Figures & data
Table 1. Description of the domains and criteria used in the multiple criteria decision analysis framework.
Figure 1. Average importance estimates for the criteria considered in the decision-making context of OMP public financing. Legend: Error bars represent 95% confidence intervals; Criteria related to the Disease Burden, Therapeutic Value and Economic Value are painted in green, blue and red, respectively.
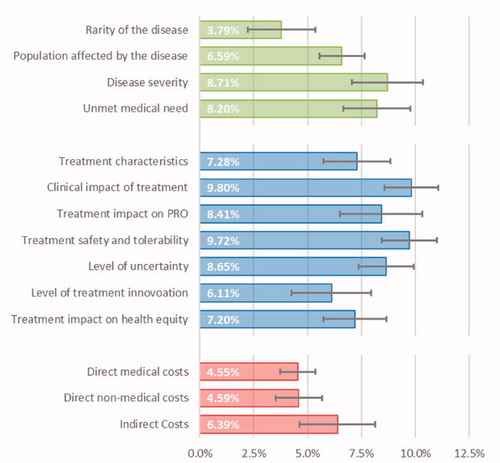
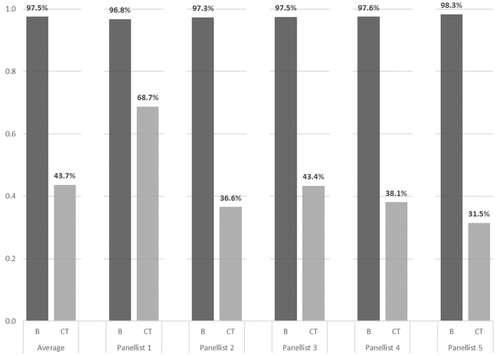
Figure 2. Overall socio-economic and therapeutic value for burosumab (B) and conventional therapy (CT). Legend: Superimposed are the portions of the overall value attributed to the Disease Burden (green), Therapeutic Value (blue) and Economic Burden (red).
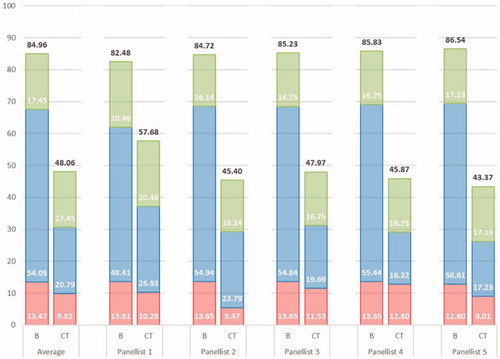
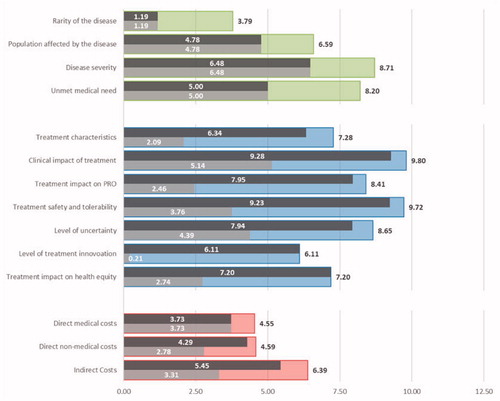
Figure 3. Breakdown of the overall socio-economic and therapeutic value for burosumab and conventional therapy. Legend: Dark grey – Burosumab; Light grey – Conventional therapy; As a reference, the average importance estimates are presented for the criteria related to the Disease Burden (green), Therapeutic Value (blue) and Economic Burden (red).
Supplemental Material
Download MS Word (122 KB)Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.