Figures & data
Figure 1. Study consort diagram. Definitions of protocol deviations: for the PK set, protocol deviations were defined as exiting the study before a serum natalizumab level below the lower limit of quantification was reached or two visits were missed before levels had returned to baseline. For the PD α4-integrin sensitivity set, protocol deviation was defined as three consecutive missed visits ahead of %RS returning to baseline. For the PD α4-integrin %RS set, protocol deviation was defined as three consecutive missed visits ahead of %RS returning to baseline or analysis of samples outside of the validated stability window. JCV, John Cunningham virus; PD, pharmacodynamic; PK, pharmacokinetic; ref-NTZ, reference natalizumab; %RS, percentage receptor saturation.
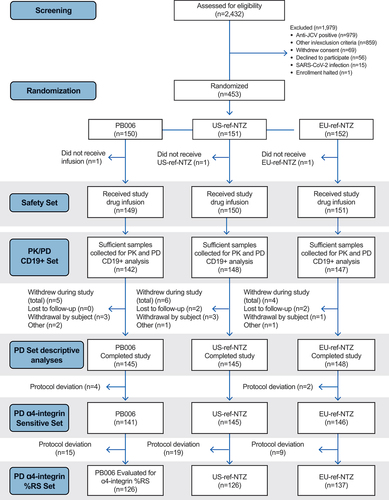
Figure 2. Study design. aSubjects were admitted to the studio center on Day −1 and discharged on Day 3, with subsequent ambulatory visits from Day 5. After the COVID-19 risk assessment, this was amended to discharge on Day 8 with ambulatory visits from Day 15. bPK/PD sampling was performed via blood sampling, once pre-infusion and then at each ambulatory visit over 85 days post-infusion. Subjects returned for ambulatory visits on days 15, 22, 29, 36, 43, 57, 71, 78, and 85. A final follow-up visit was performed to identify any new neurological symptoms potentially suggestive of PML. ADA, antidrug antibody; EOS, end of study; JCV, John Cunningham virus; PD, pharmacodynamic; PK, pharmacokinetic; ref-NTZ, reference natalizumab.
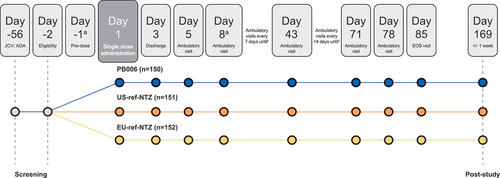
Table 1. Patient baseline characteristics (Safety Set).
Figure 3. Primary PK and PD endpoints. a) Semi-logarithmic plot of mean total serum natalizumab concentration over time for PB006 versus US-ref-NTZ and EU-ref-NTZ (PK set); b) change from baseline in mean (SD) CD19+ cell counts; c) change from baseline in mean (SD) α4-integrin %RS/RO over time for PB006 versus US-ref-NTZ and EU-ref-NTZ (PK/target receptor engagement Set) ref-NTZ, reference natalizumab; RO, receptor occupancy; RS, receptor saturation; SD, standard deviation.
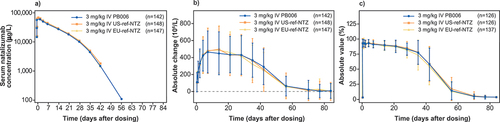
Table 2. Total natalizumab serum concentration (AUC0-inf) (PK Set).
Table 3. AUEC0-12w of baseline-corrected CD19+ cells and AUEC0-12w % α4-integrin RS.
Table 4. AUECbase-neg baseline-corrected CD34+ cells, sVCAM, and sMadcam (PD set).
Table 5. ADA and NAb rates over time across treatment arms.
EOBT PKPD of biosimilar natalizumab PB006 Supplementary Material.pdf
Download PDF (392 KB)Data availability statement
The results of the study reported herein were disclosed within the EudraCT system on 3 March 2022 (2019–003874–15).
