Figures & data
Table 1. Main parameters and their levels considered for epoxidation reaction.
Table 2. Physicochemical properties of jatropha and jojoba bio-lubricants (Cecilia et al. Citation2020; Mushtaq and Hanief Citation2021) and (Hassan, Hassan, and Youssif Citation2019.).
Table 3. Uncertainty of the instruments used in the study.
Table 4. Iodine values for different set of test samples.
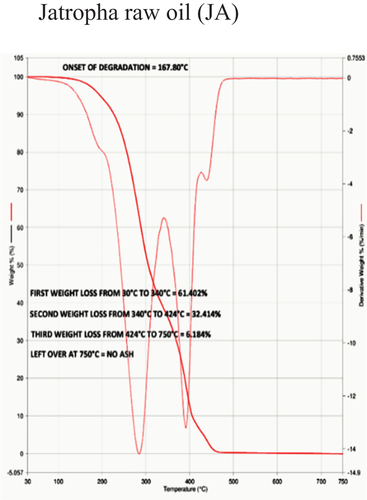
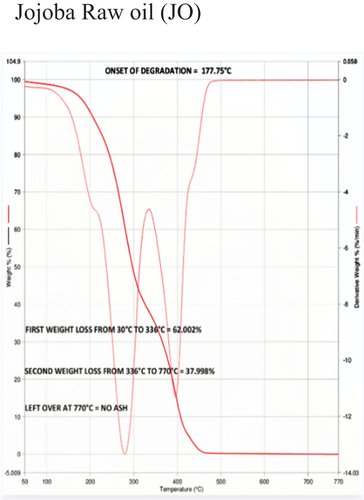
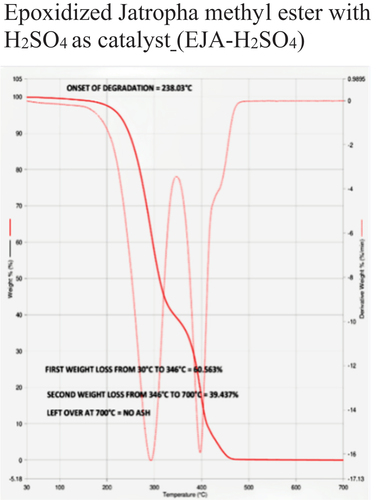
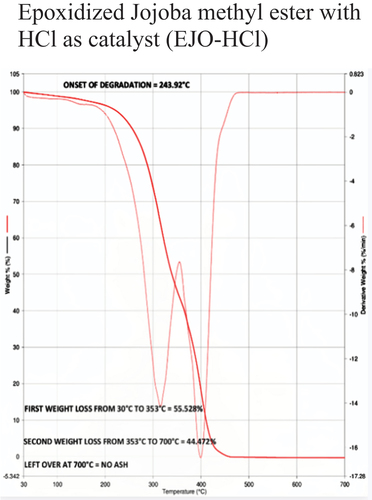
Table 5. Thermal stability data of different samples based on onset of degradation.
Table 6. Dispersion analysis of nanoparticle and TritonX-100 surfactant combinations.
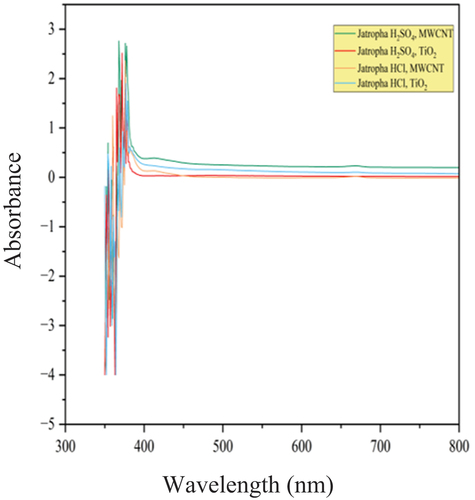
Table 7. Wavelengths of jatropha methyl ester with MWCNT and TiO2 nanoparticles.
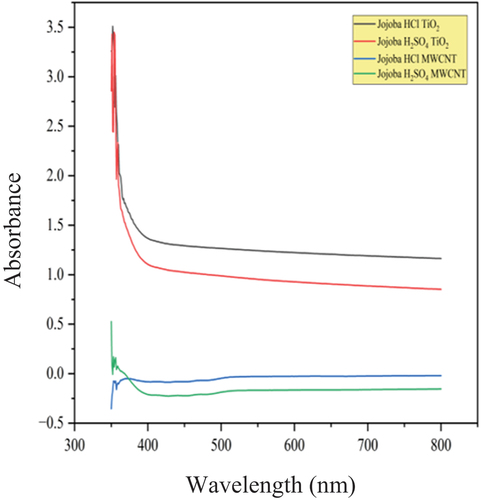
Table 8. Wavelengths of jojoba methyl ester with MWCNT and TiO2 nanoparticles.
