ABSTRACT
Autophagy has been associated with responses to chemotherapies in several types of cancer, highlighting its contribution to the development of resistance to treatments. Breast cancer (BC) is one of the most common tumors and is known for its ability to develop resistance to treatments. Doxorubicin (DXR) is a drug commonly used in BC and known to damage mitochondria. Thus, we thought to investigate if DXR treatment induces mitophagy, a selective form of autophagy specifically degrading mitochondria, in BC cells. By performing a global analysis of mitophagy-associated genes, we found a relationship between their expressions and DXR treatment. We revealed that PINK1/PARKIN-mediated mitophagy is induced following DXR treatment in different cellular BC models, such as the luminal subtype A cell line MCF7 and in the triple-negative BC cell line MDA-MB-231. By Interfering the E3 ubiquitin ligase PARKIN using miR-218-5p, we showed the efficacy in specifically targeting mitophagy in the treatment of BC. Indeed, PARKIN depletion improves cancer cells sensitivity to DXR treatment in vitro, in both stem-like and non-stem-like BC cells. Our approach, which combines two tumoricidal methods, mitophagy inhibition and chemotherapy, could therefore represent a new strategy for BC treatment.
Abbreviations: BC: breast cancer; DXR: doxorubicin; MFN1: mitofusin 1; MFN2: mitofusin 2; miRNA: micro RNA; NPs: nanoparticles; OMM: outer mitochondrial membrane; PINK1: PTEN induced kinase 1; SPATA18: spermatogenesis Associated 18; TBNC: triple negative breast cancer.
Breast cancer (BC) is the most frequently diagnosed cancer in women and its treatment remains challenging due to drug resistance. It is thus crucial to discover new players to provide new therapies for durable remission in patients. Several reports suggested that mitophagy, a selective form of autophagy selectively eliminating damaged mitochondria, contributes to drug response and resistance in several tumours such as melanoma, glioma and colorectal cancer. In the context of BC, Liensinie, an isoquinoline alkaloid, sensitizes BC cells to doxorubicin (DXR) chemotherapy by causing an accumulation autophagosomes/mitophagosomes, suggesting a putative block of autophagy/mitophagy by this agent. However, the relevance of mitophagy modulation in BC treatment remains largely unclear and this is due in part to the fact that specific inhibitors of this selective type of autophagy are lacking. One of the main stress-induced mitophagy pathways described to date is governed by the serine-threonine kinase PINK1 (PTEN induced kinase 1) and the E3 Ubiquitin ligase PARKIN. After mitochondrial depolarization, PINK1 accumulates in the outer mitochondrial membrane (OMM), and this in turn allows PINK1-dependent recruitment of PARKIN and activation of its E3 ubiquitin ligase activity, which is followed by the ubiquitination of different OMM proteins. Ubiquitination induces the recruitment of selective autophagy receptors, which recruit the autophagy machinery and mediate the sequestration of mitochondria into nascent autophagosomes to deliver them to lysosomes for turnover. In 2020, we discovered that miR-218-5p, a small non-coding RNA, is a powerful inhibitor of PINK1/PARKIN-mediated mitophagy in mammalian cells, by reducing PARKIN mRNA and protein levels and therefore affecting its E3 ubiquitin ligase activity. Following mitochondrial stress such as the combined treatment with oligomycin and antimycin, miR-218-5p expression is sufficient to reduce ubiquitynation of mitochondria and their clearance by autophagy. Interestingly, miR-218-5p expression has been found to be reduced in BC cells and its expression favours cell death of BC cells. However, its role as a putative inhibitor of mitophagy in cancer cells has not been investigated yet.
