microbes, 15(1), 2229938. https://doi.org/10.1080/19490976.2023.2229938
- George Kerry, R., Patra, J. K., Gouda, S., Park, Y., Shin, H. S., & Das, G. (2018). Benefaction of probiotics for human health: A review. Journal of Food and Drug Analysis, 26(3), 927–939. https://doi.org/10.1016/j.jfda.2018.01.002
- Giannoni, E., Baud, D., Agri, V. D., Gibson, G. R., & Reid, G. (2020). Probiotics and COVID-19. The Lancet. Gastroenterology & Hepatology, 5(8), 720–721. https://doi.org/10.1016/S2468-1253(20)30195-3
- Gohil, K., Samson, R., Dastager, S., & Dharne, M. (2021). Probiotics in the prophylaxis of COVID-19: something is better than nothing. 3 Biotech, 11(1), 1. https://doi.org/10.1007/s13205-020-02554-1
- Gouda, A. S., Adbelruhman, F. G., Sabbah Alenezi, H., & Mégarbane, B. (2021). Theoretical benefits of yogurt-derived bioactive peptides and probiotics in COVID-19 patients – A narrative review and hypotheses. Saudi Journal of Biological Sciences, 28(10), 5897–5905. https://doi.org/10.1016/j.sjbs.2021.06.046
- GRADEpro GDT. (2021). GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime [Internet]. Available from: https://www.gradepro.org/
- Gutiérrez-Castrellón, P., Gandara-Martí, T., Abreu Y Abreu, A. T., Nieto-Rufino, C. D., López-Orduña, E., Jiménez-Escobar, I., Jiménez-Gutiérrez, C., López-Velazquez, G., & Espadaler-Mazo, J. (2022). Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes, 14(1), 2018899. https://doi.org/10.1080/19490976.2021.2018899
- Hasslöf, P., Granqvist, L., Stecksén-Blicks, C., & Twetman, S. (2022). Prevention of recurrent childhood caries with probiotic supplements: a randomized controlled trial with a 12-Month Follow-Up. Probiotics and Antimicrobial Proteins, 14(2), 384–390. https://doi.org/10.1007/s12602-022-09913-9
- He, L. H., Ren, L. F., Li, J. F., Wu, Y. N., Li, X., & Zhang, L. (2020). Intestinal flora as a potential strategy to fight SARS-CoV-2 infection. Frontiers in Microbiology, 11, 1388. https://doi.org/10.3389/fmicb.2020.01388
- Heidari, Z., Tajbakhsh, A., Gheibihayat, S. M., Moattari, A., Razban, V., Berenjian, A., Savardashtaki, A., & Negahdaripour, M. (2021). Probiotics/prebiotics in viral respiratory Infections: Implication for emerging pathogens. Recent Patents on Biotechnology, 15(2), 112–136. https://doi.org/10.2174/1872208315666210419103742
- Higgins, J., & Green, S. (2011). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration [Internet]. Available from http://handbook.cochrane.org
- Higgins, J., Chandler, J., Cumpston, M., Li, T., Page, M., & Welch, V. (2021). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane 2021 [Internet]. Available from: www.training.cochrane.org/handbook
- Infusino, F., Marazzato, M., Mancone, M., Fedele, F., Mastroianni, C. M., Severino, P., Ceccarelli, G., Santinelli, L., Cavarretta, E., Marullo, A. G. M., Miraldi, F., Carnevale, R., Nocella, C., Biondi-Zoccai, G., Pagnini, C., Schiavon, S., Pugliese, F., Frati, G., & d’Ettorre, G. (2020). Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: A scoping review. Nutrients, 12(6), 1718. https://doi.org/10.3390/nu12061718
- Ivashkin, V., Fomin, V., Moiseev, S., Brovko, M., Maslennikov, R., Ulyanin, A., Sholomova, V., Vasilyeva, M., Trush, E., Shifrin, O., & Poluektova, E. (2023). Efficacy of a Probiotic Consisting of Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301 in the Treatment of Hospitalized Patients with COVID-19: a Randomized Controlled Trial. Probiotics and Antimicrobial Proteins, 15(3), 460–468.
