Figures & data
Figure 1. Patient disposition. *One patient was initially enrolled and received a dose of AVT02, and subsequently withdrew due to a positive Hepatitis B surface antigen at screening.
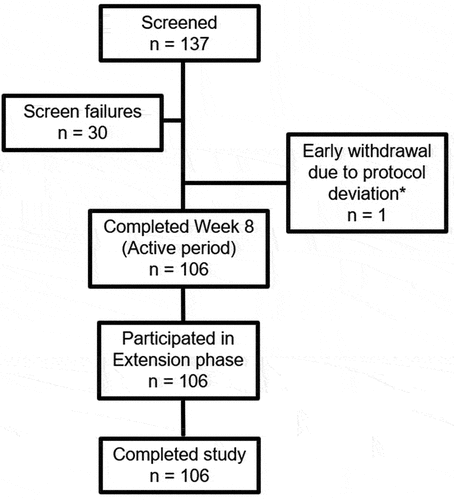
Table 1. Baseline characteristics (Safety population).
Table 2. AI performance (Full analysis population).
Table 3. Ease of use of the AI (Full analysis population).
Figure 2. Mean (± SE) of SDAI Score from baseline by visit (Full analysis set).
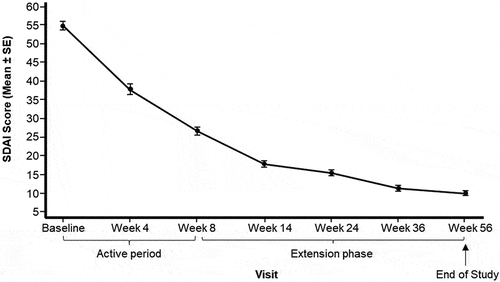
Figure 3. Mean (± SE) of DAS28 CRP from baseline by visit (Full analysis set).
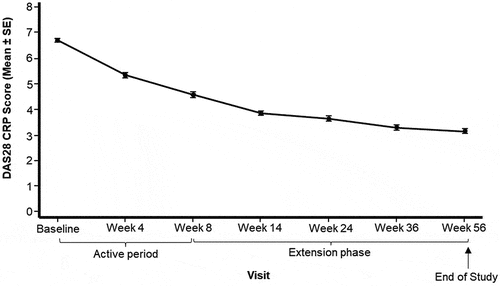
Table 4. ACR-20/-50/-70 response (Full analysis population).
Figure 4. Mean (± SD) AVT02 serum concentration from baseline by visit (Safety set).
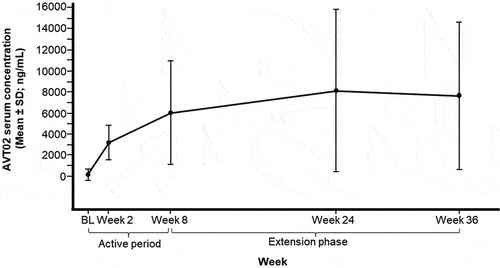
Table 5. Safety (Safety population).
Device_303_study_manuscript_supplemental.docx
Download MS Word (84.7 KB)Data availability statement
The datasets generated and/or analyzed during the current study are available from the sponsor on reasonable request.
