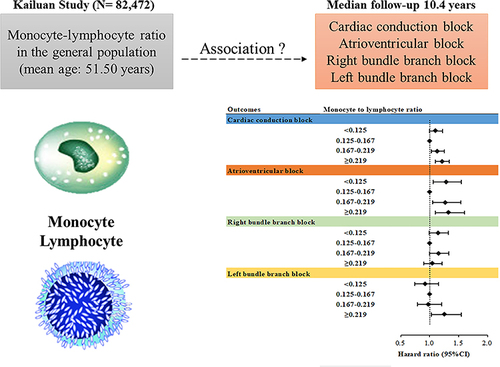
Abstract
Purpose
Inflammation plays a critical role in the development of cardiac conduction block (CCB), which is associated with an increased risk of morbidity and mortality. The monocyte-lymphocyte ratio (MLR) acts as a novel inflammatory marker; however, its association with CCB has not yet been studied. This study aimed to investigate the association between MLR and CCB risk.
Patients and Methods
In total, 82,472 CCB-free participants were identified from the Kailuan study. MLR was calculated using the monocyte count/lymphocyte count. The participants were stratified based on quartiles of MLR levels. Incident CCB and its subtypes were ascertained from electrocardiograms at biennial follow-up visits. The Cox proportional hazards model and restricted cubic spline analysis were used to investigate the association between MLR with CCB and its subtypes.
Results
During a median follow-up of 10.4 years, 3222 incident CCB cases were observed. A U-shaped association was observed between MLR and CCB risk (Pnonlinearity <0.05). After multivariate adjustment, individuals in the highest MLR quartile had a hazard ratio (HR) of 1.212 (95% CI: 1.097-1.340; Q4 vs Q2), while those in the lowest MLR quartile had an HR of 1.106 (95% CI: 1.000-1.224; Q1 vs Q2). Sensitivity and subgroup analyses yielded consistent results. The U-shaped association persisted for atrioventricular block (AVB) in subtype analyses.
Conclusion
MLR was significantly associated with an increased risk of new-onset CCB. Assessing MLR may have clinical relevance for predicting CCB risk, providing valuable insights for preventive strategies and patient management.
Pre-Registered Clinical Trial Number
The pre-registered clinical trial number is ChiCTR-TNC-11001489.
Graphical Abstract
Introduction
Cardiac conduction block (CCB) is characterised by a disease manifested as the delay or interruption in the transmission of an impulse within the conduction system, including the sinoatrial, atrioventricular block (AVB), and bundle branch block (BBB).Citation1 Regardless of the type of conduction block, disease progression in severe cases significantly increases the risk of cardiovascular morbidity and mortality.Citation2–4 Although pacemaker implantation is an effective treatment for end-stage conduction block diseases, it carries the risk of infection and thrombosis and is associated with long-term adverse cardiac remodelling.Citation5–7 Therefore, it is particularly important to identify high-risk individuals with conduction block and implement early measures to avoid illness onset.
The monocyte-lymphocyte ratio (MLR) is a novel inflammatory biomarker that can effectively reflect the balance between inherent and acquired immune responses.Citation8,Citation9 Previous epidemiological evidence suggested that MLR was an independent predictor of all-cause mortality and cardiovascular mortality in the general population.Citation10 Studies have shown that inflammation plays an important role in the initiation of CCB.Citation11,Citation12 However, data on the association of inflammatory biomarkers with CCB are relatively limited. A longitudinal cohort study found that lymphocyte count (LC) showed a U-shaped association with the risk of incident arrhythmias.Citation13 Compared with LC or monocyte count (MC) alone, MLR provides a more stable and accurate evaluation of immune status.Citation9,Citation14 Therefore, we conducted a prospective cohort study to investigate the relationship between MLR and new-onset CCB and its subtypes.