- Khaled, J. M. A. (2021). Probiotics, prebiotics, and COVID-19 infection: A review article. Saudi Journal of Biological Sciences, 28(1), 865–869. https://doi.org/10.1016/j.sjbs.2020.11.025
- Kullar, R., Johnson, S., McFarland, L. V., & Goldstein, E. J. C. (2021). Potential roles for probiotics in the treatment of COVID-19 patients and prevention of complications associated with increased antibiotic use. Antibiotics, 10(4), 408. https://doi.org/10.3390/antibiotics10040408
- Leal-Martínez, F., Abarca-Bernal, L., García-Pérez, A., González-Tolosa, D., Cruz-Cázares, G., Montell-García, M., & Ibarra, A. (2022). Effect of a nutritional support system to increase survival and reduce mortality in patients with COVID-19 in stage III and comorbidities: A blinded randomized controlled clinical trial. International Journal of Environmental Research and Public Health, 19(3), 1172. https://doi.org/10.3390/ijerph19031172
- Li, Q., Cheng, F., Xu, Q., Su, Y., Cai, X., Zeng, F., & Zhang, Y. (2021). The role of probiotics in coronavirus disease-19 infection in Wuhan: A retrospective study of 311 severe patients. International Immunopharmacology, 95, 107531. https://doi.org/10.1016/j.intimp.2021.107531
- Louca, P., Murray, B., Klaser, K., Graham, M. S., Mazidi, M., Leeming, E. R., Thompson, E., Bowyer, R., Drew, D. A., Nguyen, L. H., Merino, J., Gomez, M., Mompeo, O., Costeira, R., Sudre, C. H., Gibson, R., Steves, C. J., Wolf, J., Franks, P. W., … Menni, C. (2021). Modest effects of dietary supplements during the COVID-19 pandemic: insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutrition, Prevention & Health, 4(1), 149–157. https://doi.org/10.1136/bmjnph-2021-000250
- Mak, J. W. Y., Chan, F. K. L., & Ng, S. C. (2020). Probiotics and COVID-19: one size does not fit all. The Lancet. Gastroenterology & Hepatology, 5(7), 644–645. https://doi.org/10.1016/S2468-1253(20)30122-9
- Maldonado Galdeano, C., Cazorla, S. I., Lemme Dumit, J. M., Vélez, E., & Perdigón, G. (2019). Beneficial effects of probiotic consumption on the immune system. Annals of Nutrition & Metabolism, 74(2), 115–124. https://doi.org/10.1159/000496426
- Markowiak, P., & Śliżewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 9(9), 1021. https://doi.org/10.3390/nu9091021
- McCarty, M. F., & DiNicolantonio, J. J. (2020). May Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Progress in Cardiovascular Diseases, 63(3), 383–385. https://doi.org/10.1016/j.pcad.2020.02.007
- Morais, A. H. A., Passos, T. S., Maciel, B. L. L., & da Silva-Maia, J. K. (2020). Can Probiotics and Diet Promote Beneficial Immune Modulation and Purine Control in Coronavirus Infection? Nutrients, 12(6), 1737. https://doi.org/10.3390/nu12061737
- Nazir, Y., Hussain, S. A., Abdul Hamid, A., & Song, Y. (2018). Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. BioMed Research International, 2018, 3428417–3428437. https://doi.org/10.1155/2018/3428437
- Nistor-Cseppento, C. D., Moga, T. D., Bungau, A. F., Tit, D. M., Negrut, N., Pasca, B., Bochis, C. F., Ghitea, T. C., Jurcau, A., Purza, A. L., & Uivarosan, D. (2022). The contribution of diet therapy and probiotics in the treatment of sarcopenia induced by prolonged immobilization caused by the COVID-19 Pandemic. Nutrients, 14(21), 4701. https://doi.org/10.3390/nu14214701
