Dear editor
We read with interest the study by Garcia et al, “Common Variables That Influence Sepsis Mortality in Mice”.Citation1 Based on their own experience, authors identified some factors specific to sex and season that significantly influence sepsis mortality in the CLP model, therefore questioning the status of this model as the gold standard in preclinical research on sepsis.
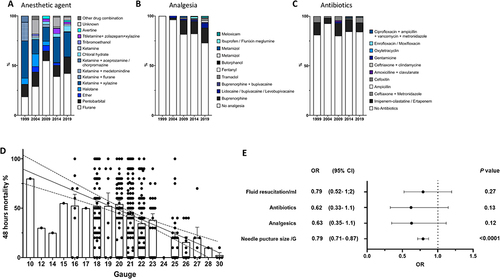
In support of this report, we performed a systematic review of 497 studies using CLP (Medline-indexed studies mentioning CLP for the years 1999, 2004, 2009, 2014, and 2019 arbitrarily) in mice aged 6 to 16 weeks old, to analyzed the surgical technique, the puncture needle size, drugs used in the protocol (class of anesthetic, analgesics, fluids, and antibiotics), and their effect on survival at 48 hours. We observed tremendous variability in procedures and outcomes: Needle size ranged from 10 to 30 gauge (21.04 ±2.8), and 15 different anesthetic regimens (single or multiple agents) were recorded. Analgesics were used in only 19.3% of studies, and their use increased in recent years (). Antibiotics and fluid resuscitation were reported in 15.5%and 62% of the studies respectively (). In multivariate analysis, the puncture needle size was the only factor significantly associated with mortality at 48h ().
Figure 1 Distribution of anesthetic agents (A), analgesic (B), and antibiotics (C) used in CLP studies in mice according to the year of publication. Respectively 16, 65, 71, 169, and 185 were analyzed for the years 1999, 2014, 2009, 2014, and 2019. (D) Puncture needle size negatively correlates with the 48h mortality, (357 studies providing survival data at 48h were analyzed; P<0.001, Slope −3.99). (E) Multivariate regression analysis of factors associated with 48h mortality (logistic regression). The dots represent the odds ratio; the line through each dot represents the 95% confidence interval. Calibration (AUC-ROC): 0.68 (0.63–0.74); P<0.0001. Goodness of fit (Hosmer-Lemeshow statistic): P value = 0.13.
In addition to these potentially standardizable parameters, we advocate that bacterial load burden, intensity and timing of caecum necrosis, occult bleeding, and inter-operator variability are non-standardizable contributors to variability in survival rate (). Indeed, CLP causes three insults to the host: surgical trauma, tissue ischemia and necrosis from the ligated cecum, and polymicrobial sepsis from fecal spillage after needle puncture(s). Subsequently, in the few studies measuring the CFU /mL in blood or peritoneal lavage using the complete spectrum of host enteric bacteria, significant differences are reported (respectively from 10^1 to 2.5*10^8 CFU/mL in the blood and from 10^2 to 10^8 CFU/mL in the peritoneal lavage at 16–24h).Citation2 Furthermore, in the CLP model, bacteria responsible for the infection are derived from the host enteric microbiome. This ensemble includes more than 400 different strains of bacteria, viruses, parasites, and fungi whose abundance, variability, and relative distribution are eminently variable. Moreover, heterogeneous peritoneal stool spillage can lead to localized or diffuse infection. Also, the germs in blood culture are extremely different from a study to another.Citation3 Overall, each step of the model is hardly standardizable and induces a risk of inter- and intra-experiment variability, which ultimately explains the literature’s lack of homogeneity and external validity.
Table 1 CLP Procedure’s Inherent Variability Factors
Finally, although the CLP model is undoubtedly valuable for studying sepsis pathophysiology, we believe it cannot meet the standard of “a rigorous, controlled, and standardized preclinical animal model” for preclinical studies investigating adjuvant therapy for early sepsis.Citation4 Therefore, one could argue that its “gold standard status”Citation5 must be challenged and finding an appropriate and reliable mouse model of sepsis and septic shock, which will lead to more successful research, must be a priority of the research agenda in the field.
Disclosure
Authors have no conflict of interest to disclose.
References
- Garcia LF, Singh V, Mireles B, Dwivedi AK, Walker WE. Common variables that influence sepsis mortality in mice. J Inflamm Res. 2023;16:1121–1134. doi:10.2147/JIR.S400115
- Fay KT, Klingensmith NJ, Chen CW, et al. The gut microbiome alters immunophenotype and survival from sepsis. FASEB J. 2019;33(10):11258–11269. doi:10.1096/fj.201802188R
- Hyde SR, Stith RD, McCallum RE. Mortality and bacteriology of sepsis following cecal ligation and puncture in aged mice. Infect Immun. 1990;58(3):619–624. doi:10.1128/iai.58.3.619-624.1990
- Osuchowski MF, Ayala A, Bahrami S, et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Shock. 2018;50(4):377–380. doi:10.1097/SHK.0000000000001212
- Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19(4):198–208. doi:10.1016/j.tim.2011.01.001