Methods
Study Design and Population
The Kailuan study is an ongoing prospective cohort study that based on a community in Tangshan. The details of this study have been described elsewhere.Citation15,Citation16 In current study, we first enrolled 101,510 individuals (81,110 men and 20,400 women, aged 18–98 years) who participated baseline examination (2006–2007). After excluding participants with missing data pertaining to the MC, LC, or electrocardiogram (ECG) (n=8274), with a history of CCB, myocardial infarction, atrial fibrillation, or heart failure (n=3974), and who did not attend any of the follow-up examinations (n=6790), a total of 82,472 participants (65,164 men and 17,308 women) were included in the current analysis (Supplementary Figure 1). These participants complete face-to-face questionnaire surveys, clinical examinations, laboratory tests and electrocardiograph (ECG) measurement during follow-up period.
This study complied with the Helsinki Declaration and was approved by the ethics committee of Kailuan General Hospital. All participants provided written informed consent prior to inclusion in the study.
Assessment of Incident CCB
CCB diagnoses were based on standard 12-lead ECGs at biennial follow-up visit.Citation17 According to the relevant US AHA guidelines,Citation18 CCB was defined as the presence of any type of heart block, including atrioventricular block (AVB), complete right bundle branch block (CRBBB), incomplete right bundle branch block (iRBBB), complete left bundle branch block (CLBBB), incomplete left bundle branch block (iLBBB), left anterior fascicular block (LAFB), left posterior fascicular block (LPFB), and nonspecific intraventricular conduction block (NS-IVCB). The right bundle branch block (RBBB) included the CRBBB and iRBBB, and the left bundle branch block (LBBB) included the CLBBB, iLBBB, LAFB, and LPFB. Detailed definitions of the endpoints are listed in Supplementary Table 1. The final diagnosis was confirmed by two cardiologists.
All participants were followed up through assessments updated every two years from the baseline examination until the onset of CCB, the date of all-cause mortality, or the last follow-up, whichever came first. The participants were monitored until December 31, 2019, for all outcomes.
Assessment of MLR
During each examination, the participants underwent venous blood collection after fasting for at least 8 hours. Routine blood tests, including MC and LC measurements, were performed using a full blood count analyzer (Sysmex XT-1800i, Sysmex Corporation). The MLR was calculated as MC/LC.
Data Collection and Definitions
Demographic data and lifestyle information, including alcohol intake, smoking status, physical activity, snoring, and medical history, were obtained through face-to-face interviews using a standardised questionnaire, as described in further detail previously.Citation19,Citation20 Anthropometric parameters and blood pressure were measured during biennial physical examinations. The detailed measurement procedures have been described previously.Citation20 Body mass index (BMI) was determined by dividing the body weight (kg) by the square of the height (m2). During the physical examination, blood samples were collected from the antecubital vein following a fasting period of at least 8 hours overnight.
Biochemical parameters, including triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), fasting blood glucose (FBG), and serum creatinine (SCr) levels, were measured using an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan). Levels of high-sensitivity C-reactive protein (hs-CRP) were assessed using an autoanalyzer (Cias Latex CRP‐H, Kanto Chemical, Tokyo, Japan). Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, utilisation of antihypertensive medication, or a self-reported history of hypertension.Citation21 Diabetes was defined as an FBG level ≥7.0 mmol/L, a history of diabetes, or utilisation of antidiabetic medication.Citation22 Hyperlipidaemia was defined as TG level ≥2.3 mmol/L, TC level ≥6.2 mmol/L, LDL-c level ≥4.1 mmol/L, HDL-c level <1.0 mmol/L, having a history of hyperlipidaemia, or usage of any lipid-lowering medication.Citation23 The estimated glomerular filtration rate (eGFR) was defined as eGFR calculated using the CKD-EPI method.Citation24
