Figures & data
Figure 1 ECSOD modulates oxidative damage and inflammation. ECSOD creates a less oxidizing environment by reducing the highly reactive superoxide radical (O2· −) to the less reactive hydrogen peroxide molecule (H2O2). Other lung antioxidants, such as catalase and peroxidases, further reduce H2O2 to water. Thus, in the presence of ECSOD and other antioxidants there are fewer reactive oxygen species, which creates an anti-inflammatory environment. However, when ECSOD is not present, a more oxidizing environment occurs because O2· − quickly forms other reactive oxygen species, i.e., hydroxyl radical (OH·), peroxynitrite (ONOO−), and bleach (HOCl). A more oxidizing environment promotes pro-inflammatory signaling that can lead to matrix degradation.
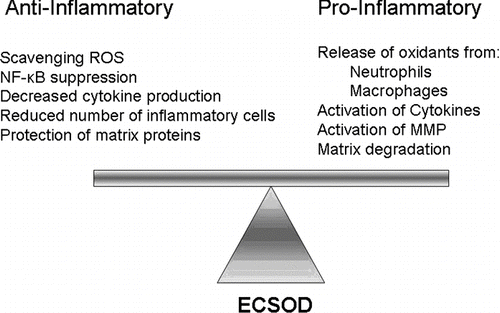
Figure 2 ECSOD protects the extracellular matrix within the lung from oxidant damage. ECSOD has a positively charged binding tail that binds to negatively charged collagen and proteoglycans found in the extracellular matrix. In lung, this results in high levels of ECSOD being associated with extracellular matrix elements found in the thick portions of the alveolar septum. ECSOD is synthesized in alveolar type II cells and is secreted into airway lining fluids. Potential sources of oxidants include inhaled airborne molecules as well as oxidants generated by cellular metabolism and those released by inflammatory cells. Protection of both airway and alveolar septa from oxidative stress is necessary to ensure that the large surface area of the lung and extensive alveolar fibroskeleton remain intact and functional. Cleavage of these structural proteins may play a role in the development of emphysema.
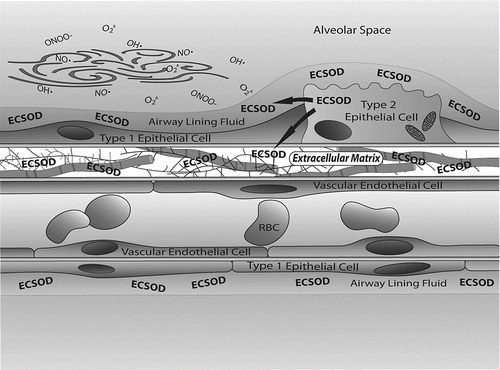
Figure 3 ECSOD functional mutation (R213G) in binding tail. ECSOD has a described mutation at amino acid number 213 in its binding tail where a C to G nucleotide substitution changes the amino acid from an arginine to a glycine. This arginine is one of a cluster of 6 positively charged amino acids in the carboxy terminus of the protein that creates strong binding affinity to heparin and other negatively charged matrix elements. The result of replacing this one arginine with glycine is that the protein has a marked decrease in affinity for the negatively charged extracellular matrix and a marked increase in its circulating levels. This mutation occurs in 4–6% of northern European populations.
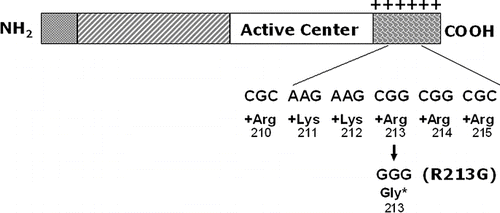
Figure 4 Effect of ECSOD binding tail mutation on COPD risk in smokers. A. This Figure is adapted from Young et al. (Citation[54]) and shows smokers who have not developed COPD have a significantly higher incidence of the G allele at the R213G locus as compared to smokers who developed COPD. This protection from the effects of smoking is presumed to be related to increased levels of ECSOD secreted into the alveolar lining fluid, and thereby, more effective antioxidant protection from airborne oxidants found in cigarette smoke. B. This figure is adapted from Juul et al. (Citation[3]). This prospective study followed individuals for an average of 24 years and found that individuals heterozygous for the ECSOD R213G mutation had significantly lower COPD morbidity and morality rates as compared to individuals that were noncarriers for this ECSOD mutation.
![Figure 4 Effect of ECSOD binding tail mutation on COPD risk in smokers. A. This Figure is adapted from Young et al. (Citation[54]) and shows smokers who have not developed COPD have a significantly higher incidence of the G allele at the R213G locus as compared to smokers who developed COPD. This protection from the effects of smoking is presumed to be related to increased levels of ECSOD secreted into the alveolar lining fluid, and thereby, more effective antioxidant protection from airborne oxidants found in cigarette smoke. B. This figure is adapted from Juul et al. (Citation[3]). This prospective study followed individuals for an average of 24 years and found that individuals heterozygous for the ECSOD R213G mutation had significantly lower COPD morbidity and morality rates as compared to individuals that were noncarriers for this ECSOD mutation.](/cms/asset/5bbb9dd3-1deb-425c-9757-8dbe3a973da0/icop_a_408692_uf0004_b.gif)
Table 1 Summary of genetic association studies of COPD with ECSOD