Figures & data
Figure 2. Schematic diagram of the respiratory mucus layer.
The mucus covers the respiratory epithelia and consists of two layers: the gel layer of secreted mucins and the periciliary layer of cell-tethered mucins. The gel layer contains soluble mucins MUC5AC and MUC5B. These soluble mucins significantly contribute to this layer’s viscosity and gel-like properties. Cilia on the epithelial surface are rich in MUC1, MUC4, MUC16, and MUC20. Host species differ in genetic makeup and mucin expression, resulting in a species-specific mucin and glycan repertoire on cell surface receptors and in the gel layer. This figure has been created with BioRender.com.
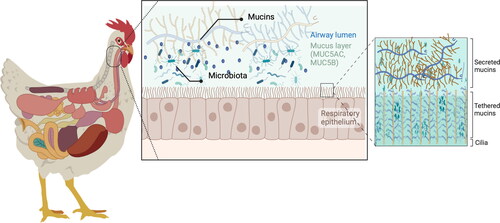
Figure 3. Barrier function, mucus production, mucociliary clearance, and host defenses in the normal and disturbed airway epithelium.
Panel 1. In a healthy stage, the mobile mucus layer forms a 3-dimensional network in the respiratory tract lumen containing secreted mucins and the residing microbiota. The heavily glycosylated and sialylated mucins (both tethered and secreted) create a barrier against pathogens, which are at risk of being expelled by mucociliary clearance after immobilization in the mucus layer.
Panel 2. Despite mucins’ various defense mechanisms, some pathogens have developed strategies to exploit or manipulate mucins (e.g. increase mucus production or modify glycan profiles) to enhance their survival or to evade host immune responses. The absence or presence of glycotopes in mucins, including sialic acid modifications, are likely to affect the infection potential of viruses. This figure has been created with BioRender.com.
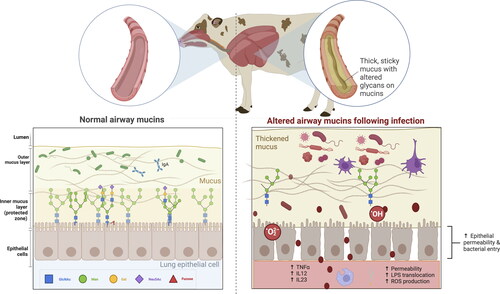
Figure 4. The microbiome-mucin axis in the respiratory tract: mucin damage matters.
Upper panel: The mutualistic relationship between the airway mucins and the microbiota and derived metabolites under eubiosis. On the one hand, the microbiota and their metabolism induce the synthesis of large gel-forming mucins, including encapsulation, glycosylation, changes in fucosylation and sialidation patterns, and thickness. On the other hand, the mucin layer serves as an environmental niche and a food source for the microbiota. The high diversity of gut mucins impacts the gut microbiota composition, diversity, and stability but also influences immune homeostasis.
Bottom panel: The mutualistic relationship between the mucin glycome and the microbiota under a disrupted airway ecosystem and environment. The deterioration of the respiratory mucosal barrier enables virus binding to the cells and the translocation of bacteria and lipopolysaccharides outside the respiratory tract, triggering immune and inflammatory responses, often resulting in increased permeability and, eventually, endotoxemia. Changes in the respiratory barrier integrity involve changes in the abundance, expression, and glycosylation of mucins, and thus immune dysregulation, dysbiosis, and risk of disease onset.
This figure has been created with BioRender.com.
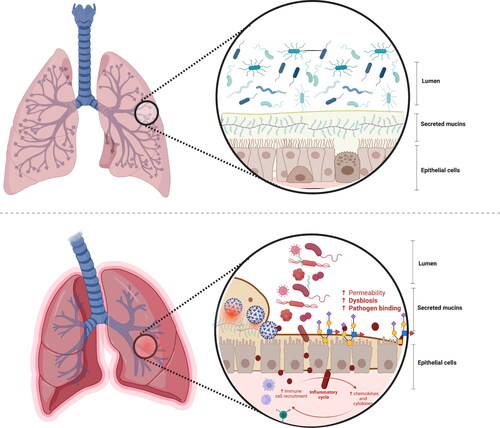
Table 1. First insights into the respiratory mucin-microbiome interplay in livestock.
The tracheal tissue samples
Table 2. Functional implications of mucin regulation by ncRNAs in livestock infected with viruses with respiratory tropism.