Statistical Analysis
Baseline characteristics are summarised as mean ± standard deviation (SD), median (25th percentile, 75th percentile), or frequency (percentages), as appropriate. Variances between groups were evaluated using one-way analysis of variance or nonparametric tests for continuous variables and chi-square tests for categorical variables. CCB probabilities were estimated using the Kaplan-Meier method and compared using the Log rank test. Cox proportional hazard regression models were used to assess the association between MLR levels and the risk of CCB and its subtypes by calculating the hazard ratio (HR) and corresponding CI (95%). The MLR quartile with the lowest incidence of CCB was selected as the reference group. Model 1 was adjusted for age (continuous variable, years) and sex (male or female). Model 2 was adjusted for age, sex, current smoking (yes or no), current drinking (yes or no), physical activity (active or inactive), and BMI (<24 kg/m2, 24–28 kg/m2, or ≥28 kg/m2).Citation25 Model 3 was adjusted for all covariates in model 2 and additionally adjusted for hypertension, diabetes, hyperlipidaemia, eGFR [continuous, mL/(min·1.73 m2)], hs-CRP (continuous, mg/L), and snoring (ever or never). Model 4 was adjusted for all covariates in model 3 and additionally adjusted for the use of antihypertensive, hypoglycaemic, and lipid-lowering medications (yes or no) at baseline. To fully assess potential nonlinear relationships, the association between the MLR and the risk of CCB and its subtypes was assessed on a continuous scale using restricted cubic splines, where the optimal df was determined by minimising the model’s Akaike information criterion.Citation26
Further analyses were conducted to assess the robustness of the association between the MLR and the risk of CCB. First, stratified analyses based on baseline age (<60 and ≥60 years) and sex were used to examine the consistency of the effect. Second, to evaluate the robustness of our results, sensitivity analyses were conducted by (1) excluding new-onset cardiac conduction block cases within the first 2 years of follow-up; (2) excluding new-onset myocardial infarction, atrial fibrillation, and heart failure during follow-up; (3) using time-dependent Cox regression models in which the level of MLR and covariates were updated at each follow-up and the most recent measurements were used to estimate risk; and (4) performing competing risk regression using the Fine-Gray model to address the potentially confounding issue of death.
Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). All tests were two-sided, and P <0.05 was considered statistically significant.
Results
The cohort consisted of 82,472 participants with a mean age of 51.50 years, and 79.01% were male. The baseline characteristics of the participants based on MLR quartiles are summarised in . Individuals with higher MLR levels were generally older; less likely to be current smokers, drinkers, or snorers; and exhibited higher body mass index and hs-CRP levels but lower eGFR levels.
Table 1 Baseline Characteristics of the Study Population
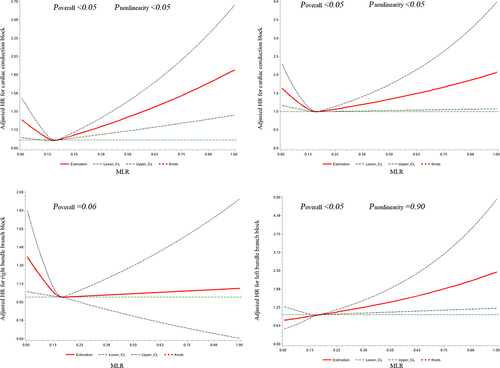
During the median follow-up of 10.4 years, 3222 new-onset CCB events occurred, corresponding to an incidence of 3.76 per 1000 person-years. For the CCB subtypes, there were 949, 1506, and 700 incident cases of AVB, RBBB, and LBBB, respectively. The cumulative incidences of CCB and its subtypes according to the MLR quartile groups are depicted in Supplementary Figure 2 (Log rank test, P<0.05). The cubic spline models () showed a U-shaped association between MLR and the risk of CCB and AVB (Pnon-linearity < 0.05) and a nearly linear association between MLR levels and LBBB risk (Pnon-linearity = 0.90).
Figure 1 Associations of MLR with the risk of cardiac conduction block and its subtypes using restricted cubic spline regression models (model 4). Point estimates (solid line) and 95% confidence intervals (dashed lines) were obtained by restricted cubic spline models.
