Figures & data
Figure 1. Protein C and protein S activation mechanisms. on the surface of endothelial cell membrane, thrombin (FIIa) binds to the thrombomodulin (TM) to form FIIa-TM complex, which activates protein C (APC). The affinity and efficiency of protein C is significantly increased after binding to endothelial protein C receptor (EPCR) and after dissociation their anticoagulant activity is increase. APC binds to protein S (PS) as a cofactor to form APC-PS complex and suppresses the tenase and prothrombinase activity by inhibiting FVa and FVIIIa and subsequently reducing the thrombin generation and thrombus formation.
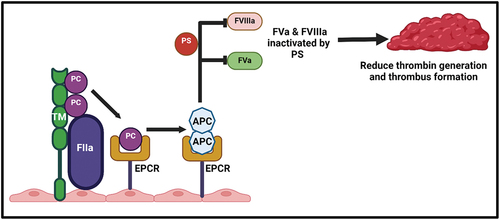
Figure 2. The molecular Structure of Protein S (PS). PS consists of several domains, including the N-terminal Gla domain, a thrombin-sensitive region (TSR), four epidermal growth factor (EGF)-like domains and a sex hormone-binding globulin (SHBG)-like region, which is composed of two laminin G-type (LG) domains. It is essential for the interaction of protein S with the other proteins.
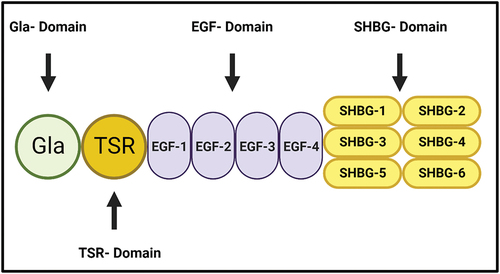
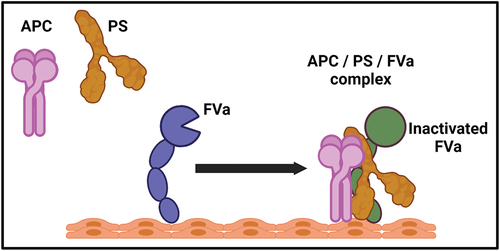
Figure 3. The enhancement of activated protein C with cofactor protein S and FVa. the efficiency of APC is significantly increase in the presence of protein S and FVa together. The recruitment of APC to the phospholipid surface required for FVa and the efficiency of APC increased in the presence of both PS and FVa. The formation of APC/PS/FVa complex is crucial for the regulation of coagulation mechanisms.