- Nobile, V., & Puoci, F. (2023). Effect of a multi-strain probiotic supplementation to manage stress during the COVID-19 pandemic: A randomized, double-blind, placebo-controlled, cross-over clinical trial. Neuropsychobiology, 82(2), 61–71. https://doi.org/10.1159/000527956
- Olaimat, A. N., Aolymat, I., Al-Holy, M., Ayyash, M., Abu Ghoush, M., Al-Nabulsi, A. A., Osaili, T., Apostolopoulos, V., Liu, S.-Q., & Shah, N. P. (2020). The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Science of Food, 4(1), 17. https://doi.org/10.1038/s41538-020-00078-9
- Paknahad, Z., & Moravejolahkami, A. R. (2021). Probiotics against viruses; COVID-19 is a paper tiger: A systematic review. Endocrine, Metabolic & Immune Disorders Drug Targets, 21(7), 1252–1260. https://doi.org/10.2174/1871530320666200917114033
- Pandey, M., Bhati, A., Priya, K., Sharma, K. K., & Singhal, B. (2022). Precision postbiotics and mental health: the management of post-COVID-19 complications. Probiotics and Antimicrobial Proteins, 14(3), 426–448. https://doi.org/10.1007/s12602-021-09875-4
- Patra, S., Saxena, S., Sahu, N., Pradhan, B., & Roychowdhury, A. (2021). Systematic network and meta-analysis on the antiviral mechanisms of probiotics: A preventive and treatment strategy to mitigate sars-Cov-2 infection. Probiotics and Antimicrobial Proteins, 13(4), 1138–1156. https://doi.org/10.1007/s12602-021-09748-w
- Peng, J., Zhang, M., Yao, G., Kwok, L. Y., & Zhang, W. (2021). Probiotics as adjunctive treatment for patients contracted COVID-19: Current understanding and future needs. Frontiers in Nutrition, 8, 669808. https://doi.org/10.3389/fnut.2021.669808
- Plaza-Diaz, J., Ruiz-Ojeda, F. J., Gil-Campos, M., & Gil, A. (2019). Mechanisms of action of probiotics. Advances in Nutrition (Bethesda, Md.), 10(suppl_1), S49–S66. https://doi.org/10.1093/advances/nmy063
- Rathi, A., Jadhav, S. B., & Shah, N. (2021). A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue. Medicines (Basel, Switzerland), 8(9), 47. https://doi.org/10.3390/medicines8090047
- Rayyan, Y. M., Agraib, L. M., Alkhatib, B., Yamani, M. I., Abu-Sneineh, A. T., & Tayyem, R. F. (2023). Does probiotic supplementation improve quality of life in mild-to-moderately active ulcerative colitis patients in Jordan? A secondary outcome of the randomized, double-blind, placebo-controlled study. European Journal of Nutrition, 62(7), 3069–3077. https://doi.org/10.1007/s00394-023-03207-8
- Reino-Gelardo, S., Palop-Cervera, M., Aparisi-Valero, N., Espinosa-San Miguel, I., Lozano-Rodríguez, N., Llop-Furquet, G., Sanchis-Artero, L., Cortés-Castell, E., Rizo-Baeza, M., & Cortés-Rizo, X. (2023). Effect of an immune-boosting, antioxidant and anti-inflammatory food supplement in hospitalized COVID-19 patients: A prospective randomized pilot study. Nutrients, 15(7), 1736. https://doi.org/10.3390/nu15071736
- Review Manager. (2014). The Cochrane Collaboration. Copenhagen: The Nordic Cochrane Centre. [Internet] Available from: https://community.cochrane.org/sites/default/files/uploads/inline-files/RevMan_5.3_User_Guide.pdf