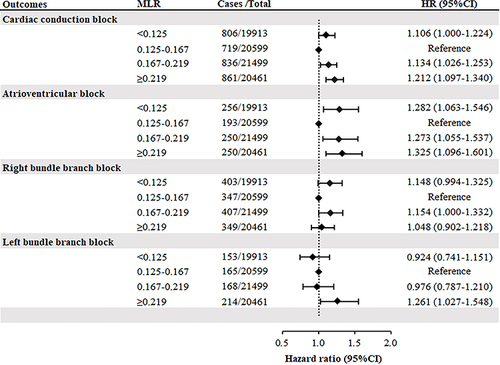
The second MLR quartile had the lowest CCB incidence. Both low and high MLR were associated with an increased risk of CCB. The multivariable adjusted hazard ratio (HR) for the Q1 group was 1.106 (95% CI, 1.000-1.224), and for Q3 and Q4, it was 1.134 (95% CI, 1.026-1.253) and 1.212 (95% CI, 1.097-1.340), respectively (; Supplementary Table 2). For the CCB subtypes, a similar trend was observed for AVB but not for RBBB and LBBB (; Supplementary Table 2). The lowest incidence of the LBBB subtype was observed in the lowest MLR quartile. In the multivariate-adjusted models, elevated MLR levels were significantly associated with an increased risk of LBBB (Supplementary Table 3).
Figure 2 Forest plot for multivariate adjusted hazard ratio for association of monocyte to lymphocyte ratio categories and cardiac conduction block and its subtypes (model 4).
The association between MC or LC alone and incident CCB was further tested in age- and sex-adjusted and multivariate-adjusted models. Neither the MC nor LC levels were associated with the risk of CCB (Supplementary Table 4).
Subgroup analyses of MLR levels and CCB risk are shown in . There were significant interactions among MLR, age, and sex (all Pinteraction<0.05). The association between MLR and new-onset CCB was more prominent in male participants and younger individuals, and these trends were also observed in the AVB subtype.
Table 2 The Associations Between the Level of MLR and the Risk of CCB and Its Subtypes Stratified by Age and Sex
A series of sensitivity analyses were performed to examine the robustness of the association between MLR levels and CCB risk, such as 1) excluding new-onset cardiac conduction block within the first two years of follow-up (n=81218), 2) excluding new-onset myocardial infarction, atrial fibrillation, and heart failure during follow-up (n=80059), and 3) using time-varying MLR as exposures. No significant changes were observed in sensitivity analysis. Meanwhile, the competing risk analysis using the Fine-Grey model showed a trend similar to that of the original analysis (Supplementary Table 5).
Discussion
Our study, based on a large prospective cohort, revealed a U-shaped relationship between MLR and overall CCB risk. Regarding the specific subtypes of CCB, an increase in MLR was significantly associated with an elevated risk of AVB and LBBB, but not RBBB. Conversely, a decrease in the MLR was significantly associated with an increased risk of developing AVB.
MLR is a novel inflammatory biomarker that is less affected by various physiological and pathological conditions and can effectively reflect the balance between inherent and acquired immune responses. Compared with MC or LC alone, MLR provides a more stable and accurate evaluation of the body’s immune status.Citation9,Citation14 In a study with a mean follow-up of 11 years, Hua et al found that MLR was a strong independent predictor of all-cause mortality and cardiovascular mortality in the general population.Citation10 In a retrospective analysis by Liu et al in China, a high MLR was predictive of the risk of ischemic stroke.Citation27 Another study conducted in the Netherlands revealed a strong relationship between MLR levels and heart failure markers and predicted heart failure hospitalisations during the follow-up period in patients with coronary artery disease.Citation28 Our study revealed a significant association between MLR and CCB risk, whereas no significant association was observed between MC or LC alone and CCB. This suggests that the immune status reflected by MLR is closely associated with CCB occurrence.