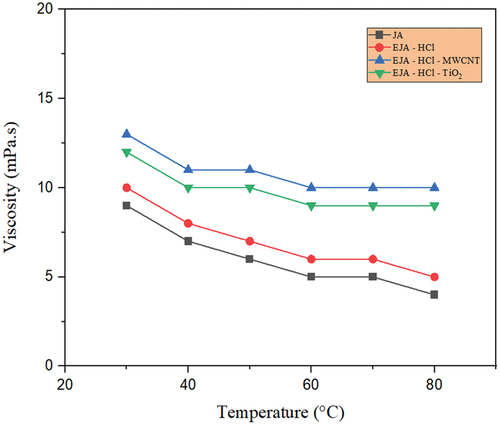
Figure 11. Temperature-dependent viscosity for JA, EJA-H2SO4,EJA - H2SO4- TiO2and EJA - H2SO4- MWCNT.
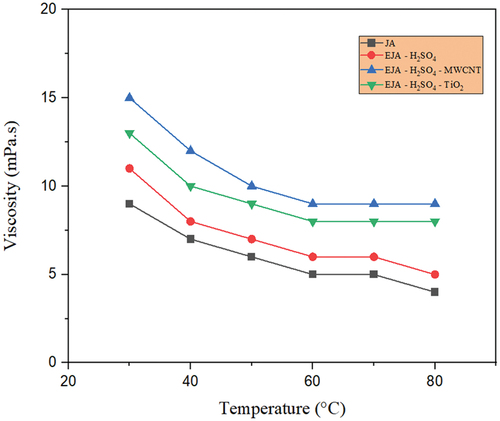
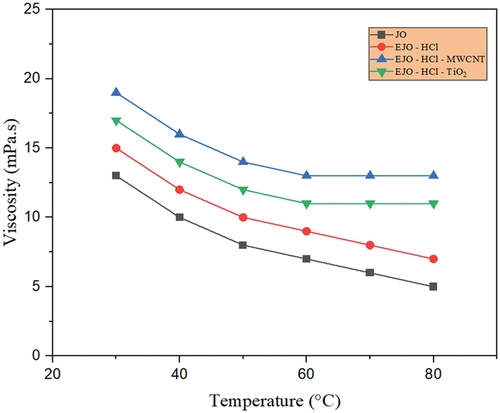
Figure 13. Temperaturedependent viscosity for JO, EJO-H2SO4,EJO – H2SO4- TiO2 and EJO- H2SO4- MWCNT.
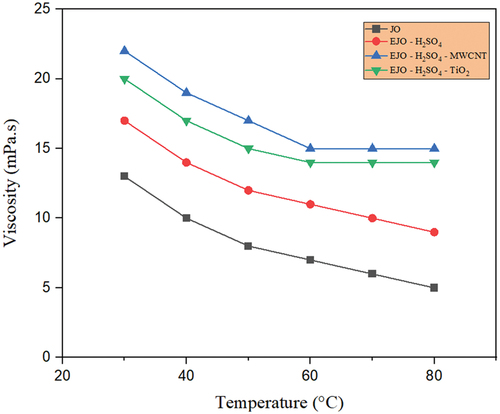
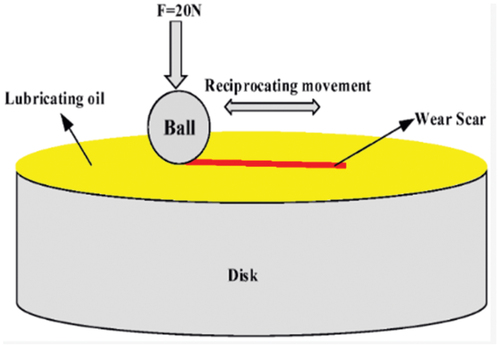
Table 9. Parameters considered for COF and wear rate analysis.
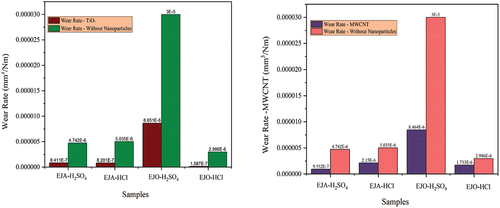
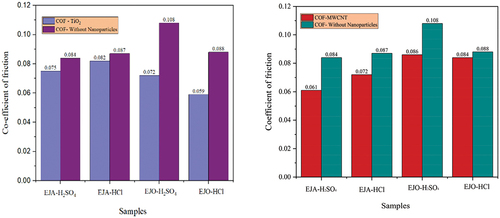
Table 10. COF and wear rate of the samples.
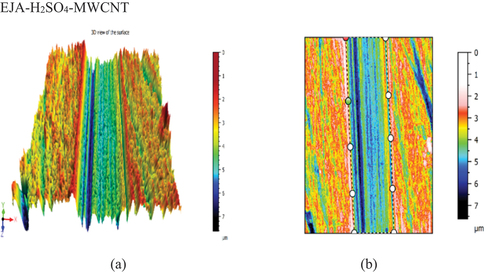
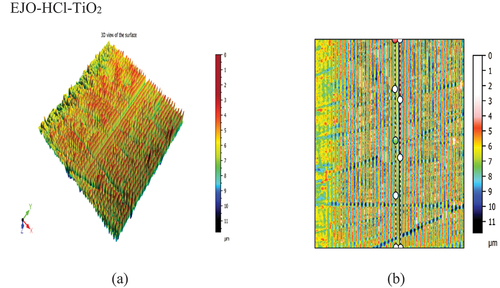
Figure 19. (a) 3Dview of the EJA-H2SO4-MWCNTsample. (b) Cross-section of the EJA-H2SO4-MWCNT sample.