- Santinelli, L., Laghi, L., Innocenti, G. P., Pinacchio, C., Vassalini, P., Celani, L., Lazzaro, A., Borrazzo, C., Marazzato, M., Tarsitani, L., Koukopoulos, A. E., Mastroianni, C. M., d’Ettorre, G., & Ceccarelli, G. (2021). Oral bacteriotherapy reduces the occurrence of chronic fatigue in COVID-19 patients. Frontiers in Nutrition, 8, 756177. https://doi.org/10.3389/fnut.2021.756177
- Sevillano-Jiménez, A., Romero-Saldaña, M., García-Mellado, J. A., Carrascal-Laso, L., García-Rodríguez, M., Molina-Luque, R., & Molina-Recio, G. (2022). Impact of high prebiotic and probiotic dietary education in the SARS-CoV-2 era: improved cardio-metabolic profile in schizophrenia spectrum disorders. BMC Psychiatry, 22(1), 781. https://doi.org/10.1186/s12888-022-04426-9
- Shahbazi, R., Yasavoli-Sharahi, H., Alsadi, N., Ismail, N., & Matar, C. (2020). Probiotics in treatment of viral respiratory infections and neuroinflammatory disorders. Molecules (Basel, Switzerland), 25(21), 4891. https://doi.org/10.3390/molecules25214891
- Slykerman, R. F., & Li, E. (2022). A randomized trial of probiotic supplementation in nurses to reduce stress and viral illness. Scientific Reports, 12(1), 14742. https://doi.org/10.1038/s41598-022-19104-9
- Slykerman, R. F., Li, E., & Mitchell, E. A. (2022). Probiotics for reduction of examination stress in students (PRESS) study: A randomized, double-blind, placebo-controlled trial of the probiotic Lacticaseibacillus rhamnosus HN001. PLoS One, 17(6), e0267778. editor https://doi.org/10.1371/journal.pone.0267778
- Stavropoulou, E., & Bezirtzoglou, E. (2020). Probiotics in medicine: A long debate. Frontiers in Immunology, 11, 2192. https://doi.org/10.3389/fimmu.2020.02192
- Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., Cates, C. J., Cheng, H.-Y., Corbett, M. S., Eldridge, S. M., Emberson, J. R., Hernán, M. A., Hopewell, S., Hróbjartsson, A., Junqueira, D. R., Jüni, P., Kirkham, J. J., Lasserson, T., Li, T., … Higgins, J. P. T. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed.), 366, l4898. https://doi.org/10.1136/bmj.l4898
- Sundararaman, A., Ray, M., Ravindra, P. V., & Halami, P. M. (2020). Role of probiotics to combat viral infections with emphasis on COVID-19. Applied Microbiology and Biotechnology, 104(19), 8089–8104. https://doi.org/10.1007/s00253-020-10832-4
- Taghinezhad-S, S., Mohseni, A. H., Bermúdez-Humarán, L. G., Casolaro, V., Cortes-Perez, N. G., Keyvani, H., & Simal-Gandara, J. (2021). Probiotic-based vaccines may provide effective protection against COVID-19 acute respiratory disease. Vaccines, 9(5), 466. https://doi.org/10.3390/vaccines9050466
- Tateno, M., Stone, B. J., Srodulski, S. J., Reedy, S., Gawriluk, T. R., Chambers, T. M., Woodward, J., Chappell, J., & Kempinski, C. F. (2020). Synthetic biology-derived triterpenes as efficacious immunomodulating adjuvants. Scientific Reports, 10(1), 17090. https://doi.org/10.1038/s41598-020-73868-6
- Vaezi, M., Ravanshad, S., Akbari Rad, M., Zarrinfar, H., & Kabiri, M. (2023). The effect of synbiotic adjunct therapy on clinical and paraclinical outcomes in hospitalized COVID‐19 patients: A randomized placebo‐controlled trial. Journal of Medical Virology, 95(2), e28463. https://doi.org/10.1002/jmv.28463