Previous evidence suggests that inflammatory responses and immune dysfunction play important roles in CCB.Citation13,Citation29 Our study demonstrated that elevated MLR was significantly associated with an increased risk of AVB and LBBB. This phenomenon can be explained using several mechanisms. First, increased chemotaxis of monocytes in the circulatory system, along with enhanced infiltration of macrophages in the heart, plays an important role in the development of cardiac fibrosis,Citation30–33 which serves as a crucial pathological basis for conduction system abnormalities.Citation29,Citation34 Second, age-related degenerative changes in the conduction system contribute to the pathogenesis of the conduction block,Citation35 and ageing is often accompanied by adaptive immune system suppression and chronic low-grade inflammation, reflected as a decreased level of T lymphocytes and an increase in circulatory monocyte level.Citation36 Additionally, a recent Mendelian randomization analysis provided evidence of a causal relationship between elevated MC and decreased LC and the deposition of abnormal cardiac proteins, which are known to be significantly associated with the occurrence of conduction blocks.Citation37,Citation38 Overall, the precise mechanisms underlying the association between high MLR and increased risk of CCB are still not fully understood.
Additionally, our study revealed a significant association between a decreased MLR and the risk of new-onset AVB. Similar results were observed in a previous study focusing on overall bradyarrhythmia events.Citation13 The specific mechanisms underlying the association between decreased MLR and increased risk of AVB remain elusive. Several factors might have contributed to this observation. Monocytes and macrophages play important roles in inflammation initiation and remission.Citation39 When their levels are too low, it may impede the inflammatory remission process, leading to a chronic low inflammatory state that can contribute to cardiac conduction system remodelling. Additionally, certain subsets of lymphocytes, such as Th17 cells, exhibit pro-inflammatory properties. The increased number of these lymphocyte subsets can directly participate in or indirectly modulate cardiac remodelling by releasing various inflammatory mediators.Citation40 Together, decreased MC or increased LC may play an etiological role in the conduction block, but the specific mechanisms require further investigation.
The results of the subgroup analyses in males were consistent with our main findings regarding the association between MLR with CCB and AVB. However, no statistically significant association was observed among females. Similar findings were previously reported by Yang et al, who found that the effect of MLR on bradyarrhythmia was more pronounced in males than in females.Citation13 This difference may be related to the effect of oestrogen, as it can reduce the risk of cardiac fibrosisCitation41 and enhance the antioxidant capacity of the myocardial tissue,Citation42 which may counteract the risk associated with MLR. Stratification analysis by age showed a significant association between higher MLR and the risk of CCB and its subtypes in participants aged < 60 years. This may be partly because ageing itself being a risk factor for developing conduction block,Citation36 which could diminish the effect of MLR in older individuals. This suggests that more attention should be paid to higher MLR in young and middle-aged individuals to prevent the occurrence of CCB.
This study had several major strengths, including a large sample size, long-term follow-up, and the use of multiple sensitivity analyses to verify the stability of the results. Outcome event data were collected through follow-ups rather than relying solely on hospitalisation reports. This approach allows for the identification of subclinical CCB, thereby avoiding underestimation of the true incidence. To the best of our knowledge, this is the first cohort study to examine the association between MLR and CCB. Despite these strengths, it is important to acknowledge the limitations of this study. First, the observational study design made it impossible to establish cause-effect relationships among the investigated variables. Further basic and Mendelian randomization studies are necessary to confirm these findings. Moreover, this was a single-centre study; despite adjusting for possible confounders, residual confounding cannot be ruled out. Finally, the study population was limited to occupational northern Chinese individuals, which may limit the generalizability of the results to other populations or regions.
Conclusion
Our study, based on a large prospective cohort, found a U-shaped association between the MLR and CCB risk. A similar association persisted for AVB; however, a linear association was observed for LBBB. Further studies are required to confirm our findings and explore the underlying mechanisms.
Ethical Approval Statement
The ethics committee of Kailuan General Hospital (approval number: 2006-05). [2006] Yilunzi No. 5.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflict of interest.