To examine whether DXR, which is known to affect mitochondria by binding cardiolipin and interfering with the electron transport chain, induces mitophagy in BC cells, we first performed a microarray analysis that allowed finding that genes involved in PINK1/PARKIN-mediated mitophagy are up-regulated after DXR treatment. In parallel, by performing a bioinformatical analysis of public datasets of gene expression microarray experiments performed in MCF7 cells treated with DXR or resistant to DXR, we found that the expression levels of some autophagy/mitophagy genes are altered in response to DXR treatment. Interestingly, the mRNA encoding for SPAT18 (spermatogenesis-associated 18 protein) was found to be strongly upregulated, supporting the intriguing idea that different pathways might be activated depending on the degree of severity of the mitochondrial injury. Indeed, SPATA18, which mediates a particular quality control of mitochondrial components, was found to be upregulated early after DXR administration, compared to our condition in which we performed prolonged acute treatment with DXR. This latter treatment induces a mitophagy that is accompanied by upregulation of both PINK1 and PARKIN, and a downregulation of both MFN1 and MFN2.
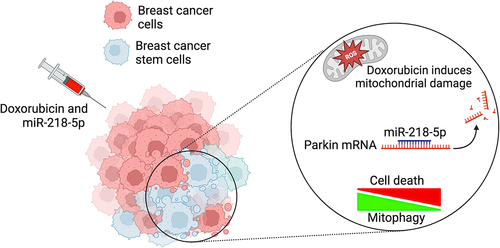
We next showed, through confocal microscopy, western-blot analysis and mitochondrial DNA content analysis, that DXR acts as an inducer of PINK1/PARKIN-mediated mitophagy in BC cells. This finding gave us the idea to test whether targeting mitophagy miR-218-5p could improve the efficacy of DXR. We indeed found that miR-218-5p re-expression is capable of blocking DXR-induced mitophagy in our BC models. It is interesting to note that miR-218-5p-mediated inhibition of mitophagy was effective in MCF7 cells but also in MDA-MB-231 cells, a more aggressive type of BC cells, as well as in mammosphere that mimic the “cancer stem-like cell” population. These observations suggest that this combinatorial approach could be suitable independently from the sub-type of the tumour. Altogether, these results highlight PARKIN and possibly other factors of the same mitophagy pathway, as an interesting target in BC in response to DXR treatment, despite its low expression levels in these cells ().
Figure 1. Co-administration of miR-218-5p improves the efficacy of the chemotherapeutic drug DXR in stem and non-stem breast cancer cells. DXR treatment induces production of ROS and the mitophagic pathway mediated by PINK1 and PARKIN in BC cells. miR-218-5p, by inhibiting this mitophagy through PARKIN depletion, favours cytotoxicity of DXR. Created with BioRender.com.
A number of questions remain unanswered: what is the relationship between PINK1-mediated mitophagy and DXR resistance in BC? Is mitophagy directly involved in the development of the resistance? Which are the consequences of impairing this process in DXR-resistant cells? Can other mitophagy proteins be targeted? We can speculate that an induction of mitophagy in resistant cells might facilitate BC cells to degrade damaged mitochondria and thus to reduce oxidative stress, supporting their survival under the chemotherapeutic treatment. It could also be a way of promoting metabolic rewiring, as described recently in drug-tolerant persistent lung adenocarcinoma cells under MAPK inhibitor treatment. Further work is required to better understand this aspect.
MiRNA-based therapy to treat cancer has attracted a lot of attention for the treatment of malignant tumours. Nanoparticles (NPs), in particular, offer an excellent opportunity for the cancer cell-specific delivery of miRNAs but also chemotherapeutic agents since they are non-toxic and have a high binding capacity for miRNAs. Thus, the next step could be to synthetise miR-218-5p/DXR NPs and to intratumorally inject them in BC mouse models to explore potential in vivo anticancer synergisms of this combinatorial treatment. By using miR-218-5p/DXR NPs, one could target both the tumour and its resident stem cells, which may have the potential to improve patient survival. In conclusion, this study highlighted PINK1/PARKIN-mediated mitophagy as a therapeutic target for improving a standard chemotherapy, enhancing its effects and extending its spectrum of action to different subtypes of BC, which are generally characterised by a high heterogeneity in molecular characteristics and therapeutic response.
Disclosure statement
No potential conflict of interest was reported by the author(s).