- Vázquez-Frias, R., Consuelo-Sánchez, A., Acosta-Rodríguez-Bueno, C. P., Blanco-Montero, A., Robles, D. C., Cohen, V., Márquez, D., & Perez, M. (2023). Efficacy and safety of the adjuvant use of probiotic Bacillus clausii strains in pediatric irritable bowel syndrome: A randomized, double-blind, Placebo-Controlled Study. Paediatric Drugs, 25(1), 115–126. https://doi.org/10.1007/s40272-022-00536-9
- Wong, M. C. S., Zhang, L., Ching, J. Y. L., Mak, J. W. Y., Huang, J., Wang, S., Mok, C. K. P., Wong, A., Chiu, O.-L., Fung, Y.-T., Cheong, P.-K., Tun, H.-M., Ng, S. C., & Chan, F. K. L. (2023). Effects of gut microbiome modulation on reducing adverse health outcomes among elderly and diabetes patients during the COVID-19 Pandemic: A Randomised, Double-Blind, Placebo-Controlled Trial (IMPACT Study). Nutrients, 15(8), 1982. https://doi.org/10.3390/nu15081982
Appendices
Appendix 1
Search strategy for PubMed
The search strategy for PubMed: ((‘probiotic*’[Title/Abstract] OR ‘pro biotic*’[Title/Abstract] OR ‘prebiotic*’[Title/Abstract] OR ‘pre biotic*’[Title/Abstract] OR ‘synbiotic*’[Title/Abstract] OR ‘lactobacill*’[Title/Abstract] OR ‘lacto bacill*’[Title/Abstract] OR ‘bifidobacteri*’[Title/Abstract] OR ‘bifidus*’[Title/Abstract] OR ‘streptococcus thermophil*’[Title/Abstract] OR ‘saccharomyce*’[Title/Abstract] OR ‘probiotics’[MeSH Terms] OR ‘lactobacillus’[MeSH Terms] OR ‘bifidobacterium’[MeSH Terms] OR ‘streptococcus thermophilus’[MeSH Terms] OR ‘saccharomyces’[MeSH Terms]) AND (‘covid*’[Title/Abstract] OR ‘corona-virus’[Title/Abstract] OR ‘corona-virus’[Title/Abstract] OR ‘cov’[Title/Abstract] OR ‘ncov*’[Title/Abstract] OR ‘n cov*’[Title/Abstract] OR ‘sars*’[Title/Abstract] OR ‘covid*’[Title/Abstract] OR ‘covid 19’[MeSH Terms])) AND (2019:2023[pdat])
Search Strategy for CENTRAL
CENTRAL via Cochrane Library, issue 9, 2023
ID Search
#1 covid*
#2 corona*
#3 n-cov*
#4 ncov*
#5 sars*
#6 MeSH descriptor: [COVID-19] explode all trees
#7 #1 OR #2 OR #3 OR #4 OR #5 OR #6
#8 probiotic*
#9 ‘pro biotic*’
#10 pro-biotic*
#11 prebiotic*
#12 ‘pre biotic*’
#13 pre-biotic*
#14 synbiotic*
#15 lactobacill*
#16 bifidobacteri*
#17 bifidus*
#18 ‘streptococcus thermophil*’
#19 saccharomyce*
#20 MeSH descriptor: [Probiotics] explode all trees
#21 MeSH descriptor: [Prebiotics] explode all trees
#22 MeSH descriptor: [Synbiotics] explode all trees
#23 MeSH descriptor: [Lactobacillus] explode all trees
#24 MeSH descriptor: [Bifidobacterium] explode all trees
#25 MeSH descriptor: [Streptococcus thermophilus] explode all trees
#26 MeSH descriptor: [Saccharomyces] explode all trees
#27 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26
#28 #7 AND #27
Search Strategy for WHO Covid-19 Databasetw:(probiotic* OR prebiotic* OR synbiotic* OR lactobacill* OR bifidobacteri* OR bifidus* OR ‘streptococcus thermophil*’ OR saccharomyce*) AND type_of_study:(‘clinical_trials’) AND la:(‘en’)
Appendix 2
Summary of results of dichotomous and continuous outcomes reported in individual studies
A2 Table 1: Summary of results of dichotomous outcomes reported in individual studies.
A2 Table 2: Summary of results of continuous outcomes reported in individual studies.