Acknowledgments
We thank all the survey teams of the Kailuan Study Group for their contribution and the study participants who contributed their information.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Smits JP, Veldkamp MW, Wilde AA. Mechanisms of inherited cardiac conduction disease. Europace. 2005;7(2):122–137. doi:10.1016/j.eupc.2004.11.004
- Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation. 1998;98(22):2494–2500. doi:10.1161/01.CIR.98.22.2494
- Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB, Prescott E. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J. 2013;34(2):138–146. doi:10.1093/eurheartj/ehs291
- Liu M, Du Z, Sun Y. Prognostic significance of first-degree atrioventricular block in a large Asian population: a prospective cohort study. BMJ Open. 2022;12(4):e062005. doi:10.1136/bmjopen-2022-062005
- Kerola T, Eranti A, Aro AL, et al. Risk factors associated with atrioventricular block. JAMA Netw Open. 2019;2(5):e194176. doi:10.1001/jamanetworkopen.2019.4176
- Stockburger M, Boveda S, Moreno J, et al. Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J. 2015;36(3):151–157. doi:10.1093/eurheartj/ehu336
- Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32(8):991–998. doi:10.1093/eurheartj/ehq497
- Chen L, Liu C, Liang T, et al. Monocyte-to-lymphocyte ratio was an independent factor of the severity of spinal tuberculosis. Oxid Med Cell Longev. 2022;2022:7340330. doi:10.1155/2022/7340330
- Ji H, Niu X, Yin L, et al. Ratio of immune response to tumor burden predicts survival via regulating functions of lymphocytes and monocytes in diffuse large B-cell lymphoma. Cell Physiol Biochem. 2018;45(3):951–961. doi:10.1159/000487288
- Hua Y, Sun JY, Lou YX, Sun W, Kong XQ. Monocyte-to-lymphocyte ratio predicts mortality and cardiovascular mortality in the general population. Int J Cardiol. 2023;379:118–126. doi:10.1016/j.ijcard.2023.03.016
- Lv X, Sun Y, Tan W, et al. NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block. Open Life Sci. 2021;16(1):1240–1251. doi:10.1515/biol-2021-0106
- Nguyen MN, Kiriazis H, Gao XM, Du XJ. Cardiac fibrosis and arrhythmogenesis. Compr Physiol. 2017;7(3):1009–1049.
- Yang X, Zhao S, Wang S, et al. Systemic inflammation indicators and risk of incident arrhythmias in 478,524 individuals: evidence from the UK Biobank cohort. BMC Med. 2023;21(1):76. doi:10.1186/s12916-023-02770-5
- Wang H, Guo Z, Xu Y. Association of monocyte-lymphocyte ratio and proliferative diabetic retinopathy in the U.S. population with type 2 diabetes. J Transl Med. 2022;20(1):219. doi:10.1186/s12967-022-03425-4
- Xu W, Zhao H, Han X, et al. Relationship between early-onset stroke and triglyceride-glucose index among young Chinese adults. Lipids Health Dis. 2023;22(1):3. doi:10.1186/s12944-023-01773-8
- Li J, He D, Yu J, et al. Dynamic status of SII and SIRI alters the risk of cardiovascular diseases: evidence from Kailuan Cohort Study. J Inflamm Res. 2022;15:5945–5957. doi:10.2147/JIR.S378309
- Wu L, Wu M, Zhao D, et al. Elevated high-sensitivity C-reactive protein levels increase the risk of new-onset cardiac conduction disorders. Cardiovasc Diabetol. 2023;22(1):268. doi:10.1186/s12933-023-01987-1
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–e62. doi:10.1016/j.jacc.2008.02.032
- Li J, Huang Z, Hou J, et al. Sleep and CKD in Chinese adults: a cross-sectional study. Clin J Am Soc Nephrol. 2017;12(6):885–892. doi:10.2215/CJN.09270816
- Li H, Qian F, Zuo Y, et al. U-shaped relationship of high-density lipoprotein cholesterol and incidence of total, ischemic and hemorrhagic stroke: a prospective cohort study. Stroke. 2022;53(5):1624–1632. doi:10.1161/STROKEAHA.121.034393
