Figures & data
Figure 1. Matched cohort creation for all data sources. aPatients eligible for matching are those with prescription of mirabegron or antimuscarinic medications that met all study inclusion criteria (i.e. not excluded due to the listed exclusion criteria). bMatched patients are those that met all study inclusion/exclusion criteria and were PS-matched at a 1:4 ratio. cOnly 1:1 matching was performed in Denmark. For all other data sources, 1:1 up to 1:4 matching was performed. dAfter case adjudication, 2 patients with adjudicated cancer dates before the index date (mirabegron cohort) were removed, together with their 4 matches, from the final analytic file. Abbreviations. CPRD, Clinical Practice Research Datalink; OAB, overactive bladder.
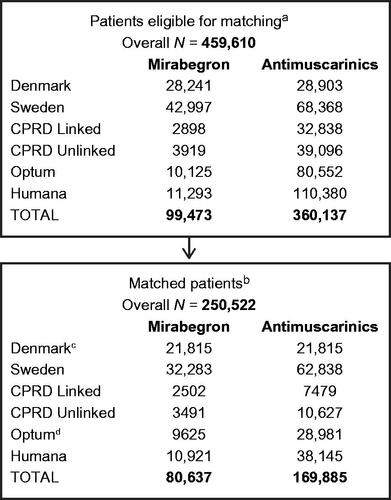
Table 1. Baseline characteristics of mirabegron and antimuscarinic initiators in propensity score matched cohorts, all study populations.
Table 2. Meta-analysis results for hazard ratios of sex-specific composite cancer endpoints, propensity-score matched cohorts, all ages, all study populations by time since treatment initiation.
Figure 2. Summary of incidence rates for bladder cancer in women and men and prostate cancer in men, propensity-score matched cohorts, stratified by time since treatment initiation, all data sources. Abbreviation. IR, incidence rate.
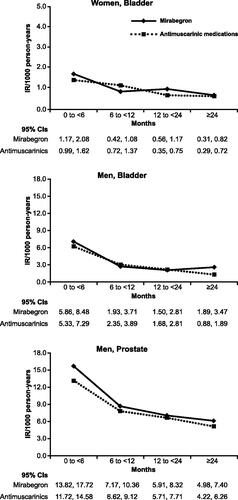
Table 3. Hazard ratios of sex-specific composite cancer endpoints, a comparison within tertiles of mirabegron cumulative dose.
Supplemental Material
Download MS Word (57.4 KB)Supplemental Material Figure S3
Download PDF (603.8 KB)Supplemental Material Figure S2
Download PDF (604.9 KB)Supplemental Material Figure S1
Download PDF (253.4 KB)Data availability statement
This observational study is based on individual patient data. Therefore, we are not able to make this data fully available to the public.
