Figures & data
Table 1. Demographics and disease characteristics at parent study baseline of patients with NfL measurements.
Figure 2. Integrated changes in mNIS+7 during the parent studies and the Global OLE. (A) APOLLO. (B) Phase II OLE. Data are integrated change from parent study baseline in mNIS+7. (C) Integrated change in Norfolk QOL-DN score during the parent studies and the Global OLE from the APOLLO study. aFor APOLLO patients initiating alternative ATTRv amyloidosis treatment, mNIS+7 assessments after alternative treatment are treated as missing. The APOLLO-placebo arm began patisiran treatment at the start of the Global OLE. APOLLO mNIS+7 parent study baseline (mean (SD)): APOLLO-placebo = 74.6 (37.0); APOLLO-patisiran = 80.9 (41.5) [Citation6]. bPhase II OLE mNIS+7 parent study baseline (mean (SD)): 53.0 (35.6). cData are integrated change from parent study baseline in Norfolk QOL-DN. The APOLLO-placebo arm began patisiran treatment at the start of the Global OLE. APOLLO Norfolk QOL-DN parent study baseline (mean (SD)): APOLLO-placebo = 55.5 (24.3); APOLLO-patisiran = 59.6 (28.2) [Citation6]. ATTRv: hereditary transthyretin (v for variant); BL: baseline; CI: confidence interval; mNIS+7: modified Neuropathy Impairment Score + 7; Norfolk QOL-DN: Norfolk Quality of Life-Diabetic Neuropathy questionnaire; OLE: open-label extension; SD: standard deviation.
![Figure 2. Integrated changes in mNIS+7 during the parent studies and the Global OLE. (A) APOLLO. (B) Phase II OLE. Data are integrated change from parent study baseline in mNIS+7. (C) Integrated change in Norfolk QOL-DN score during the parent studies and the Global OLE from the APOLLO study. aFor APOLLO patients initiating alternative ATTRv amyloidosis treatment, mNIS+7 assessments after alternative treatment are treated as missing. The APOLLO-placebo arm began patisiran treatment at the start of the Global OLE. APOLLO mNIS+7 parent study baseline (mean (SD)): APOLLO-placebo = 74.6 (37.0); APOLLO-patisiran = 80.9 (41.5) [Citation6]. bPhase II OLE mNIS+7 parent study baseline (mean (SD)): 53.0 (35.6). cData are integrated change from parent study baseline in Norfolk QOL-DN. The APOLLO-placebo arm began patisiran treatment at the start of the Global OLE. APOLLO Norfolk QOL-DN parent study baseline (mean (SD)): APOLLO-placebo = 55.5 (24.3); APOLLO-patisiran = 59.6 (28.2) [Citation6]. ATTRv: hereditary transthyretin (v for variant); BL: baseline; CI: confidence interval; mNIS+7: modified Neuropathy Impairment Score + 7; Norfolk QOL-DN: Norfolk Quality of Life-Diabetic Neuropathy questionnaire; OLE: open-label extension; SD: standard deviation.](/cms/asset/b4e102da-4738-41b2-a9af-270046e602c7/iamy_a_2232520_f0002_c.jpg)
Table 2. Change in PND score from parent study baseline and Global OLE enrolment to Global OLE 24 months.
Figure 3. Integrated change in NfL levels during the parent studies and the Global OLE. (A) APOLLO. (B) Phase II OLE. Data are integrated change from parent study baseline in NfL level. The APOLLO-placebo arm began patisiran treatment at the start of the Global OLE. BL: baseline; CI: confidence interval; NfL: neurofilament light chain; OLE: open-label extension.
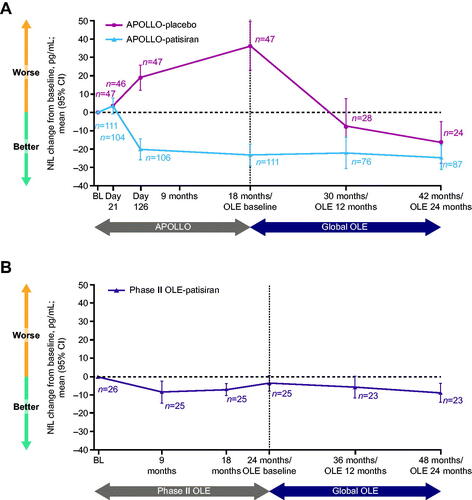
Table 3. Mean NfL levels (SD) in the APOLLO and Global OLE studies.
Table 4. Mean NfL levels (SD) in the phase II OLE and Global OLE studies.
Table 5. Exposure and overall safety in the Global OLE.
Table 6. Integrated exposure-adjusted mortality rates in the parent and Global OLE studies.
Supplemental Material
Download MS Word (243.8 KB)Data availability statement
Anonymized individual participant data that support these results would be made available in a secure-access environment 12 months after study completion and when the product and indication have been approved for no less than 12 months in the US and/or the EU.
Access will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.