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi:10.1161/01.HYP.0000107251.49515.c2
- Sattar N, Rawshani A, Franzén S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139(19):2228–2237. doi:10.1161/CIRCULATIONAHA.118.037885
- Zou Q, Su C, Du W, et al. Longitudinal association between physical activity, blood lipids, and risk of dyslipidemia among Chinese adults: findings from the China Health and Nutrition Surveys in 2009 and 2015. Nutrients. 2023;15(2):341. doi:10.3390/nu15020341
- Rognant N, Lemoine S, Laville M, Hadj-Aïssa A, Dubourg L. Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes Care. 2011;34(6):1320–1322. doi:10.2337/dc11-0203
- Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
- Meira-Machado L, Cadarso-Suárez C, Gude F, Araújo A. smoothHR: an R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med. 2013;2013:745742. doi:10.1155/2013/745742
- Liu H, Zhan F, Wang Y. Evaluation of monocyte-to-high-density lipoprotein cholesterol ratio and monocyte-to-lymphocyte ratio in ischemic stroke. J Int Med Res. 2020;48(7):300060520933806. doi:10.1177/0300060520933806
- Gijsberts CM, Ellenbroek G, Ten Berg MJ, et al. Effect of monocyte-to-lymphocyte ratio on heart failure characteristics and hospitalizations in a coronary angiography cohort. Am J Cardiol. 2017;120(6):911–916. doi:10.1016/j.amjcard.2017.06.020
- Frimodt-Møller EK, Gottdiener JS, Soliman EZ, et al. Inflammation and incident conduction disease. J Am Heart Assoc. 2023;12(1):e027247. doi:10.1161/JAHA.122.027247
- Refai A, Gritli S, Barbouche MR, Essafi M. Mycobacterium tuberculosis virulent factor ESAT-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front Cell Infect Microbiol. 2018;8:327. doi:10.3389/fcimb.2018.00327
- Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi:10.1016/j.immuni.2016.02.015
- Cheng B, Chen HC, Chou IW, Tang TW, Hsieh PC. Harnessing the early post-injury inflammatory responses for cardiac regeneration. J Biomed Sci. 2017;24(1):7. doi:10.1186/s12929-017-0315-2
- Demoulin JC, Simar LJ, Kulbertus HE. Quantitative study of left bundle branch fibrosis in left anterior hemiblock: a stereologic approach. Am J Cardiol. 1975;36(6):751–756. doi:10.1016/0002-9149(75)90456-7
- Frimodt-Møller EK, Soliman EZ, Kizer JR, et al. Lifestyle habits associated with cardiac conduction disease. Eur Heart J. 2023;44(12):1058–1066. doi:10.1093/eurheartj/ehac799
- Vedantham V. New approaches to biological pacemakers: links to sinoatrial node development. Trends Mol Med. 2015;21(12):749–761. doi:10.1016/j.molmed.2015.10.002
- Berben L, Floris G, Kenis C, et al. Age-related remodelling of the blood immunological portrait and the local tumor immune response in patients with luminal breast cancer. Clin Transl Immunol. 2020;9(10):e1184. doi:10.1002/cti2.1184
- Saunders CN, Chattopadhyay S, Huhn S, et al. Search for AL amyloidosis risk factors using Mendelian randomization. Blood Adv. 2021;5(13):2725–2731. doi:10.1182/bloodadvances.2021004423
- Dostal D, Glaser S, Baudino TA. Cardiac fibroblast physiology and pathology. Compr Physiol. 2015;5(2):887–909.
- Fischer HJ, Finck TLK, Pellkofer HL, Reichardt HM, Lühder F. Glucocorticoid therapy of multiple sclerosis patients induces anti-inflammatory polarization and increased chemotaxis of monocytes. Front Immunol. 2019;10:1200. doi:10.3389/fimmu.2019.01200
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi:10.1016/j.cell.2010.02.021
- Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res. 2003;57(2):388–394. doi:10.1016/S0008-6363(02)00705-8
- Borrás C, Sastre J, García-Sala D, Lloret A, Pallardó FV, Viña J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34(5):546–552. doi:10.1016/S0891-5849(02)01356-4
